|
|
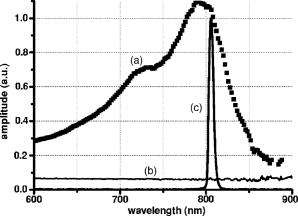
1.IntroductionLaser welding of the cornea is a technique that has been proposed since 19921 as a replacement for or as a supporting tool to conventional suturing procedure in ophthalmic surgery. It has been used experimentally in cataract surgery for the sealing of the corneal cut, and it was applied clinically for the first time in a transplant of the cornea in lieu of the continuous suture.2 The laser procedure showed remarkable advantages compared to conventional suturing: it reduces postoperative inflammations and foreign body reactions; it provides an immediate sealing of the wound, thus preventing endophthalmitis; and it improves the subsequent healing process, as demonstrated in previous works on animal models.3, 4 So far, various lasers have been proposed for corneal laser welding. Most of them had emission wavelengths in the near-infrared (e.g., erbium fiber laser,5 diode laser6, 7) and in the infrared spectral regions ( laser8, 9) that are characterized by limited optical penetration depths in the corneal stroma, due to the high absorption of the water content of the tissue. Our approach is quite different, being based on the use of near-infrared diode laser radiation at in association with the topical application of a solution of Indocyanine Green (ICG) to the corneal wound to be repaired. This dye is characterized by high optical absorption around ,10 while the stroma is almost transparent at this wavelength. Photothermal activation of the stromal collagen is thus induced by laser radiation only in the presence of ICG, resulting in a selective welding effect that produces an immediate sealing of the wound edges and good mechanical strength. The laser welding technique is based on an essentially photothermal process: local heating due to laser irradiation induces structural modifications and∕or denaturation of the collagen fibers, followed by the formation of new bonds and interactions with adjacent proteins.11 It is still not clear which mechanism leads to these new bonds and what kind of bonds are involved in this process, since both strongly depend on the type of tissue and on the temperature reached during the process. What is well known is that laser exposure time and temperature distribution inside tissue play primary roles in inducing collagen denaturation and a closure effect with good mechanical resistance and minimal side effects.12 In order to promote a comprehensive analysis of the laser welding mechanism, it is thus very important to account for the temperature enhancement during laser treatment inside the tissue, by describing the spatial and temporal evolution of the associated thermal process. In this paper, we present an experimental and model thermal analysis of laser welding of the cornea. We develop a mathematical model based on the bio-heat equation13 which is solved by using the Finite Element Method (FEM). The predictive accuracy is verified by comparing the post-processing description of the temperature behavior at the air∕cornea boundary surface with the results obtained from an ex vivo experimental study in which we set up infrared thermocamera measurements of the temperature rise on the external surface of a porcine cornea during a diode laser-welding procedure. After this verification, the model is used to describe the temperature rise inside the corneal wound and to estimate the thermal damage in the irradiated area and in adjacent tissues. 2.Materials and Methods2.1.Laser Welding ProcedureExperimental and clinical experiences on diode laser welding of the cornea3, 14, 15, 16 have indicated that, after staining of the wound edges with a high-concentration solution of ICG in sterile water , effective tissue closure was achieved at very low laser power densities, typically around in porcine cornea, emitted by an AlGaAs diode laser. Overall laser irradiation time was found to be about for a cut length (i.e., the typical perimeter of a transplanted corneal button). Laser light was delivered to the external perimeter of the cut by means of contiguous spots obtained by keeping the tip of a -core fiber at a distance of about from the surface of the cornea, with an exposure time of about for each spot. The technique that we used to deliver light energy was the so-called side irradiation, performed by keeping the fiber tip at a small angle with respect to the corneal surface. In doing so, the laser beam was able to penetrate deeply into the stroma (which is transparent to the diode laser wavelength), in a direction almost perpendicular to the wound walls, thus producing homogeneous irradiation and a welding of the cut edges. 2.2.Optical Properties of ICGIn order to study the temperature rise in the wound volume, the absorption coefficient of the pharmaceutical formulation of ICG (IC-GREEN, Akorn, Buffalo Grove, Illinois), which is used to enhance laser thermal effect, had to be measured. The ICG absorption curve depends greatly on concentration and on solvent10; thus, the absorption curve may change when the solution is absorbed by the stromal collagen. Ex vivo measurements were performed in order to estimate the ICG absorption coefficient value in corneal tissue at the diode laser wavelength and to characterize the spectral behavior of the solution. A full thickness cut in length was produced by a precalibrated knife in freshly enucleated pig eyes. The cut was stained with the ICG solution, the preparation was left in place for , and the wound was then washed with abundant water, as is done typically during surgical procedures. The corneal portion around the cut was excised, positioned on a quartz sample holder, and studied with a spectrophotometer (Model V-560, Jasco Corporation, Tokyo, Japan). The optical absorption curve of the ICG-stained cornea showed a maximum at , which matched the diode laser wavelength quite well, as shown in Fig. 1 . A reference spectra of the nonstained cornea was also acquired. Assuming that ICG penetrated in a thickness of about inside the corneal collagen at the wound edges, we used this measured curve to estimate an ICG absorption coefficient of in the corneal cut. This value was used in our FEM model. 2.3.Infrared Camera MeasurementsEx vivo measurements were performed on 15 porcine eyes obtained less then post mortem from a local slaughterhouse. Particular care was taken to enucleate the pig eyes before the usual washing procedure at , as this could affect our measurements. A full thickness cut in length was produced in each eye using a precalibrated knife in the periphery around the external perimeter of the corneal button. The cut was stained with the ICG solution and irradiated by laser light. The fiber tip ( core diameter, with a numerical aperture of 0.24) was kept at a constant distance of from the external surface of the cornea, as in typical surgical operations. A single spot was illuminated for , maintaining diode laser power at a constant value. Three different laser power emissions were tested: , , and , corresponding to power densities of , , and on the corneal surface, respectively. The intermediate value corresponded to the upper limit employed in surgical corneal laser welding. Two measurements were performed in each eye: (a) the three laser power densities were used to irradiate three different spots of the ICG-stained wound, and (b) the same irradiation conditions were used to illuminate a nonstained area, which was thus used as a control of the effect of the diode laser illumination on native stroma. During the measurements, the eyes were kept in a water bath at a constant temperature of , and the posterior part of the eyeball was completely immersed in the water, while the cornea was kept in free contact with ambient air. This configuration simulated in vivo conditions and enabled direct measurement of surface temperature. An infrared thermocamera (ThermoVision A20, FLIR Systems, Inc., Wilsonville, Oregon) was used to measure the temperature rise on the external surface of the cornea during the treatment. The camera was equipped with a focal length germanium lens, which allowed a minimum working distance of , resulting in a spatial resolution of . The camera was controlled via computer, by the use of the ThermaCam Researcher Software. Once the object parameters were provided, such as the eye surface emissivity, direct measurement of the temperature was visualized and then stored for processing in the computer. The resulting thermal sensitivity of the system was at . 2.4.Thermal ModelThe temperature rise inside the cornea during the laser welding treatment was evaluated by means of a model based on the solution of partial differential equations that describe heat transfer to the tissue and thermal propagation inside the cornea. The problem was solved with the FEM, by using commercial software (Comsol Multiphysics 3.2, Comsol AB, Sweden). The parameters and the coefficient values used in the model17, 18 are listed in Table 1 . Table 1List of model parameters and coefficients [Cornea parameters ρ , Cp , k , Ea , and ΔS are taken from the literature (Refs. 17, 18)].
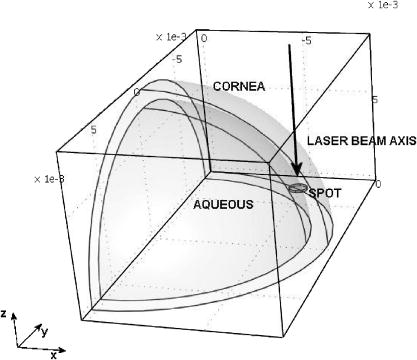
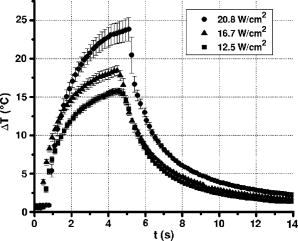
A three-dimensional (3D) eye model was set up by assuming a spherical eye to be divided into three subdomains: cornea ( inner radius and outer radius), aqueous, and ICG-stained wound (positioned inside the cornea subdomain). Free convection at the interface with ambient air at room temperature was modeled. We supposed that the laser light propagated in a direction perpendicular to the corneal cut, as occurs in the “side irradiation” technique (see Fig. 2 ). The bio-heat equation in space and time described the temperature profile in the eye: where is temperature (°C), is time (s), is density , (J∕kg°C) is heat capacity, and (W∕m°C) is thermal conductivity of the subdomain. is the heat source term, i.e., is supposed to be null everywhere except in the ICG layer, being:where is the measured absorption coefficient of the ICG solution, (W) is the diode laser power output, is the spot area, and (m) is the propagation length of the laser light. In Eq. 1, we omitted the heat source terms due to blood perfusion (the cornea is a nonvascularized tissue) and to metabolism (which is negligible, compared to laser heating).17, 19 The absorption coefficient was assumed to be constant during the laser treatment.We considered that cornea, aqueous, and ICG-stained wound were at an initial temperature , while the air was at room temperature . The optothermal parameters of cornea and aqueous were assumed to be the same. We modeled free convection at the cornea-air boundary (with a convection constant ) and at the cornea-aqueous interface (convection constant ).17, 19 The other external boundaries were considered to be thermally insulated, while continuity was imposed between internal boundaries. Moreover, the following assumptions were adopted: thermal radiation emission at the tissue-air interface and reflection of laser light from the external surface of the cornea were disregarded; the thermo-optical parameters were considered to be constant during the process. Calculations were also carried out to account for the heat damage occurring at the considered conditions of laser irradiation. By assuming that damage consists of the thermal denaturation of proteins, it is possible to estimate the damage function in terms of the Arrhenius integral18, 20: where is the time duration of the heating treatment, is the original concentration of undamaged tissue, is the remaining concentration of undamaged tissue after time , is the constant of gases, is the temperature, (J∕mol) is an empirically determined activation energy barrier, and is an empirically valued coefficient that is approximately:where is the Boltzmann constant, is the Planck constant, and (J∕mol°C) is the entropy of activation. Values for and used in the model are listed in Table 1.An estimate of the Arrhenius integral thus gives an estimate of the damage induced in the tissue. Conventionally, indicates complete necrosis of the tissue and irreversible thermal damage. 3.Results3.1.Infrared Camera MeasurementsMeasurements were first performed on nonstained porcine corneas: at a power density of (the upper limit of clinical procedures) and after a exposure time, a temperature rise as low as was recorded (averaged on 15 measurements) on the corneal surface. Slightly visible heat damage (evidenced by some tissue whitening) was found to occur when laser power greater than was used and laser irradiation was maintained on the same area for longer than . The corresponding temperature enhancement was , as measured on the external surface. In ICG-stained corneal wounds, all thermal images collected during laser irradiation showed heat confinement in close proximity to the irradiated area, as the heated zone radius was about twice the illumination spot, as measured at . For each laser power density (12.5, 16.7, and ), 15 measurements of the temperature rise within the laser spot were detected. (Maximum values are reported in Table 2 .) Averaged behaviors are illustrated in Fig. 3 . 3.2.Thermal Model ResultsThe bio-heat equation was solved by using the FEM. The results obtained for external corneal surface were compared with experimental data. There was excellent agreement between the experimental and calculated data (Fig. 4 ), confirming the assumptions made in the model. The model was thus used to study the temperature enhancement inside the wound, since it was impossible to perform direct measurement there. The analysis of the temperature distribution inside the eye indicated that the temperature radial distribution had a peak value located at about from the corneal surface; it then dropped down toward the aqueous in about (Fig. 5 ). Thermal damage due to laser irradiation was studied: the Arrhenius integral [eq. 3] was calculated during laser treatment time for an exposure time . The values (see Table 2) were found to be below the threshold of irreversible damage for clinically employed power density values.20 The heat affected zone was well confined in the ICG-stained cut. Fig. 4Temperature rise of a laser-welded cornea external surface, calculated within the mathematical model (continuous line). Mean value experimental data are shown (symbols), as a reference. Calculations and data refer to a treatment time with laser power density. 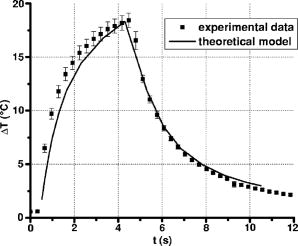 Fig. 5Temperature rise distribution along radial distance from the center of the eye toward external ambient air. These calculated data refer to laser power density and treatment time. The maximum value is inside the cornea, in correspondence with the laser spot center. 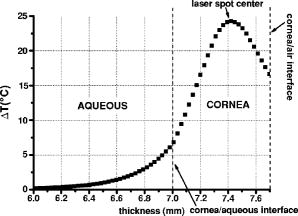 In order to find whether and when the heating process could reach saturation point during laser welding procedures, we applied the model to a longer exposure time of up to . In doing this, we assumed that ICG solution was stable under laser light. Calculated data showed that the induced temperature reached a plateau value after of irradiation (see Fig. 6 ). Fig. 6Calculated temperature rise for a irradiation time of an ICG-stained cornea at a power density of . Data refer to the maximum temperature inside the corneal tissue. 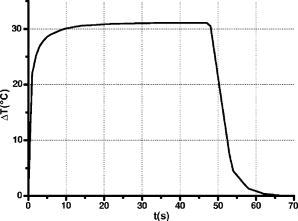 Table 2Maximum temperature rise inside the ICG-stained cut and on the external surface, for different laser power densities and treatment times: calculated and measured data (mean value on fifteen measurements) and solutions of Arrhenius integral Ω (2s) are reported.
4.DiscussionExperimental and simulation analysis of the temperature rise during laser welding of the cornea was carried out. Low power diode laser interaction with a porcine cornea was modeled, to reproduce the operative conditions of the laser-induced suturing of corneal wounds.3 The present study enabled us to evaluate the temperature on the corneal surface in the region of the stained wound: we could compare the results of experimental tests performed with an infrared (IR) camera, with simulations of the process, obtained by using the FEM. The results were found to be in excellent agreement, giving us the opportunity to characterize this particular thermal process on the whole and to study the effects of laser welding inside the tissue, where a direct measurement is unfeasible. Both the thermocamera measurements and the predictive analysis showed a relatively modest temperature increase, which reached its maximum value inside the ICG-stained region. For a mean temperature of in the living cornea, the maximum temperature peak due to photothermal interaction was in the range, as calculated inside the stained wound for the operative irradiation parameters which, according to our experience, gave the best welding results in clinical procedures (i.e., laser power of , corresponding to power density of in the irradiation spot, with of irradiation time on each laser spot). These temperature values were very close to the point at which early changes in the mechanical properties of the cornea can occur.18, 21 A consideration of the thermal denaturation processes of collagen thus has to be made. The main component of the corneal stroma is type I collagen, the temperature-dependent denaturation mechanism and melting temperature of which have been studied over the past few years. Experimental studies18, 21, 22, 23, 24 have shown the presence of more than one denaturation point of type I collagen, which is associated with structural changes in the stromal fibers. In particular, differential scanning calorimetry (DSC) data18 and second harmonic generation (SHG) imaging microscopy of porcine corneas,21 supported by standard histological analysis, have pointed out that the collagen fibers of the corneal stroma undergo four distinct structural changes in the temperature range between . The first change was found in the region between and and then at , , and around . Researchers21 suggested that, at these four denaturation points, a thermal disruption of stromal cross-links occurs, characterized by different order transitions, and that the reorganization of corneal collagen takes place in three distinct phases between and , resulting in a different strength of cross-links within corneal collagen. In this temperature range, collagen fibers are organized into bundles, which become denser as the temperature increases. The formation of these bundles, separated by cavities of a size that increases with the temperature, may be interpreted as being a consequence of the thermal disruption of cross-linking elements of the stroma. At temperatures higher than , the collagen is completely denatured, and its structure is completely disorganized. In view of this, our analysis indicated that an important factor for the success of laser welding procedures is the induction of structural modifications in native collagen fibers that correspond to low-temperature denaturation phases well below the limit of collagen disorganization. As was shown by our experimental observations and confirmed by the present photothermal model, temperatures near the second and third denaturation points are reached during typical surgery conditions ( laser power densities), and the objective result is an immediate sealing effect. When using higher laser power, higher temperatures are developed (e.g., about with a power density); macroscopically, this corresponds to a poorer welding effect, with evident whitening and lack of transparency in the corneal stroma. It should be noted that this behavior is a direct consequence of the association of ICG staining with diode laser irradiation. The temperature dynamics would be completely different in the case of corneal welding induced by an infrared laser wavelength mainly absorbed by the water content of the stroma, without the application of any exogenous chromophore. It is clear that in such a case, the optical absorption would be localized in the proximity of the corneal surface, with a higher risk of superficial heat damage and lesser homogeneous temperature distribution in the corneal thickness. On the contrary, the application of ICG solution only in the cut edges resulted in a selective and localized welding effect, thus preventing temperature enhancement and, possibly, thermal damage in all those areas not stained with the ICG solution. Direct thermocamera measurements on diode laser irradiation of unstained corneal tissue confirmed this figure. In the presence of ICG, our experimentally validated model indicated that the temperature rise was spatially well-confined in the ICG-stained wound and that local heat release was reliably controlled. As regards the latter, when solving the Arrhenius integral, we found that the fraction of damaged tissue was well below the threshold indicating irreversible thermal damage. This result confirmed previously reported studies on animal models:3 microscopic observations and histological analyses never revealed the occurrence of thermal injuries in laser-welded rabbit corneas. A microscopy study on laser-welded porcine corneas is in progress: preliminary image analysis evidenced the absence of heat damage to the irradiated area. When considering very long irradiation times on the same spot, a constant temperature value was reached in about , indicating that ICG-mediated laser welding induced a heating process that leads to saturation. This dynamics of transient temperature is similar to the one studied in IR-laser irradiated collagen samples.25, 26 This behavior could be exploited in order to further increase the safety of the welding technique, by using even lower laser powers, but at the expense of longer application times. For example, a power density of could ensure that the temperature rise remains below , but exposure times of per spot would be necessary in order to induce an effective corneal welding in each irradiated spot. The cooling time history was also studied, showing that the cooling rate is relatively fast compared with the typical application times: in the case of retreatment, as sometimes occurs in operative conditions, the risk of heat accumulation is thus negligible. 5.ConclusionsThis thermal analysis on the diode laser welding of porcine corneas has enabled us to quantify, in terms of space distribution and time evolution of the induced temperature rise, the encouraging phenomenological findings that we recorded in previous experimental and clinical studies regarding the effectiveness and safety of corneal laser welding procedures. In addition, the analysis could represent a starting point for forthcoming studies aimed at investigating, at a microscopic level, the mechanism of laser-induced welding of corneal tissue, in order to better characterize the structural modification that occurs near the second and third denaturation points of type I collagen (i.e., in the range) under laser irradiation. The practical consequence would be a more precise definition of the operative parameters, which could favor the diffusion of the clinical use of this procedure. AcknowledgmentsThe authors acknowledge the “OPTOWELD” Project of Azienda USL 4 (Public Health Service) of Prato, Italy, for supporting this study. We would also like to thank Dr. Stefano Pardini for preparing the eye samples. ReferencesN. L. Burstein,
J. M. Williams,
M. J. Nowicki,
D. E. Johnson, and
W. Q. Jeffers,
“Corneal welding using hydrogen fluoride laser,”
Arch. Ophthalmol. (Chicago), 110
(1), 12
–13
(1992). 0003-9950 Google Scholar
R. Pini,
L. Menabuoni, and
L. Starnotti,
“First application of laser welding in clinical transplantation of the cornea,”
Proc. SPIE, 4244 266
–271
(2001). https://doi.org/10.1117/12.427800 0277-786X Google Scholar
F. Rossi,
R. Pini,
L. Menabuoni,
R. Mencucci,
U. Menchini,
S. Ambrosini, and
G. Vannelli,
“Experimental study on the healing process following laser welding of the cornea,”
J. Biomed. Opt., 10
(2), 24004
(2005). 1083-3668 Google Scholar
R. Pini,
V. Basile,
S. Ambrosini,
G. Vannelli,
F. Rossi,
L. Menabuoni,
R. Pratesi, and
M. Monici,
“Healing process study of laser-welded corneal tissue by Multispectral Imaging Autofluorescence Microscopy (MIAM),”
Proc. SPIE, 6138 404
–409
(2006). 0277-786X Google Scholar
H. E. Savage,
R. K. Halder,
U. Kartazayeu,
R. B. Rosen,
T. Gayen,
S. A. McCormick,
N. S. Patel,
A. Katz,
H. D. Perry,
M. Paul, and
R. R. Alfano,
“NIR laser tissue welding of in vitro porcine cornea and sclera tissue,”
Lasers Surg. Med., 35
(4), 293
–303
(2004). https://doi.org/10.1002/lsm.20094 0196-8092 Google Scholar
G. Trabucchi,
P. G. Gobbi,
R. Brancato,
F. Carones,
A. G. Resti,
A. Jansen, and
R. Pini,
“Laser welding of corneal tissue: preliminary experiences using and diode laser,”
Proc. SPIE, 2623 380
–387
(1996). https://doi.org/10.1117/12.230353 0277-786X Google Scholar
T. J. Desmettre,
S. R. Mordon, and
V. Mitchell,
“Tissue welding for corneal wound suture with CW 1.9 micro diode laser: an in vivo preliminary study,”
Proc. SPIE, 2623 372
–379
(1996). https://doi.org/10.1117/12.230351 0277-786X Google Scholar
A. Barak,
O. Eyal,
M. Rosner,
E. Belotserkousky,
A. Solomon,
M. Belkin, and
A. Katzir,
“Temperature-controlled CO2 laser tissue welding of ocular tissues,”
Surv. Ophthalmol., 42 S77
–S81
(1997). 0039-6257 Google Scholar
E. Strassmann,
N. Loya,
D. D. Gaton,
A. Ravid,
N. Kariv,
D. Weinberger, and
A. Katzir,
“Temperature controlled CO2 laser soldering of pig cornea,”
Proc. SPIE, 4609 222
–228
(2002). https://doi.org/10.1117/12.433863 0277-786X Google Scholar
M. L. J. Landsman,
G. Kwant,
G. A. Mook, and
W. G. Zijlstra,
“Light-absorbing properties, stability, and spectral stabilization of indocyanine green,”
J. Appl. Physiol., 40 575
–583
(1976). 0021-8987 Google Scholar
K. M. McNally-Heintzelman,
“Laser tissue welding,”
Biomedical Photonics Handbook, 39/10
–39/11 CRC Press, Boca Raton, FL
(2003). Google Scholar
R. Brinkmann,
B. Radt,
C. Flamm,
J. Kampmeier,
N. Koop, and
R. Birngruber,
“Influence of temperature and time on thermally induced forces in corneal collagen and the effect on laser thermokeratoplasty,”
J. Cataract Refractive Surg., 26
(5), 744
–754
(2000). https://doi.org/10.1016/S0886-3350(00)00310-2 0886-3350 Google Scholar
V. V. Tuchin,
“Light-tissue interactions,”
Biomedical Photonics Handbook, 3–11
–3–13 CRC Press, Boca Raton, FL
(2003). Google Scholar
L. Menabuoni,
B. Dragoni, and
R. Pini,
“Preliminary experiences on diode laser welding in corneal transplantation,”
Proc. SPIE, 2922 449
–452
(1996). https://doi.org/10.1117/12.260709 0277-786X Google Scholar
L. Menabuoni,
F. Mincione,
B. Dragoni,
G. P. Mincione, and
R. Pini,
“Laser welding to assist penetrating keratoplasty: in vivo studies,”
Proc. SPIE, 3195 25
–28
(1998). https://doi.org/10.1117/12.297915 0277-786X Google Scholar
R. Pini,
F. Rossi,
L. Menabuoni,
R. Mencucci,
U. Menchini,
S. Ambrosini, and
G. Vannelli,
“Diode laser welding for cornea suturing: an experimental study for the repair process,”
Proc. SPIE, 5314 245
–252
(2003). https://doi.org/10.1117/12.529067 0277-786X Google Scholar
F. Manns,
D. Borja,
J. M. Parel,
W. Smiddy, and
W. Culbertson,
“Semianalytical thermal model for subablative laser heating of homogeneous nonperfused biological tissue: application to laser thermokeratoplasty,”
IEEE Trans. Biomed. Eng., 51
(8), 288
–297
(2003). 0018-9294 Google Scholar
J. Kampmeier,
B. Radt,
R. Birngruber, and
R. Brinkmann,
“Thermal and biomechanical parameters of porcine cornea,”
Cornea, 19
(3), 355
–363
(2003). 0277-3740 Google Scholar
K. Gosalia,
J. Weiland,
M. Humayun, and
G. Lazzi,
“Thermal elevation in the human eye and head due to the operation of a retinal prosthesis,”
IEEE Trans. Biomed. Eng., 51
(8), 1469
–1477
(2004). https://doi.org/10.1109/TBME.2004.827548 0018-9294 Google Scholar
J. Welch,
“The thermal response of laser irradiated tissue,”
IEEE J. Quantum Electron., QE-20 1471
–1481
(1984). https://doi.org/10.1109/JQE.1984.1072339 0018-9197 Google Scholar
H. Y. Tan,
S. W. Teng,
W. Lo,
W. C. Lin,
S. J. Lin,
S. H. Jee, and
C. Y. Dong,
“Characterizing the thermally induced structural changes to intact porcine eye, part 1: second harmonic generation imaging of cornea stroma,”
J. Biomed. Opt., 10
(5), 054019
(2005). https://doi.org/10.1117/1.2012987 1083-3668 Google Scholar
R. Dong,
X. Yan,
X. Pang, and
S. Liu,
“Temperature-dependent Raman spectra of collagen and DNA,”
Spectrochim. Acta, Part A, 60 557
–561
(2004). https://doi.org/10.1016/S1386-1425(03)00262-2 0584-8539 Google Scholar
S. J. Lin,
C. Y. Hsiao,
Y. Sun,
W. Lo,
W. C. Lin,
G. J. Jan,
S. H. Jee, and
C. Y. Dong,
“Monitoring the thermally induced structural transitions of collagen by use of second-harmonic generation microscopy,”
Opt. Lett., 30
(6), 622
–624
(2005). https://doi.org/10.1364/OL.30.000622 0146-9592 Google Scholar
T. Theodossiou,
G. S. Rapti,
V. Hovhannisyan,
E. Georgiou,
K. Politopoulos, and
D. Yova,
“Thermally induced irreversible conformational changes in collagen probed by optical second harmonic generation and laser-induced fluorescence,”
Lasers Med. Sci., 17
(1), 34
–41
(2002). 0268-8921 Google Scholar
N. Y. Ignatieva,
V. V. Lunin,
S. V. Averkiev,
A. F. Maiorova,
V. N. Bagratashvili, and
E. N. Sobol,
“DSC investigation of connective tissues treated by IR-laser radiation,”
Thermochim. Acta, 422
(1–2), 43
–48
(2004). https://doi.org/10.1016/j.tca.2004.04.030 0040-6031 Google Scholar
W. Small IV, N. J. Heredia,
D. J. Maitland,
D. C. Eder,
P. M. Celliers,
L. B. Da Silva,
R. A. London, and
D. L. Matthews,
“Experimental and computational laser tissue welding using a protein patch,”
J. Biomed. Opt., 3
(1), 96
–101
(1998). https://doi.org/10.1117/1.429866 1083-3668 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||