|
|
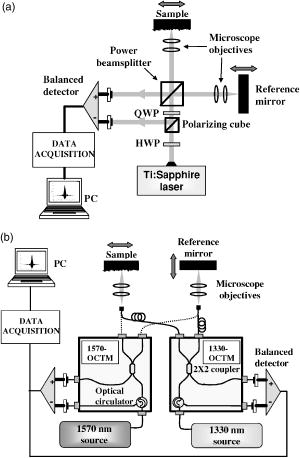
1.IntroductionOptical coherence tomography (OCT) is an imaging technique that appeared about ago.1 It is based on the interferometric analysis of low-coherence light backscattered from inhomogeneities inside a tissue. It can provide in-depth profiles of tissues with a resolution of the order2 of . Since it offers a noninvasive and noncontact approach, OCT is gaining acceptance as a prominent medical imaging technique especially for ophthalmic applications. Recent developments in the OCT domain aimed at improving several characteristics such as resolution, signal-to-noise ratio (SNR), or penetration depth. These are key parameters for improving OCT efficiency in the detection of pathologies such as tumors inside highly scattering organs and tissues (e.g., skin, heart, or liver). These parameters are strongly related to the light source characteristics, especially to its emission wavelength and bandwidth . As shown by the equation , a light source with a broader bandwidth results in an improvement of the OCT axial resolution (also called the coherence length of the light source). As there is a strong spectral dependency of tissue optical properties such as absorption and scattering coefficients, OCT penetration depth and contrast are mainly related to the emission wavelength of the source. Previous studies were based on the comparison of images obtained with two different wavelengths, mostly at both 800 and , showing3, 4 a better penetration depth at . Several authors predicted penetration depths in several tissues for various optical sources, but these results were not confirmed experimentally.5 In this paper, we propose a more complete investigation with comparison of images obtained with three different optical sources at 810-, 1330-, and emission wavelengths with a better coverage of the near-IR band. Along with a qualitative comparison of images, we used a graphical method6 to measure attenuation and backscattering coefficients from OCT in-depth profiles of miscellaneous in vitro biological samples. From these measurements, we deduced comparisons of the performance of each emission wavelength, especially on the penetration depth and backscattering intensity for the studied samples. The influence of coherence length is then taken into account to make a more detailed comparison of the sources used in this experiment. 2.Experimental SetupOur OCT setup is based on three different sources used with different Michelson interferometers. The first source is a Kerr-lens mode-locking, ultrashort pulsed Ti:sapphire laser that gives a maximum bandwidth of at an about central wavelength. In this configuration, we measure a coherence length of . The second one uses nonlinearly transformed light from a high-power laser to produce broadband, high-power continuous light in the near-IR region (BBS series from MPB Communications, Inc.). Its emission spectrum is centered near with a bandwidth, leading to a coherence length of . The third source is a superluminescent diode (SLD) from JDS Uniphase, Inc. that emits a bandwidth spectrum at a central wavelength. Its coherence length is equal to . Spectra of the sources are shown in Fig. 1 , along with the OCT response of each system measured with a mirror surface used as a sample inside the interferometers. Note here that although the coherence lengths of each source were measured from the FWHM of the OCT traces, the presence of sidelobes on each side may deteriorate the tissue profile quality. These sidelobes are related to the deviation of the source spectrum from a perfect Gaussian shape. We can see from Fig. 1b that sidelobes are large for the source (where the sidelobe amplitude is almost 30% of the total OCT amplitude) and for the SLD (where the width of each sidelobe is about ). For the Ti:sapphire laser, the spectrum is close to a Gaussian such that the sidelobes are narrower than for the other sources. Fig. 1(a) Spectra of the sources used for experiments, (b) OCT response obtained with each source and setup, and (c) OCT response of each setup treated with the deconvolution method presented in Sec. 3.  The layout of the setup is depicted in Fig. 2 . A free-space interferometer is used with the Ti:sapphire laser. For the 1330- and sources, we use customized fiber interferometer modules (1330-OCTM and 1570-OCTM). SMF-28 fibers with polarization controllers were used. Each of these modules is optimized to fit the spectral domain of each source. For each source light is focused onto the samples with near-IR, achromatic low-numerical aperture (NA, 0.25 NA, magnification) microscope objectives in order to get a wide depth of field. Data acquisition consists of balanced detection, analog envelope detection and analog-to-digital (A∕D) conversion with a digital multimeter. For efficient balanced detection, the two incoming waves on the detector must be in phase quadrature. This condition is achieved with a polarizing cube coupled with a HWP and a QWP in the free-space interferometer, and with a broadband fiber circulator in the fiber interferometer. The sample under test is covered with a thin glass coverslip. It is then placed inside a chamber filled with saline solution for better preservation. To match the SNRs of each source as closely as possible, the following procedure was implemented before the experiments. First, we inserted a biological tissue in the sample arm of the interferometer. The reflected power from the reference and the sample arm were then approximately equalized by inserting neutral density filters in both arms. Care was taken that the thickness of density filters was the same in both arms in order to limit the dispersion mismatch. We checked that this ratio was roughly the same for each source. Then, the OCT response of each system was measured by placing in the sample arm a totally reflective gold mirror whose reflectivity was assumed to be the same for each wavelength used throughout. The input power of each source was independently modified with neutral density filters to achieve the same OCT response amplitude for each system, thus removing the wavelength dependence of the detector quantum efficiency. Hence, under the shot-noise limit induced by balanced detection, the SNR difference between the three systems is minimized. 3.Results and DiscussionLight propagation inside the sample is assumed to be described by the Beer-Lambert law: where and are the incident optical power and the transmitted power after propagating through a medium thickness of , respectively. The coefficient represents the whole attenuation of the medium. For shallow depths in turbid tissue, the approximation can be made that the absorption and scattering coefficients and are the main contributions to attenuation:6 Obviously, the penetration depth is defined as the reciprocal of the attenuation coefficient.For a qualitative comparison of penetration depth and contrast, images of in vitro samples were made with these three different sources. The samples were placed in such a way that the focus is located in the middle of the scan. For better visibility we chose inhomogeneous tissue. On the top row of Fig. 3 we show images from a fresh chicken heart ventricle wall. The bottom row shows a profile from a mouse earlobe that was fixed in formalin just after sacrifice. Because of the numerous discontinuities generated by cartilage layers inside this sample, the “ringing” effect induced by important sidelobes for 1330- and sources can mask a lot of details. As a matter of fact, sidelobes can be a significant issue especially in the case of inhomogeneous tissues where discontinuities with various reflection coefficients are located very close to each other: sidelobes from strongly reflected signals can mask weakly reflected signals. Thus, we treated the mouse ear images obtained at 1330 and with a deconvolution method to remove the sidelobes from the OCT signal and thus improve image sharpness.7 While this method serves the same purpose as spectral shaping methods,8 it is based on an iterative deconvolution of the raw profile with the system OCT responses that are shown in Fig. 1b. As shown in Fig. 1c, where the deconvolution method was applied to the OCT traces of each source and system, it can bring an efficient sidelobe extinction of at least , while the main OCT signal amplitude remains unchanged. The chicken heart sample is more homogeneous: within the sample, the masking effect induced by the sidelobes is weak and needs no special treatment. On the left, the images obtained with the Ti:sapphire laser show accurate details at shallow depths not only because the depth resolution is higher than with the other sources, but also because the backscattering seems to be higher. As these tissue are inhomogeneous, the nature of the backscattered power cannot be clearly defined: it mainly comes on the one hand from diffuse reflection generated by a large-scale index mismatch at inhomogeneities,9 and on the other hand from the statistical process of light scattering by an homogeneous ensemble of particles. Nevertheless we can notice that the 1330- and wavelengths still benefit from higher penetration depths (i.e., lower attenuations) despite the fact that absorption is assumed to be higher for these wavelengths: as can be seen in the images, the second layer of the ventricle wall is more visible at 1330 and , and the backscattered intensity is quite homogeneous over the whole mouse ear thickness at 1330 and , while it slightly decreases5, 10 with depth at . This statement implies via Eq. 2 that the sum of statistical and diffuse scattering decreases as wavelength increases and that its contribution to may be more important than absorption. The semiempirical law stating that statistical scattering behaves as an inverse power of the wavelength may be partly responsible for this.4, 11 Nevertheless, there is also a strong contribution from diffuse reflections, so that we cannot draw an unambiguous conclusion about the backscattered light behavior as a function of wavelength. Fig. 3OCT profiles of (a), (b), and (c) a chicken heart ventricle wall and (d), (e), and (f) mouse earlobe. From left to right, Ti:sapphire laser, broadband source, and SLD. Each source was assigned a different color map that refers to its emission wavelength. Light is incident from the left. 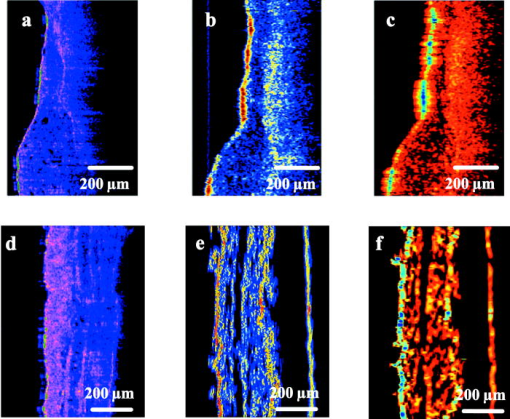 In fact, a qualitative comparison is obviously limited by numerous factors. From these images, it is very difficult to compare the penetration depths for 1330- and sources and to define which one is the lowest. Furthermore, the aperture function of the microscope objectives is not taken into account. In other words, because the beam is focused inside the sample, the mixing of the probe and the reference beams in the interferometer is less efficient for in-depth cells that are far from focus, giving rise to a reduced OCT signal. The Rayleigh range is especially important for comparisons in Fig. 3, since it gives the distance from the focused beam waist for which the OCT amplitude decreases by a factor. Since the same microscope objectives were used for the three sources, one might expect a lower Rayleigh range for . As a consequence, it may be partly responsible for lower penetration depths observed on the images made with Ti:sapphire laser. Speckle noise is also a limiting factor since it introduces a granular image aspect that depends on the source coherence length. It is thus very difficult to make clear and definitive conclusions, and someprecise measurements of attenuation and backscattering are necessary for more accurate comparisons. For this, we apply Eq. 1 to the reference OCT power measured from the glass-sample interface and the OCT power measured from an optical depth inside the sample. We obtain the following formula:3 where is the backscattering coefficient, and and are the reflection and transmission coefficients of the glass-sample interface and are given byThe refractive index of glass is assumed to be equal to 1.51 for and 1.50 for 1330 and . The refractive index of the sample is measured for each source by placing the tissue that was previously soaked in a saline solution inside a cell made of a front glass coverslip and an end mirror. We then compared the OCT-measured optical thicknesses of the same cell with and without the tissue.12 The refractive indices of the tissues were obtained by averaging the thickness ratio over the whole cell. For tissues studied in this paper, typical values between 1.39 and 1.42 were obtained with a typical error of 1%. An important point in Eq. 4 it that depends on the coherence length . In a turbid media, adjacent scattering centers are separated by distances much shorter than the coherence length. Because of the width of the OCT trace assigned to the reflection of each scattering center the total OCT signal amplitude measured at one point of the depth scan arises not only from the scattering center at this point, but also from contributions of all the scattering centers that are located at distances less than from this point.6 When the coherence length is much less than the photon mean free path in the tissue, and under the approximation of the Gaussian shape of the OCT function by a rectangular function of width , this assertion is expressed through the factor in Eq. 3. Consequently, the measured backscattered intensity from one point inside the tissue depends on the coherence length. Note that and are corrective factors that represent the aperture function of the interferometer at and at the glass-sample interface, respectively. Hence, is the ratio of the reduced OCT amplitude measured at and the maximum OCT amplitude measured at the focus point. This was estimated in free space by measuring OCT traces from a hard reflection off a totally reflective mirror that was inserted in the sample arm. First, we measure the amplitude of the OCT signal that is obtained when the mirror is placed at the focus of the objective. The OCT amplitude is then measured for different positions of this mirror from the beam focus. Measurements were made for each source. In the Gaussian beam approximation, the measured data points can be approximated by the following formula:where is the Rayleigh range, and is the waist of the focused beam. These parameters are then deduced from data extrapolation with the form in Eq. 5 and are reported in Table 1 . An aperture correction function in the sample is obtained straightforwardly by replacing by , or by noting that the equivalent Rayleigh range in the tissue is . Typical errors in the aperture correction arise from the standard deviations of the fit and from errors on refractive index measurements. They are found to be less than 5%. If we add the measurement errors that arise from and , typical errors in and run between 10 and 15%.Table 1Rayleigh range in free space and focused beam diameter of the sources used in the experiments.
We can then rewrite Eq. 3 in the form whereAttenuation and backscattering measurements come straightforwardly by plotting as a function of optical depth for each OCT in-depth scan. For relatively shallow depths in the sample, the obtained curve is easily fitted by a straight line whose slope and offset give and , respectively. Figure 4a shows a typical curve calculated from a single in-depth scan. As we go deeper into the sample, the curve slope tends to level off until becomes a constant. We believe that this may be explained by the fact that the single-scattering assumption is no longer valid: due to multiple scattering effects the beam looses its spatial coherence and the aperture function is modified from its ideal Gaussian shape measured in free space.13 More precisely, the probability for a photon scattered once to be backscattered toward the detector can no longer neglected. This is why the single-scattering model of Eq. 6 underestimates the backreflected power from deep centers. Nevertheless, the photon mean-free path exceeds several hundreds of micrometer in most biological tissues: even for highly scattering tissues such as skin, this degradation seldom occurs3 for depths less than . Care was taken to check that the straightline fitting procedure was done over a region where the curve has a roughly constant slope. Another cause of the OCT signal degradation may be the dispersion induced by the sample. As an approximation to biological samples, the effect of water-induced dispersion on OCT traces broadening has been investigated with various optical sources in a recent work.14 From this work, for thicknesses under , we can predict for our sources characteristics a very weak broadening of the OCT trace. Dispersion effect is therefore neglected in our calculations.Fig. 4Comparison of OCT profiles for (a) a single in-depth scan and (b) a spatially and temporally averaged in-depth scan. The pronounced peaks in the constant signal region are artifacts. 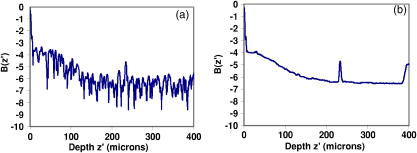 Measurements of attenuation and backscattering coefficients were carried out on several in vitro tissue samples: chicken aorta intimal surface, chicken myocardium outer surface, chicken liver, and human epidermis in the finger tip region. These samples were more homogeneous than those chosen in Fig. 3. Aorta, myocardium, and liver were placed after sacrifice inside a refrigerator for several hours before experimental study. Skin was taken from an in vivo human finger tip and was studied immediately after excision. In-depth scans were performed on 150 equidistant transverse sites over a transverse range of . This means that two adjacent measured sites are separated by a distance of . Although this distance is lower than the focused beam diameter, the overlap between two adjacent probed volumes is limited, and each transverse scan is considered to be independent from each other. Spatial averaging over the whole transverse range is thus possible for speckle noise reduction.15, 16 Moreover, this procedure further reduces the local irregularities induced by inhomogeneities. Each in-depth scan was also repeated five times and then temporally averaged for electronic noise reduction.16 This resulted in an efficient noise reduction of roughly , as shown in Fig. 4. Attenuation and backscattering coefficients were obtained from Eqs. 6, 7 and are given in Table 2 . Mean values are obtained from fitting the spatially and temporally averaged in-depth scan for each sample. Standard deviations are calculated from statistics of the coefficients measured at each transverse point. Hence, they provide useful information concerning tissue structure and homogeneity. Liver tissue is the most homogeneous of the four samples studied in this paper. This is confirmed by the weak standard deviations measured for this sample. Skin tissue has a more stratified nature, while myocardium is made of striated tissue. These two samples give the highest standard deviations compared to the mean value. The samples of the artery wall were taken from intima and media layers. These layers are made out of elastic fibers and lamellae disposed between collagen fibers and smooth muscle cells. Nevertheless standard deviations are lower than those of skin and myocardium, which means that coefficients measured at different points are less dispersed. Therefore, cellular structures in the aorta sample are larger than those in the skin and myocardium samples. The general trend from these measurements shows that the attenuation and backscattering coefficients decrease as wavelength increases. This is particularly true for skin, in which the attenuation decreases continuously from at at . For other samples, attenuation decreases abruptly between 810 and , but this trend is less pronounced between 1330 and , as expected from the results collected by Slainter 5 It is not easy to deduce from Table 2 which source and which wavelength would give the best OCT images in terms of penetration depth and backscattering. Instead of directly comparing the measured attenuation and backscattering, these coefficients were reintroduced in a simplified form of Eq. 3: The main advantage of proceeding in this manner is that we neglect the aperture effects as they mainly depend on the microscope objectives and thus can be altered independently from the source. The effect of the coherence length is also taken out by removing the factor. As a consequence, the parameter represents the ratio of the backscattered power at depth over the incident power obtained with sources that are free from focusing effects [ , ]s and with equal coherence lengths. Thus, we can establish a clear comparison of the sources only in terms of their emission wavelength. We compare the behavior of for each source and for each sample. The results are shown in Fig. 5 , where we plot the parameter on a logarithmic scale as a function of depth . One expects that a low attenuation combined with a high backscattering would give the best performance for OCT imaging. Thus, high values of are required to achieve a good SNR. Penetration depths are evaluated by comparing the slopes of the curves in Fig. 5. First, the results show that the broadband source gives the best results for liver and aorta. For shallow depths in human skin and myocardium, the Ti:sapphire laser gives the highest values. Nevertheless, as stated by Sainter, 5 its attenuation is higher than for the 1330- and wavelengths. Surprisingly, the source never gives the highest values, except for long depths in human skin. Although the penetration depth at is higher than for the other wavelengths, this result shows that the wavelength is not necessarily the best wavelength for OCT experiments, except in the case of human skin. The assessment made by Bouma that sources are well suited for this type of measurement was based on a qualitative study of penetration depth, but did not include the backscattering amplitude in the comparison.10 An important limiting factor for sources may be the absorption induced by water inside the tissue and the chamber. As a matter of fact, water absorption is much more important at (around ) than at (around ) and at , where it is very weak . As a consequence, this factor limits the penetration depth of the SLD much more than the two other sources. Without this limiting factor, the wavelength would give by far the best penetration depths.Fig. 5(a) to (d) Comparison of the parameter as a function of depth for several samples at 810-, 1330-, and sources. Inserts in each graph show the case where coherence length is taken into account. 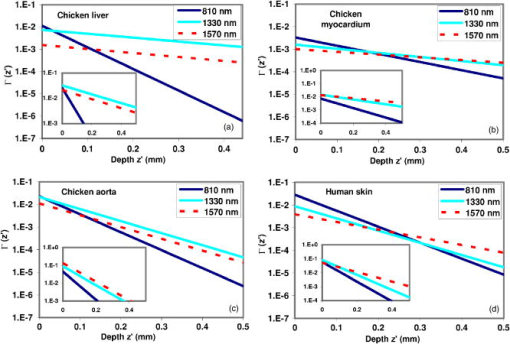 Table 2Optical coefficients of several samples measured by OCT with 810= , 1330= , and 1570=nm sources.
Note that these comparisons hold for the emission wavelengths only, and do not take into account the coherence length of each source. Taking into account the factor from Eq. 3 and inserting it into Eq. 8 would leave the penetration depth unchanged, but would bring a change in the backreflection amplitude. Subplots to this effect are inserted for each sample in Fig. 5. It turns out that a source with a short coherence length (thus giving a high axial resolution) would give a lower parameter and vice versa. The subplots show that for all the samples under test, the source and the SLD benefit from higher values compared to the Ti:sapphire laser. This, combined with the high penetration depths already stated, would give a pronounced advantage for these two sources, especially for the SLD in the case of myocardium, skin, and aorta. Still, the source or the Ti:sapphire laser may be chosen in cases where axial resolution takes precedence over the SNR: a coherence length under is often necessary to obtain high-quality images. Nevertheless, when this condition is achieved, a somewhat larger coherence length may be sometimes preferable because it yields higher backscattering amplitude. As is often the case, trade-offs may be required. 4.ConclusionThe graphical method described in this paper gives a detailed comparison of the OCT performances of these sources and the results emphasize the influence of emission wavelength on penetration depth and backscattering efficiency, on the one hand, and the influence of coherence length, on the other hand. When the coherence length is neglected, our measurements show the advantage of working with emission wavelength for good backscattering amplitude and contrast, while sources emitting at give good penetration depth. The sources provide a good compromise between the two. Note that the coherence length can substantially modify the choice of an adequate source in so far as backscattering amplitude can be changed. AcknowledgmentsThis work was supported by the Canadian Institute for Photonic Innovations (CIPI) project BP5 and contributions from CIPI Affiliates Bookham Technology and MPB Communications Inc. Other fundings from the CFI and FEMTOTECH programs are also acknowledged. ReferencesD. Huang,
E. A. Swanson,
C. P. Lin,
J. S. Schuman,
W. G. Stinson,
W. Chang,
M. R. Hee,
T. Flotte,
K. Gregory,
K. Puliafito, and
J. G. Fujimoto,
“Optical coherence tomography,”
Science, 254 1178
–1181
(1991). https://doi.org/10.1126/science.1957169 0036-8075 Google Scholar
Handbook of Optical Coherence Tomography, Marcel Dekker, New York
(2002). Google Scholar
J. M. Schmitt,
A. Knüttel,
M. Yadlowsky, and
M. A. Eckhaus,
“Optical-coherence tomography of a dense tissue: statistics of attenuation and backscattering,”
Phys. Med. Biol., 39 1705
–1720
(1994). https://doi.org/10.1088/0031-9155/39/10/013 0031-9155 Google Scholar
Y. Pan and
D. L. Farkas,
“Noninvasive imaging of living human skin with dual-wavelength optical coherence tomography in two and three dimensions,”
J. Biomed. Opt., 3 446
–455
(1998). https://doi.org/10.1117/1.429897 1083-3668 Google Scholar
A. W. Sainter,
T. A. King, and
M. R. Dickinson,
“Effect of target biological tissue and choice of light source on penetration depth and resolution in optical coherence tomography,”
J. Biomed. Opt., 9 193
–199
(2004). https://doi.org/10.1117/1.1628243 1083-3668 Google Scholar
J. M. Schmitt,
A. Knüttel, and
R. F. Bonner,
“Measurement of optical properties of biological tissues by low-coherence reflectometry,”
Appl. Opt., 32 6032
–6042
(1993). 0003-6935 Google Scholar
R. W. Schafer,
R. M. Mersereau, and
M. A. Richards,
“Constrained iterative restoration algorithms,”
Proc. IEEE, 69 432
–450
(1981). 0018-9219 Google Scholar
A. C. Akcay,
J. P. Rolland, and
J. M. Eichenholz,
“Spectral shaping to improve the point spread function in optical coherence tomography,”
Opt. Lett., 28 1921
–1923
(2003). 0146-9592 Google Scholar
A. K. Popp,
M. T. Valentine,
P. D. Kaplan, and
D. A. Weitz,
“Microscopic origin of light scattering in tissue,”
Appl. Opt., 42 2871
–2880
(2003). 0003-6935 Google Scholar
B. E. Bouma,
L. E. Nelson,
G. J. Tearney,
D. J. Jones,
M. E. Brezinski, and
J. G. Fujimoto,
“Optical coherence tomographic imaging of human tissue at and using Er- and Tm-doped fiber sources,”
J. Biomed. Opt., 3 76
–79
(1998). https://doi.org/10.1117/1.429898 1083-3668 Google Scholar
Y. T. Pan,
R. Birngruber,
J. Rosperich, and
R. Engelhardt,
“Measurement of optical-interaction coefficients of intralipid in visible and NIR range,”
Proc. SPIE, 2134 354
–363
(1994). 0277-786X Google Scholar
G. J. Tearney,
M. E. Brezinski,
J. F. Southern,
B. E. Bouma,
M. R. Hee, and
J. G. Fujimoto,
“Determination of the refractive index of highly scattering human tissue by optical coherence tomography,”
Opt. Lett., 20 2258
–2260
(1995). 0146-9592 Google Scholar
J. M. Schmitt,
A. Knüttel, and
M. Yadlowsky,
“Confocal microscopy in turbid media,”
J. Opt. Soc. Am. A, A11 2226
–2235
(1994). 0740-3232 Google Scholar
T. R. Hillman and
D. D. Sampson,
“The effect of water dispersion and absorption on axial resolution in ultrahigh-resolution optical coherence tomography,”
Opt. Express, 13 1860
–1874
(2005). https://doi.org/10.1364/OPEX.13.001860 1094-4087 Google Scholar
J. M. Schmitt,
“Speckle in optical coherence tomography,”
J. Biomed. Opt., 4 95
–105
(1999). https://doi.org/10.1117/1.429925 1083-3668 Google Scholar
A. I. Kholodnykh,
I. Y. Petrova,
K. V. Larin,
M. Motamedi, and
R. O. Esenaliev,
“Precision of measurement of tissue optical properties with optical coherence tomography,”
Appl. Opt., 42 3027
–3037
(2003). 0003-6935 Google Scholar
|