|
|
1.IntroductionThe heart is an energy-demanding organ. Experimental estimation of myocardial mechanical power ranged between 0.1 to from rodents to humans.1, 2 The delicacy of energy balance of the heart relies heavily on adequate perfusion via the coronary arteries. Insufficient oxygen supply, as in the case of coronary artery disease, could result in a 34% increase of myocardial waste heat production and 14% loss of mechanical efficiency.3 On the other hand, oxygen surge can also result in excessive reactive oxygen species (ROS) production, with myocardial damage, as in the case of reperfusion after prolonged ischemia. As ROS might modify biologically important lipids and proteins, reperfusion injury manifests as impaired mechanical performance, ventricular arrhythmia, and permanent myocardial damage. However, the highly reactive nature of ROS makes direct observation of their kinetics at the tissue level very difficult. Recently, optical imaging methods became attractive techniques for myocardium characterization. In such techniques, the light-tissue interactions are measured with a light source, including photon scattering,4 absorption,5 polarization,6 coherence,7 and fluorescence.8, 9, 10 Fluorescence measurements could be applied to indicate many physiologic parameters in cells and tissues, such as intrinsic fluorescence changes,11 oxygenation,12 membrane potential,13 and ROS.14, 15 In this work, we demonstrated an optical imaging technique based on recordings of myocardial oxidative metabolism with an intracellular fluorescent indicator [dihydroethidium (DHE)] in a 2-D format. We applied this method to study the spatial distribution and temporal evolution of ROS dynamics in an isolated perfused rat heart throughout global ischemia reperfusion. In addition, we determined the time sequence of intracellular ROS dynamics and cardiac performance with simultaneous recordings of ECG and intracardiac pressure. 2.MethodologyThe work was conducted in accordance with Taiwan’s. Animal Protection Law (Scientific Application of Animals) of 1998. For preparation of isolated perfused rat hearts 300-g male Wistar rats were heparinized (500 IU) and anaesthetized with an intraperitoneal bolus of pentobarbital . The heart was quickly removed and placed on a Langendorf perfusion apparatus. We used standard Tyrode’s solution with supplementation of oxygen and to perfuse the heart at a constant flow (3-ml/100-g body weight) with a rolling pump. The heart was then submerged in a warm tissue bath at 37.5°C throughout the experiment. The optical mapping setup is shown in Fig. 1 . A Verdi laser was used to provide at as a light source. The laser beam was directed on the anterior surface of the heart with an optic fiber. The reflection of longer wavelengths from the heart were collected through a long-pass filter and captured with a charge-coupled device (CCD) camera. An image stream contained 1000 -pixel frames and was recorded at an average rate of . The spatial resolution was in each image. Image streams were processed with an in-house developing software based on a LabVIEW platform. Fig. 1Setup of optical mapping system. (a) Side and (b) front views of the optical mapping system, consisting of a high-speed CCD camera with a 600-nm long-pass filter (C), a pair of optical fibers to deliver laser (Of), and heart (H) mounted on a Langendorf perfusion apparatus (L). The left ventricle constituted a major part of (c) epicardial mapping surface (d) during the experiment. 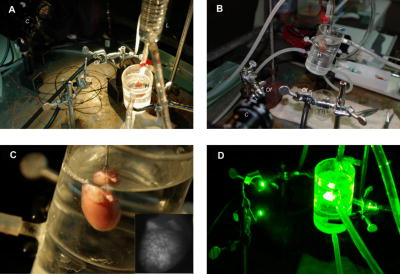 To record intracellular ROS dynamics throughout the global ischemia reperfusion, we adopted dihydroethidium (DHE) for probing intracellular ROS generation. DHE was reported to reflect enhanced oxidative metabolism in hypothermic guinea pig hearts.16 Direct penetration of the chemical into the cell results in cytoplasmic distribution. ROS would oxidize DHE to yield ethidium, which intercalates DNA and stains cell nuclei with red fluorescence (absorption peak of , emission peak of ). Ethidium bound to genomic DNA from calf thymus at an apparent Kd of 17 has been reported. Since the redox equilibrium between DHE and ethidium is determined by cellular ROS, the ethidium-DNA complex-derived red fluorescence would dynamically reflect the concentration of cellular ROS, given that the cellular concentration of ethidium approaches the Kd of the ethidium-DNA interaction. In the experiment, the heart was loaded with DHE in of Tyrode’s solution for , and allowed to stand for 20 to 25 minutes for stable fluorescence measurements. To test the temperature modulation of ROS production, we cooled down the temperature of the perfusate and water bath to for , and then rewarmed them to for another . Image streams were recorded in baseline, hypothermia, and rewarm periods. To record ROS production throughout global ischemia reperfusion, the rolling pump was turned off for of global ischemia and turned on again for of reperfusion. Image streams were recorded at 30, 60, 120, 240, 360, 480, 600, 900, 1200, 1500, and in both ischemia and reperfusion periods. In the control experiment, isolated perfused hearts were subjected to the same imaging scheme for . To study the relationship of DHE fluorescence and cardiac function, we performed another series of experiments to simultaneously record epicardial DHE fluorescence, myocardial electrical activity, and left ventricular pressure. After the heart was mounted to a Langendorf apparatus, a pair of platinum electrodes was positioned in the posterior wall of the left ventricle (LV) to record cardiac electrical activity. A microtip pressure transducer was inserted into the LV via an apical stab wound. Both signals were sampled at , digitized with a digitizer (Digidata 1322, Axon Instruments) and stored for further analysis. In the data analysis, the mean fluorescence intensity (MFI) was used to estimate relative ROS production. MFI is defined as the pixel adjusted average of fluorescence intensity within an image. The average of MFI from 100 images in a stream was assigned to represent that series. The fluorescence signals from a single heart were normalized to their own baseline conditions and expressed as a percentage of baseline. Statistical significance was defined as using the student’s -test. 3.ResultsWe loaded the heart with DHE and did not find any significant changes in ventricular pressure, diastolic pressure, or R-R interval. Epicardial coronary tributaries were unstained, and as a result, the mapping surface had a mosaic pattern. However, no obvious spatial inhomogeneity could be found within the cardiac muscle. Also, the photobleaching effects of the laser on the DHE stain in isolated perfused rat hearts were tested. We did not find any significant fluctuation ( of baseline MFI) of red fluorescence within of continuous excitation. Since the perfusate temperature was reported to modulate cardiac ROS production,16 we adopted this method to test the reversibility of DHE staining. We cooled down the perfusate to for and observed a significant increase of fluorescence intensity. Warming up the perfusate to brought the fluorescence intensity back to baseline (Fig. 2 ). Thus, DHE could dynamically reflect intracellular ROS levels in isolated rat heart preparations. Fig. 2Dynamic response of DHE to temperature modulation. DHE would dynamically reflect the intracellular level of reactive oxygen species (ROS). Temperature modulation of ROS production in isolated perfused heart could be demonstrated with DHE staining. Induction of ROS surge, as indicated by enhanced fluorescence intensity, with hypothermic perfusion (27°) could be reversed with reperfusion with normothermic Tyrode’s solution (see upper row for representative maps, and lower bar diagram for MFI quantification. * indicates using the student’s -test. Data were obtained from four rat hearts). 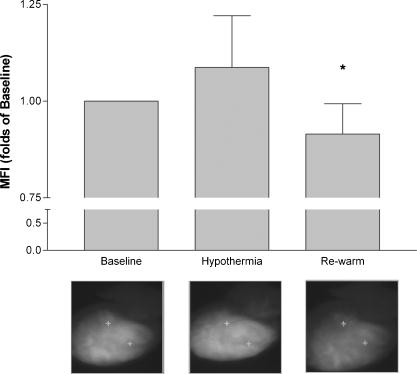 We applied this technique to record temporal sequences of ROS production throughout the global ischemia reperfusion (Fig. 3 ). Ischemia would result in a gradual decrease of MFI. After of ischemia, the MFI reached a steady state ( of baseline, ). Reperfusion would immediately elicit a ROS burst. The peak of burst ( of baseline, ) lasted for and gradually faded out. After of reperfusion, there were no significant differences of MFI compared with the baseline. Fig. 3DHE fluorescence response to ischemia reperfusion. Results of (a) control experiment were obtained from three hearts, showing resistance to photobleaching and small fluctuation in the 60-min recording period. Throughout global ischemia and reperfusion, (b) ischemia would depress epicardial DHE fluorescence, while reperfusion would immediately induce a transient surge. Image streams were recorded at 30, 60, 120, 240, 360, 480, 600, 900, 1200, 1500, and in both ischemia and reperfusion periods. Mean fluorescence intensity was normalized to baseline and expressed in relative folds. Data were acquired from eight hearts. 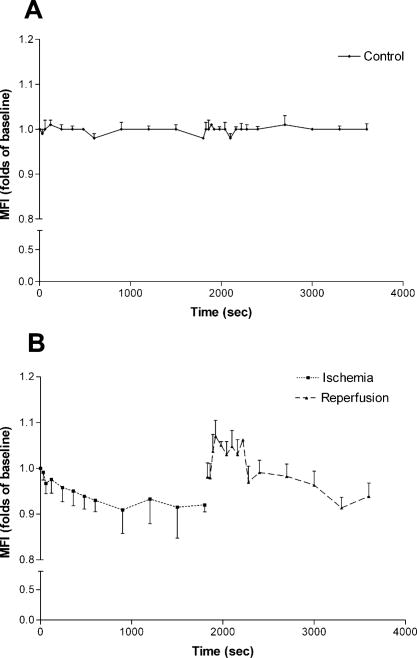 Simultaneous monitoring of ventricular function and ROS production throughout the reperfusion of global ischemia could give us further insight into the role of ROS in the pathogenesis of reperfusion injury. Global ischemia rapidly resulted in decreased developed pressure and left ventricular end systolic pressure (LVESP) within . Meanwhile, left ventricular end diastolic pressure (LVEDP) gradually increased. The effect was accompanied with a prolonged R-R interval and finally electrical silence after . DHE fluorescence also gradually decreased [Fig. 3b], but the process took longer to reach a steady state in spite of the earlier cessation of spontaneous electrical activity and contraction. On reperfusion, the heart immediately broke electrical silence into fibrillating activity and a homogenous DHE fluorescence elevation could be seen immediately. At the end of of reperfusion, the restoration of ventricular contraction and the organization of ventricular tachycardia were accompanied with the normalization of DHE fluorescence. A typical series is shown in Fig. 4 . Fig. 4Cardiac function and DHE fluorescence. A representative series of simultaneous intraventricular pressure, electric activity, and epicardial DHE fluorescence recording throughout global ischemia reperfusion was demonstrated. The left column is the timing of recording; the middle column is the DHE fluorescence intensity maps; the right column is the pressure (upper half) and ECG (lower half) traces. Reperfusion for would return DHE fluorescence to the baseline level, but it would only partially reverse the ischemia-induced acute cardiac dysfunction. 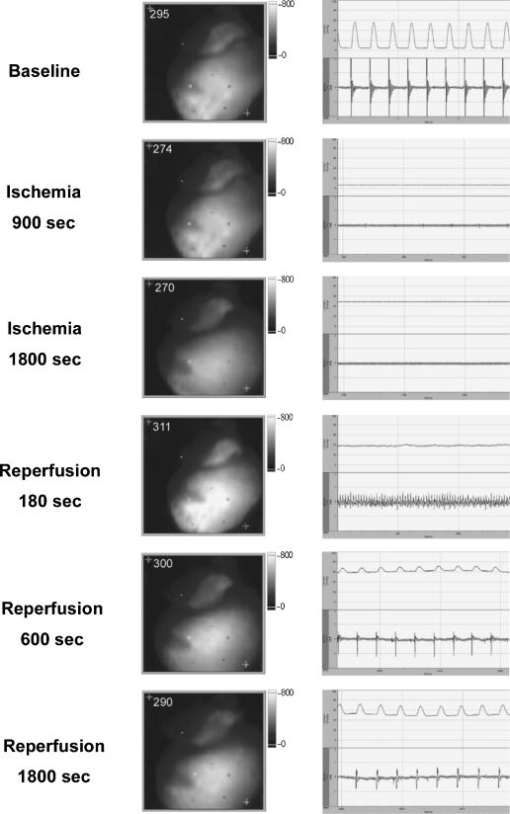 4.DiscussionIn the present study, we demonstrated that optical mapping of myocardial ROS production is feasible. Also, we demonstrated the time sequence of cardiac functional alteration and ROS production throughout global ischemia reperfusion in isolated perfused rat hearts. We found that after reperfusion, the initiation of ventricular arrhythmia was accompanied with an elevation of ROS production. Attenuated ROS surge could be seen after of reperfusion, and acute cardiac dysfunction, such as deterioration of pressure generation and electrical instability, was also partially alleviated. ROS played an important role in cardiovascular biology. All of the cell types in the cardiovascular system are capable of generating ROS. The source of cellular ROS includes mitochondrial oxidative phosphorylation, activity of NADPH oxidase and xanthine oxidase, auto-oxidation of catecholamines, and uncoupling of synthase.18 Their reactive nature could result in oxidative modification of biomolecules and initiate signal cascades to modify cellular function. ROS have been reported to modulate ion flux, calcium handling, energy homeostasis, vasomotor function, hypertrophy, gene expression, and the signaling pathways of growth factors and cytokines,19 and therefore have great impacts on cardiac function. Numerous experimental data suggested the association between an increased oxidative burden and various cardiovascular pathologies, including atherosclerosis, ischemic heart disease, myocardial stunning, reperfusion injury, and heart failure.19 However, a lack of consistent efficacy of antioxidants in large-scale clinical trials 20, 21, 22, 23 raised questions about the pathogenic role of ROS. To solve the controversy, direct measurement of tissue ROS production throughout the disease process would be of great value. Currently available methods to analyze tissue oxidative burdens rely on quantification of biomarkers, such as thiobarbituric acid reactive species (TBARS) and 8-epi-isoprostane. Although widely used, there are still concerns in the preparation, analysis, specificity, and sensitivity of these methods.24 In addition, these methods could not demonstrate physiologic or pharmacologic responses in a single preparation. Optical imaging methods represent another approach to address this issue. The optical mapping technique was traditionally used to study the behavior of electric excitation wavefronts in the heart. As fluorescent indicators of ROS production were widely used to address free-radical biology at the cellular level,25, 26 their application in tissue levels has been explored with optic fiber spectrofluorophotometry.15 In our study, we extended to the 2-D analysis of spatial distribution and temporal evolution of ROS production. Motion-related registration artifacts have been minimized by a high sampling rate. Reduced energy demand for electric-contraction coupling and basal metabolism might account for decreased MFI during the of ischemia. Reoxygenation-associated mitochondrial respiratory bursts might attribute to excessive ROS production during the of reperfusion. Normalization of ROS production could only partially reverse the reperfusion-induced acute cardiac dysfunction. This suggests that factors other than excessive ROS production could be involved in the pathogenic mechanism of reperfusion injury. There are some limitations of this study. First, the spatial resolution of the system was about , which meant each pixel contained 10 to 30 cardiomyocytes. Reflective confocal intravital microscopy would provide much better planar resolution and would enable us to study the contribution of delicate structures, such as endothelial cells and leukocytes. Second, we excluded leukocytes and platelets in the perfusate, which might attenuate the magnitude of reperfusion injury.27, 28 Finally, ethidium was a potential carcinogen, and therefore, its application for diagnostic use in humans would be limited. AcknowledgmentsThis work was supported by grants NSC92-2314-B-002-289 and NSC92-2314-B-002-220 from the Taiwan National Science Council. The authors also wish to extend their heartfelt gratitude to T. Z. Wu for her technical assistance. ReferencesC. L. Gibbs,
“Cardiac energetics: sense and nonsense,”
Clin. Exp. Pharmacol. Physiol., 30 598
–603
(2003). 0305-1870 Google Scholar
D. S. Loiselle and
C. L. Gibbs,
“Species differences in cardiac energetics,”
Am. J. Physiol., 237 H90
–H98
(1979). 0002-9513 Google Scholar
J. T. Stewart,
L. A. Simpson,
R. E. Smith,
C. Callicott, and
A. I. Camm,
“Left ventricular energetics: heat production by the human heart,”
Cardiovasc. Res., 27 1024
–1032
(1993). 0008-6363 Google Scholar
Y. M. Wang,
C. W. Sun,
C. K. Lee,
C. W. Lu,
“Comparisons of optical scattering properties between biological tissues and a phantom with time, aperture, and angle gating,”
Opt. Express, 12 1157
–1168
(2004). https://doi.org/10.1364/OPEX.12.001157 1094-4087 Google Scholar
S. P. Nighswander-Rempel,
R. A. Shaw,
B. Kuzio, and
V. V. Kupriyanov,
“Detection of myocardial cell damage in isolated rat hearts with near-infrared spectroscopy,”
J. Biomed. Opt., 9 779
–787
(2004). https://doi.org/10.1117/1.1751125 1083-3668 Google Scholar
C. W. Sun,
L. S. Lu,
C. C. Yang,
Y. W. Kiang, and
M. I. Su,
“Myocardial tissue characterization based on the time-resolved Stokes-Mueller formalism,”
Opt. Express, 10 1347
–1353
(2002). 1094-4087 Google Scholar
I. K. Jang,
G. L. Tearney,
B. MacNeill,
M. Takano,
“In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography,”
Circulation, 111 1551
–1555
(2005). https://doi.org/10.1161/01.CIR.0000159354.43778.69 0009-7322 Google Scholar
I. R. Efimov,
V. P. Nikolski, and
G. Salama,
“Optical imaging of the heart,”
Circulation, 94 21
–23
(2004). 0009-7322 Google Scholar
V. K. Ramshesh and
S. B. Knisley,
“Spatial localization of cardiac optical mapping with multiphoton excition,”
J. Biomed. Opt., 8
(2), 253
–259
(2003). https://doi.org/10.1117/1.1559831 1083-3668 Google Scholar
G. A. MacGowan,
C. Du,
V. Glonty,
J. P. Suhan,
“Rhod-2 based measurements of intracellular calcium in the perfused mouse heart: Cellular and subcellular localization and response to positive inotrop,”
J. Biomed. Opt., 6
(1), 23
–30
(2001). https://doi.org/10.1117/1.1316091 1083-3668 Google Scholar
G. Salama,
R. Lombardi, and
J. Elson,
“Maps of optical action potentials and NADH fluorescence in intact working hearts,”
Am. J. Physiol., 252 H384
–H394
(1987). 0002-9513 Google Scholar
B. Chance,
“On the mechanism of the reaction of cytochrome oxidase with oxygen,”
Ann. N.Y. Acad. Sci., 244 163
–173
(1975). 0077-8923 Google Scholar
M. Morad and
G. Salama,
“Optical probes of membrane potential in heart muscle,”
J. Physiol. (London), 292 267
–295
(1979). 0022-3751 Google Scholar
C. P. LeBel,
H. Ischiropoulos, and
S. C. Bondy,
“Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress,”
Chem. Res. Toxicol., 5 227
–231
(1992). https://doi.org/10.1021/tx00026a012 0893-228X Google Scholar
L. G. Kevin,
A. K. Camara,
M. L. Riess,
E. Novalija, and
D. F. Stowe,
“Ischemic preconditioning alters real-time measure of radicals in intact hearts with ischemia and reperfusion,”
Am. J. Physiol. Heart Circ. Physiol., 284 H566
–H574
(2003). 0363-6135 Google Scholar
A. K. Camara,
M. L. Riess,
L. G. Kevin,
E. Novalija, and
D. F. Stowe,
“Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart,”
Am. J. Physiol. Heart Circ. Physiol., 286 H1289
–H1299
(2004). 0363-6135 Google Scholar
N. W. Luedtke,
J. S. Hwang,
E. Nava,
“The DNA and RNA specificity of eilatin complexes as compared to eilatin and ethidium bromide,”
Nucleic Acids Res., 31 5732
–5740
(2003). 0305-1048 Google Scholar
C. F. Mueller,
K. Laude,
J. S. McNally, and
D. G. Harrison,
“Redox mechanisms in blood vessels,”
Arterioscler., Thromb., Vasc. Biol., 25 274
–278
(2005). 1079-5642 Google Scholar
F. J. Giordano,
“Oxygen, oxidative stress, hypoxia, and heart failure,”
J. Clin. Invest., 115 500
–508
(2005). 0021-9738 Google Scholar
C. H. Hennekens,
J. E. Buring,
J. E. Manson,
M. Stampfer,
“Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease,”
N. Engl. J. Med., 334 1145
–1149
(1996). 0028-4793 Google Scholar
D. D. Waters,
E. L. Alderman,
J. Hsia,
B. V. Howard,
“Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial,”
JAMA, J. Am. Med. Assoc., 288 2432
–2440
(2002). 0098-7484 Google Scholar
M. Boaz,
S. Smetana,
T. Weinstein,
Z. Matas,
“Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease: randomised placebo-controlled trial,”
Lancet, 356 1213
–1218
(2000). 0140-6736 Google Scholar
R. M. Salonen,
K. Nyyssonen,
J. Kaikkonen,
E. Porkkala-Sarataho,
“Antioxidant supplementation in atherosclerosis prevention study: Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression,”
Circulation, 107 947
–953
(2003). 0009-7322 Google Scholar
K. K. Griendling and
G. A. FitzGrerald,
“Oxidative stress and cardiovascular injury,”
Circulation, 108 1912
–1916
(2003). 0009-7322 Google Scholar
Z. Xie,
P. Kometiani,
J. Liu,
“Intracellular reactive oxygen species mediate the linkage of to hypertrophy and its marker genes in cardiac myocytes,”
J. Biol. Chem., 274 19323
–19328
(1999). 0021-9258 Google Scholar
E. A. Konorev,
H. Zhang,
J. Joseph,
M. C. Kennedy, and
B. Kalyanaraman,
“Bicarbonate exacerbates oxidative injury induced by antitumor antibiotic doxorubicin in cardiomycytes,”
Am. J. Physiol. Heart Circ. Physiol., 279 H2424
–H2430
(2000). 0363-6135 Google Scholar
A. M. Lefer,
B. Campbell, and
Y. K. Shin,
“Effects of a metalloproteinase that truncates P-selectin glycoprotein ligand on neutrophil-induced cardiac dysfunction in ischemia/reperfusion,”
J. Mol. Cell. Cardiol., 30 2561
–2566
(1998). 0022-2828 Google Scholar
J. G. Kingma Jr., S. Plante, and
P. Bogaty,
“Platelet GPIIb/IIIa receptor blockade reduces infarct size in a canine model of ischemia-reperfusion,”
J. Am. Coll. Cardiol., 36 2317
–2324
(2000). 0735-1097 Google Scholar
|

