1.IntroductionThe macular pigments (MP) in the human eye are composed of the carotenoid species lutein and zeaxanthin1 2 and are located in the retinal region about 5 mm in diameter that is responsible for viewing with the highest acuity (see Fig. 1). These pigments are thought to play an important role in the treatment and prevention of age-related macular degeneration (AMD), the leading cause of blindness in the elderly in the Western world.3 4 Supportive epidemiological studies analyzing carotenoid levels via serum assays have shown that dietary intakes and blood levels of lutein and zeaxanthin are inversely associated with risk of advanced AMD.5 6 For example, it was shown that individuals who have high intakes of lutein and zeaxanthin have 42 lower rates of the wet form of AMD.6 The recent Age-Related Eye Disease Study (AREDS), carried out by the National Eye Institute, one of the Federal government’s National Institutes of Health, has demonstrated that an antioxidant supplement combination containing high levels of zinc, vitamin E, vitamin C, and β-carotene can slow the progression of moderate AMD,7 but no prospective interventional studies of lutein and/or zeaxanthin have been performed on a similar scale to date. It has also been demonstrated that MP levels are lower in autopsy eyes from patients with AMD.8 9 Figure 1Fundus photograph of healthy human retina, showing optic nerve head (bright spot at left) and macula [dark shaded area outlining macular pigment (MP) distribution]. 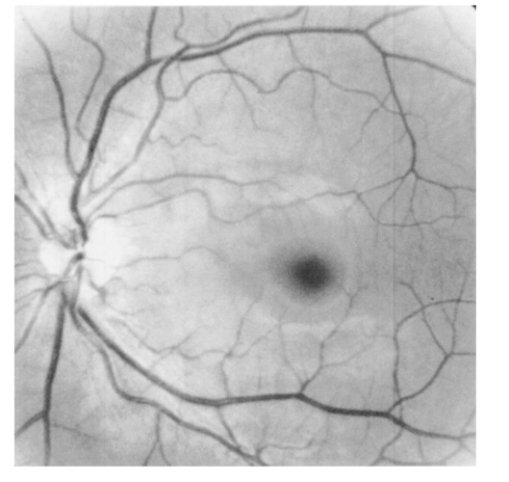 In order to advance our understanding of MPs with a view to preventing AMD, more reliable data on carotenoid levels in the living human eye are needed. Postmortem studies have significant limitations since historical information on clinical history and risk factors of the donors is generally unavailable. Noninvasive methods of assessment of MP levels in living humans would be powerful tools in epidemiological research on AMD, and these methods can also be expected to be very useful for monitoring studies of dietary and/or nutritional interventions designed to raise MP levels. The spatial distribution of lutein and zeaxanthin in the human and monkey retina has been extensively investigated in the past. Spectroscopic studies of tissue sections of primate maculae indicate that there are very high concentrations of carotenoid pigments in the Henle fiber layer of the fovea and smaller amounts in the inner plexiform layer.10 Biochemical studies have demonstrated that in the foveal area, zeaxanthin predominates over lutein by a 2:1 ratio, while in the peripheral retina, the concentration of lutein and zeaxanthin drops up to 100-fold relative to the center of the fovea, and the zeaxanthin-to-lutein ratio reverses to 1:2.11 12 It is thought that the majority of the carotenoids in the foveal region are associated either with cones13 or Mu¨ller cells14 and that the carotenoids of the peripheral retina are present in the rod outer segments.15 16 High spatial-resolution psychophysical studies correlate well with these findings, showing a peak of macular pigment density with an average width of 1.03 deg at half maximum in normal subjects.17 The absolute concentrations of macular pigments are extremely high compared with other tissue sites, and typically correspond to 10 to 30 ng per macular punch biopsy. The spectral absorption behavior is shown in Fig. 2(a) for an excised macula and reveals that optical density levels in the peak of the absorption near 460 nm are typically very strong. In spite of this very thin tissue layer, the optical density measured in an ∼1 mm diameter spot reaches an average value of about 0.3 above background, which explains the origin of the strong yellow coloration of the macula. Comparing the optical absorption of the tissue with lutein and zeaxanthin solutions, one finds that the absorption behavior is remarkably similar, including the appearance of vibronic substructure, and that there is little overlap with any other, potentially confounding, chromophores—at least in the “intact” retina. Indeed, all currently existing noninvasive detection concepts attempt to take advantage of this MP-specific optical absorption feature. Figure 2Absorption spectra of excised, flat-mounted human retina (a) and lutein and zeaxanthin solutions in methanol (b). The spectral properties of carotenoids are very similar in tissue and in solution. 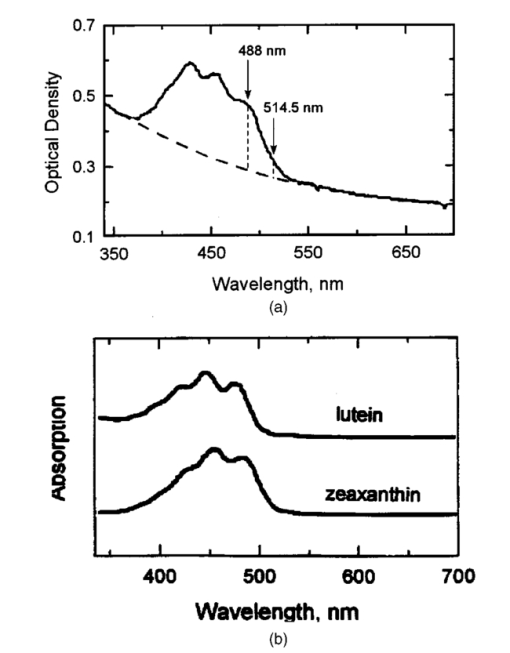 The mechanisms by which these two macular pigments, derived exclusively from dietary sources such as green leafy vegetables and orange and yellow fruits and vegetables, might protect against AMD is still unclear. Also, it is not known why only lutein and zeaxanthin are accumulated in the macula, and no other, similarly structured carotenoids (see Fig. 3 for the molecular structures of lutein, zeaxanthin, and β-carotene). Obviously, OH― groups, existing only in lutein and zeaxanthin in their end rings, are required to anchor these carotenoids to specific binding proteins. Regarding their function, the MPs are known to be excellent free radical scavenging antioxidants in a tissue at high risk of oxidative damage from high levels of light exposure and abundant highly unsaturated lipids.18 19 3 4 In addition, since these molecules absorb in the blue-green spectral range, they act as filters that may attenuate photochemical damage and/or image degradation caused by short-wavelength visible light reaching the retina.20 Figure 3Molecular structures of the carotenoid pigment species β-carotene, lutein, and zeaxanthin. All molecules feature a linear, chainlike conjugated carbon backbone consisting of nine alternating carbon single (C―C) and double bonds (C=C) and four methyl groups attached as side groups. The structural differences between the molecules originate from the end groups attached to the carbon backbone. These end groups, termed ionone rings, contain an attached OH molecule in lutein and zeaxanthin, and they also differ in the location of an additional C=C bond in each ring. In lutein this leads to an effective conjugation length of ten C=C bonds and in zeaxanthin to eleven C=C bonds. 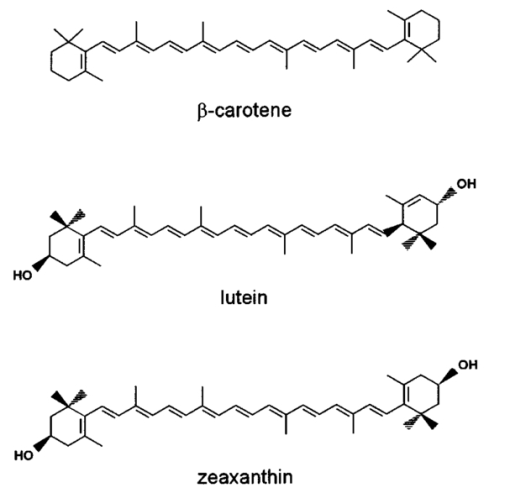 2.Psychophysical Pigment Measurements, Reflectance, and Autofluorescence TechniquesCurrently, the most commonly used noninvasive method for measuring human macular pigment levels is a psychophysical heterochromatic flicker photometry (HFP) test. This subjective test involves color intensity matching of flickering blue and green light aimed at the fovea in relation to the same lights aimed at the perifoveal area. The required optical instrumentation is schematically shown in Fig. 4. HFP was initially described by several investigators in the 1970s, and at that time it usually required rather extensive apparatus utilizing Maxwellian view optics, xenon arc lamps, and fixed optical tables.21 22 In recent years, newer versions using free-view optics and simpler light sources, such as light-emitting diodes or projector lamps, have been described that allow construction of portable flicker devices suitable for clinical studies at multiple sites.23 24 Figure 4Experimental setup for heterochromatic flicker photometry (a) and light stimulus configuration (b) showing flicker spot and peripheral fixation point (located above the ficker spot). Light from a tungsten lamp is routed toward the retina via two channels filtered to provide blue and green light, respectively, and recombined to project a blue-green flickering stimulus. The subject adjusts the intensities to achieve flicker fusion for foveal and peripheral retinal positions. The optical density of the MP is inferred from the required attenuation. 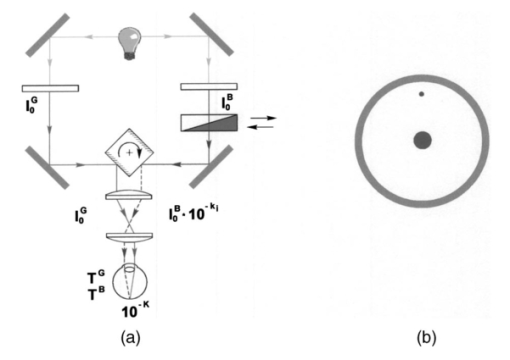 HFP infers the concentration of carotenoids in the macula by determining the perceived optical density of the filtering effects of these yellow pigments at different wavelengths. The subject initially fixates on a spot of light rapidly alternating between a blue color near the absorption maximum of the macular pigment (∼460 nm) and a green color in a region of the visible spectrum in which the macular carotenoid pigments do not absorb significantly (∼540 nm). The subject adjusts the relative intensities of the blue and green lights until the sensation of flickering is minimized or eliminated. The subject then repeats the task using eccentric fixation on a region of the macula where the concentration of the macular pigment is assumed to be so low that its absorption of blue light is negligible. Typical eccentric fixation points are 4 to 9 deg from fixation (1.2 to 2.6 mm at the retinal surface). After repeated measurements using central and eccentric fixation, the perceived optical density of the macular carotenoids at the edge of the central flickering spot is then calculated according to the following formula where macular pigment optical density (MP OD) is equal to the logarithm of the energy of blue light necessary to minimize flicker at the test location (B fov) relative to the energy needed at the extrafoveal reference point25: Some investigators utilize flickering spots or rings of varying diameters to map out the macular pigment distribution at multiple points, typically along the horizontal meridian.17 In some cases, these results yield symmetric MP densities, as shown in Fig. 5, but in other cases evidence for more complex, asymmetric MP distributions with secondary MP peaks was shown as well.17 Multiple wavelengths may be used in an attempt to recreate the macular pigment absorption curve in order to confirm that the subject is performing the task properly.17 Figure 5Comparison of MP density measured along the horizontal (solid squares) and vertical (open circles) meridians. The close correspondence between the two curves suggests that the MP density for this subject can be considered symmetric. (Reproduced from Ref. 17 with permission.)  Numerous epidemiological HFP studies of macular pigment density have been published in recent years. Many of these investigations have focused on young healthy populations in order to ascertain correlations between macular pigment and various putative risk factors for AMD, such as age, smoking, gender, iris color, body mass index, blood carotenoid levels, and dietary carotenoid intake.26 27 28 29 The correlations have generally been of modest significance and sometimes even contradictory, owing in part to the wide range of macular pigment levels in the population and the complex nature of carotenoid homeostasis, as well as differences in HFP methodologies among research groups. Relatively few HFP studies have been performed on patients with significant macular pathology, and these must all be interpreted with caution since some of the critical assumptions of HFP are undoubtedly violated in these subjects. The most notable of these studies demonstrated lower macular pigment levels in the clinically normal fellow eye of patients with unilateral exudative AMD,29 while another group found that retinitis pigmentosa patients appear to have normal macular pigment levels.30 HFP has also been employed in some small-scale dietary supplementation trials.31 32 33 Responses to interventions with lutein supplements or dietary modification were quite variable, with some subjects showing no change at all despite several months of intervention.32 33 Possible explanations include poor subject compliance or the presence of saturated carotenoid binding sites in the macula. Also, since HFP measures macular carotenoid levels relative to a peripheral zero point, it is possible that a significant rise in carotenoids in the peripheral retina in response to supplementation could mask a response centrally. As a subjective psychophysical test, HFP measurements can never be directly correlated with the “gold standard” of high pressure liquid chromatography (HPLC) analysis of carotenoids in the macula. Thus there has been considerable interest by some carotenoid researchers in developing objective optical methods capable of measuring macular pigment noninvasively. These methods, based on fundus reflection or fundus fluorescence (autofluorescence) spectroscopy, might require less reliance on subject training and attentiveness, and there is the potential to perform direct correlations with HPLC in human cadaver eyes or in animal model systems. Therefore, these optical methods would be likely to have wider applications in subjects with significant macular pathology relative to HFP. Furthermore, these methods lend themselves to quantitative imaging of the whole spatial MP distribution. The first efforts to measure macular pigment objectively were photographically based reflectance techniques. Delori and colleagues34 took a series of fundus photographs using a variety of narrow-wavelength illumination filters. They found that photographs taken at 470 nm, which is near the absorption maximum of the macular pigment, exhibited a dark central spot corresponding to the peak of the distribution of the macular carotenoids. Other photographs taken at wavelengths longer than 500 nm showed a much less dense central spot that appeared to originate from increased foveal melanin density rather than macular carotenoids. A comparison of the on-peak and off-peak photographs allowed a qualitative estimate of the macular carotenoids since it was assumed that other chromophores make insignificant contributions to foveal absorbance. Quantitative assessments of macular pigment were not feasible, owing to limitations of the photographic technology at that time since subtle variations in subject alignment and flash intensity could lead to large picture-to-picture variations in exposure. Also, the filters used were probably not sufficiently monochromatic, and image digitization technology and registration algorithms were not adequately advanced. More recently, with the advent of high-resolution digital fundus cameras and scanning laser ophthalmoscope (SLO) systems, there has been a resurgence of interest in this reflectance technology. Modern computing methods greatly simplify image registration and digital subtraction. Argon laser lines at 488 and 514 nm can be used for monochromatic on-peak and off-peak images, and confocal optics can be incorporated.35 36 The MP OD is then calculated according to the following equation where Cλ is a constant that depends on the absorption coefficients of the macular pigment, and Ref values are the measured reflectances at the fovea and parafovea (14 deg outside the fovea) at 488 and 514 nm36: A closely related technique called quantitative fundus reflectometry has been employed by several groups. In its typical form, the foveal region is illuminated successively with a series of narrow bandpass filters on a rotating wheel, and reflected light is collected by a photomultiplier37 or a video camera.38 Then, using a sophisticated optical model, the average spectral contribution of absorbance by macular carotenoids is calculated for the region illuminated.39 Since the sclera and the cone photoreceptor disks are the primary reflectors in this technique, the incident and reflected light must pass through multiple absorptive and reflective structures, making the optical model quite complex, and it is unclear how much the model will have to be altered in the face of significant macular pathology. In its current form, image acquisition time is long enough that precise head alignment and fixation may be required. Berendschot and colleagues35 have compared this technique with a digital subtraction SLO method in a lutein supplementation study. They found that SLO provided the most reliable data and estimated that macular pigment optical density rises in the range of 5 per month in response to 10 mg of daily lutein supplement. More recently, the same group used reflectometry on an elderly cohort with normal maculae and compared them with a cohort with early age-related maculopathy.40 They could detect no difference in macular pigment levels between the two cohorts. Delori et al.;41 42 have developed a technique to measure macular pigment density based on attenuation of lipofuscin fluorescence originating from the retinal pigment epithelium (RPE). The major fluorophore of lipofuscin is A2E, a compound formed by the condensation of two retinaldehyde molecules with phosphatidyl ethanolamine.43 A2E builds up with age in RPE cells in a more or less even distribution across the posterior pole of the eye.44 A2E has a fluorescence excitation spectrum that partially overlaps with the absorption spectrum of the macular carotenoids, and its emission spectrum is broad and at wavelengths well beyond the absorption of carotenoids.43 In Delori’s technique, the foveal region is illuminated with two different wavelengths of near monochromatic light, one within the absorption range of both the macular pigment and lipofuscin, and one within the absorption range of lipofuscin alone. The relative fluorescence under these two conditions is then compared, incorporating appropriate correction factors for the quantum fluorescence efficiency at the two different excitation wavelengths at foveal and extrafoveal sites. The mean macular pigment optical density attenuates the excitation of lipofuscin fluorescence at the fovea is calculated, using the extrafoveal site as a zero point, according to the following equation42: In this model, the two excitation wavelengths Λ1 (470 nm) and Λ2 (550 nm) are in the high and low absorption ranges of the macular pigment, and the lipofuscin fluorescence is measured at the same emission wavelength λ (710 nm). FF and FP represent the autofluorescence of all layers located posteriorly to the macular pigment at the fovea and perifovea (7 deg temporal to the fovea), respectively, and KMP is the extinction coefficient relative to that at 460 nm. A critical but as yet unproven assumption of this technique is a requirement that no other fluorophores contribute significant fluorescence unless their spatial distributions precisely match that of A2E.42The lipofuscin fluorescence attenuation technique is not yet widely used. One report of a comparison with HFP and reflectometry has been published which demonstrated reasonable correlations, although with some systematic differences.42 Studies on normal and AMD subjects are reportedly in progress. 3.Resonance Raman Scattering of CarotenoidsCarotenoids are π-electron conjugated carbon-chain molecules (C 40 H 56) and are similar to polyenes in their structure and optical properties. Lutein and zeaxanthin contain nine alternating conjugated carbon double and single bonds comprising a polyene backbone, with additional conjugation into one or two double bonds provided by ionone rings, which terminate the carbon backbone on each end (see Fig. 3). Four methyl groups (CH 3) are attached to the backbone as side groups. Strong, electric dipole allowed optical absorption occurs between the molecules’ delocalized π orbitals, and the resulting absorption spectra are virtually the same for lutein and zeaxanthin (see Fig. 2). Raman scattering can be used to detect the vibrational frequencies of the polyene backbone as well as the methyl side groups. For lutein and zeaxanthin dissolved in tetrahydrofuran (THF), these Raman Stokes lines appear at 1525 cm−1 (C=C stretch), 1159 cm−1 (C―C stretch), and 1008 cm−1 (C―CH 3 rocking motions),45 as shown in Fig. 6 for a solution of lutein in tetrahydrofuran. Figure 6Resonant Raman spectrum of a solution of lutein in tetrahydrofuran (THF), showing the three most prominent carotenoid Raman peaks at 1008, 1159, and 1525 cm−1. The peaks correspond, respectively, to the rocking motions of the methyl components (C―CH 3), and the stretch vibrations of the carbon-carbon single bonds (C―C) and double bonds (C=C). The side peak at 1030 cm−1 is an artifact and corresponds to THF. Laser excitation was at 488 nm. 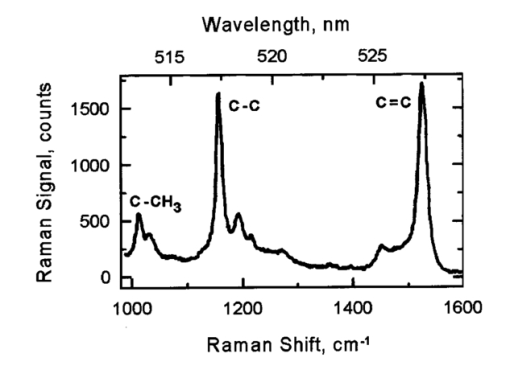 Ordinarily the Raman scattering process is weak in intensity, requiring intense illumination, long acquisition times, and high sensitivity detectors, and in biological systems the spectra tend to be very complex because of the diversity of compounds present. This scenario changes drastically if the compounds exhibit absorption bands that are due to electronic dipole transitions of the molecules, particularly if these are located in the visible wavelength range. When illuminated with monochromatic light overlapping one of these absorption bands, the Raman scattered light will exhibit a substantial resonance enhancement. In the case of the macular carotenoids, 488-nm argon laser light provides an extraordinarily high resonant enhancement of the Raman signals—on the order of 105 (Ref. 45). No other biological molecules found in significant concentrations in human ocular tissues exhibit similar resonant enhancement at this excitation wavelength, so in vivo carotenoid resonance Raman spectra are remarkably free of confounding Raman responses. Raman scattering is a linear spectroscopy, meaning that the Raman scattering intensity (IS) scales linearly with the intensity of the incident light (IL). Furthermore, at a fixed incident light intensity, the Raman response scales with the population density of the scatterers N(Ei) in a linear fashion determined by the Raman scattering cross-section σR(i→f) (a fixed constant determined by the excitation and collection geometries) as long as the scatterers can be considered as optically thin:48 In vivo resonance Raman spectroscopy in the eye takes advantage of several favorable anatomical properties of the tissue structures encountered in the light-scattering pathways. First, the major site of macular carotenoid deposition in the Henle fiber layer is on the order of only 100 μm in thickness.10 This provides a chromophore distribution very closely resembling an optically thin film having no significant self-absorption of the illuminated or scattered light. Second, the ocular media (cornea, lens, vitreous) are generally of sufficient clarity not to attenuate the signal, and they should require appropriate correction factors only in cases of substantial pathology, such as visually significant cataracts. Third, since the macular carotenoids are situated anteriorly in the optical pathway through the retina, the illuminating light and the backscattered light never encounter any highly absorptive pigments, such as photoreceptor rhodopsin and RPE melanin, while the light unabsorbed by the macular carotenoids and the forward- and side-scattered light will be efficiently absorbed by these pigments.48 Initial ocular resonance Raman studies were performed on flat-mounted human cadaver retinas, human eye cups, and whole frog eyes using a laboratory-grade Raman spectrometer and an argon laser. HPLC analysis was performed to confirm the linearity of response, and the ability to achieve a spatial resolution on the order of 100 μm was shown.46 An instrument suitable for clinical use in living humans and nonhuman primates was then developed,47 48 as shown schematically in Fig. 7(a). It consisted of a low-power argon laser that projected a 1-mm, 0.5-mW, 488-nm spot onto the foveal region through a pharmacologically dilated pupil for 0.5 s. The 180-deg backscattered light was then collected, and Rayleigh-scattered light was rejected through the use of high-efficiency bandpass filters before being routed via fiber optics to a Raman spectrometer and Peltier-cooled CCD camera interfaced to a personal computer equipped with custom-designed analysis software. Laser illumination levels on the retina were well within established safety standards. Living humans fixated on a suitable target to ensure alignment, while monkey experiments employed a video camera and red laser aiming beam to confirm foveal targeting. Using living monkey eyes, the linearity of response at “eye safe” laser illumination levels could again be established on this system relative to HPLC analysis of MP levels after enucleation, and it was possible to rapidly measure a relatively large population of human volunteers.48 49 Details of the Raman instrumentation are published in a separate paper in this issue.50 Figure 7(a) Experimental setup showing light delivery and collection optics of a fiber-based Raman spectrometer. (b) and (c) A typical Raman measurement on the computer monitor. (b) The unprocessed spectral response (sharp Raman peaks superimposed on broad fluorescence background). (c) The Raman response of macular pigment after a polynomial fit of the fluorescent background and subtraction of that background. 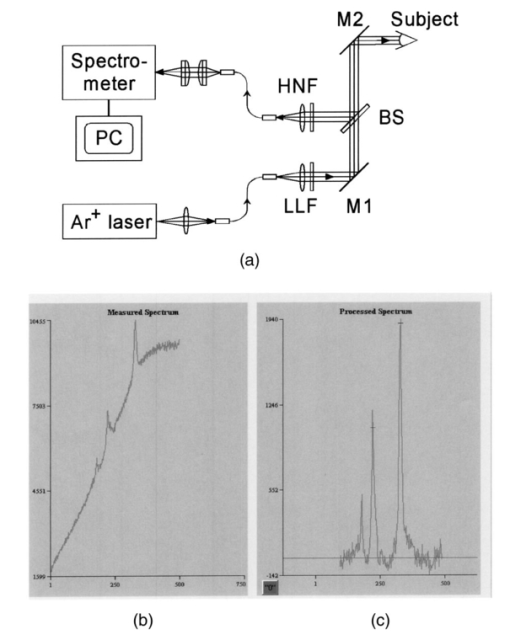 The Raman spectra from the macula of a healthy human volunteer, measured with dilated pupil, are shown in Figs. 7(b) and 7(c). Figure 7(b) shows a typical spectrum obtained from a single measurement and clearly reveals carotenoid Raman signals superimposed on a spectrally broad fluorescence background. This background is caused partially by the intrinsic fluorescence of carotenoid and partially by lipofuscin fluorophors deposited in the retinal pigment epithelial layer. The ratio between the intensities of the carotenoid C=C Raman peak and the fluorescence background is high enough (∼0.25) that it is easily possible to quantify the amplitude of the C=C peak after digitally subtracting the background [Fig. 7(c)]. Initial experience with the resonance Raman scanner in a clinical setting revealed that it was sensitive, specific, and well accepted by the subjects. Intersession and intrasession repeatability was in the range of ±10,48 49 matching or exceeding other in vivo macular pigment measurement techniques, objective or subjective. Since the clinical version relies on foveal alignment on a fixation target, 20/80 or better acuity is ordinarily required. Subjects with dense media opacities such as visually significant cataracts or with poor pharmacologic pupillary dilation (<6 mm) were generally excluded from studies since their readings might be artifactually low. A survey of a large population of clinically examined normal individuals ranging from 21 to 84 years old showed that there was an approximately 10-fold range of macular pigment in each decade and that there is a steady decline of average macular pigment readings with increasing age until the readings level off at a steady low level past age 60 (Ref. 48). This decline cannot be explained by yellowing of the lens with age because nearly half of the elderly subjects had had prior cataract surgery and now had optically clear prosthetic intraocular lenses, but their macular pigment levels were consistently lower than those of the young subjects with natural lenses. Macular carotenoids were 32 lower in AMD patients who did not regularly consume high doses of lutein supplements (⩾4 mg/day) relative to age-matched controls who did not consume supplements.49 51 AMD patients who began to consume high-dose lutein supplements regularly after their initial diagnosis of AMD had levels in the normal range for their age. These findings are very supportive of the hypothesis that low macular carotenoid levels are a risk factor for AMD and that macular pigment levels can be modified through supplements, even in an elderly population with significant macular pathology. Measurements made with resonance Raman scattering (RRS) are not directly comparable to HFP since RRS in its current form measures absolute amounts of carotenoids in the entire area illuminated with the laser,46 47 48 while HFP measures perceived optical density only at the edge of the illumination spot relative to a peripheral site.52 Nevertheless, an initial comparison study has been performed using HFP with 1.5-deg macular illumination and RRS with a 1-mm laser spot.53 Baseline studies on 40 healthy individuals below age 61 revealed a significant correlation between the two methods, but intrasession and intersession variability was much lower for RRS. Both methods displayed an inverse correlation with increasing age, but only the decline revealed by RRS was statistically significant, which was due in part to HFP’s higher variability. 4.Raman ImagingRecently we extended our carotenoid Raman spectroscopy to an imaging mode.54 This approach can map the spatial profiles of MP distributions with micron-scale resolution and at the same time assess their concentrations while maintaining the high specificity for caroteniods. Using excised human eye cups as initial test samples and resonant excitation of the pigment molecules with narrow-bandwidth blue light from a mercury arc lamp, we recorded Raman images originating from the carbon-carbon double-bond stretch vibrations of the molecules. The experimental setup is shown schematically in Fig. 8. Light from a mercury arc lamp is routed via a fiber bundle into a light delivery and collection module. Inside the module, the light is sent through a diffuser (not shown); collimated by a condenser lens, L1; spectrally filtered at 488 nm with a 1-nm bandpass filter, F1; further filtered via reflection from a notch-type holographic beamsplitter, BS; and imaged by lens L2 (30-mm focal length) onto a spot about 4 mm in diameter centered on the fovea of the excised eye cup (EC). The light scattered back from the retina is collected by lens L2, transmitted through the beamsplitter, and filtered at the C=C stretch frequency (527 nm in the case of 488-nm excitation). The filtering is achieved with a combination of narrowband interference filters (F2 and F3 with bandwidths of 1 and 10 nm, respectively). Camera lens L3 is used to image the Raman-scattered light onto the pixel array of a CCD camera, permitting digital image acquisition with 16 bits of gray scale. Filter F2 is angle tuned to alternately transmit the Raman-scattered light at 527 nm (on-peak position) or to transmit the background light at a wavelength position of 525 nm, just missing the Raman peak (off-peak position). The bandwidth of filter F2 is chosen so that it matches the bandwidth of the excitation light for the maximum Raman signal throughput. The beamsplitter BS and filters F2 and F3 combine to provide an extinction of 10−6 at the Raman excitation wavelength (488 nm). Figure 8Experimental setup used for Raman imaging. Filtered light from a mercury light source is projected onto an excised eye cup and the Raman-scattered light is imaged onto a CCD camera array. See text for discussion. 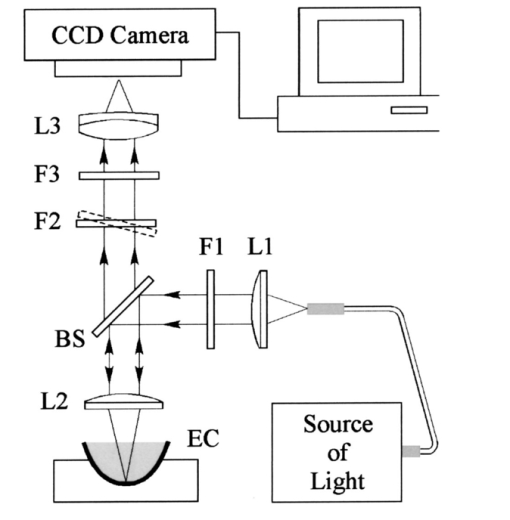 To generate a Raman image, we used the difference between two image datasets. A first dataset was obtained with filter F2 tuned to the on-peak position and another set with the filter tuned to the off-peak position. The eye cup was placed in a hemispherical holder so that its natural shape would be preserved, and eyes with discernable ocular pathology or postmortem artifacts (e.g., hemorrhages, retinal detachment, macular holes) were rejected. For both datasets, identical exposure times (typically 50 s) and imaging conditions were used. All datasets were processed with software obtained from the National Institutes of Health (NIH Image 1.62), allowing one to display the Raman signal levels received by the CCD pixel array versus spatial position as en face maps and/or as topographical representations (“surface plots”) in pseudo (false) colors or shades of gray. We measured a total of twelve excised postmortem eye cups from donors aged 7 to 60 years with no known history of ocular pathology, all of which yielded significantly varying Raman images. Typical results for macular pigment distributions are shown in Fig. 9. The eye cups differed dramatically in spatial widths of MP, symmetry, and absolute levels. The Raman image for the eye cup shown in Fig. 9(b) clearly reveals an asymmetric, cone-shaped pigment distribution, with high pigment levels concentrated over a small-diameter central area (FWHM, ∼1 mm) and rapidly decreasing levels toward the wings of the cone. The center of the distribution appears to have a depletion in the pigment density about 250-μm in diameter, with a hole depth of roughly half the peak pigment concentration. The Raman image for the eye cup in Fig. 9(a) reveals a much narrower distribution (FWHM, ∼250 μm, corresponding to an approximately fourfold reduction in width) and no central hole. Line plots, obtained from the measured en face images by plotting the pixel intensities along a line running through the center of the distributions, can be used to quantify the variations in MP widths and hole depths in any desired axis running through the center of the distributions [Figs. 9(c) and 9(d)]. These results demonstrate that resonance Raman spectroscopy is capable of imaging physiological MP distributions in human eye cups with a good signal-to-noise ratio, even when using Raman excitation with a nonlaser light source. Figure 9Raman images and line plots of two excised eye cups. (a) and (b) Surface plots emphasizing the topology of the MP distribution. Note the asymmetric MP distribution, strong and narrow ridge of the eye cup in (a), and the symmetric volcanolike MP distribution with central depression of the eye cup in (b). (c) and (d) Line scans of respective MP distributions obtained by plotting Raman intensities along a line running through the center of the MP distributions. 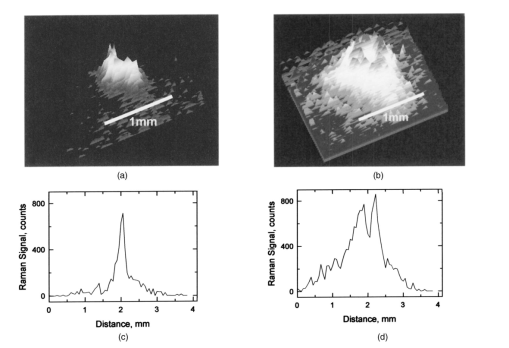 Our Raman image results appear to be in agreement with results published by other groups relating to macular pigment distributions in healthy living retinas. These include SLO measurements57 where drastic individual variations in macular pigment distributions were observed, including the observation of a central “hole” in pigment levels, and small-stimulus heterochromatic flicker photometry17 with a reported sevenfold variation in individual macular pigment widths. Efforts are under way to modify our instrument for use in living eyes. The integral of the area illuminated in this imaging technique can be correlated with the previous single-spot resonance Raman method or with extraction and HPLC analysis. There is a wide range of peak widths at half maximum, but it is clear that a 1-mm diameter spot is sufficient to encompass the entire foveal peak of macular pigment in all eyes examined so far. It is anticipated that a similar instrument suitable for use in living human eyes will continue to provide new insights on the correlations of macular carotenoid levels and distributions with various macular pathological states. 5.DiscussionThe following is a discussion of the advantages and disadvantages of the current technologies used to noninvasively assess MP status. HFP is a psychophysical test, and as a subjective technology it has to rely on a number of assumptions. The subject must understand the assigned task and perform it with a high degree of attention to fixation, especially when working with an eccentric target. Thus it is usually wise to provide adequate training to subjects and to confirm reliability by repetitive measurements at separate sessions. Despite extensive training, some subjects (∼5) never learn to perform HFP reliably, particularly when viewing the eccentric target, which is subject to perceptual (Troxler) fading.25 In our experience, elderly subjects tend to have more trouble performing HFP than younger subjects, especially if they have significant macular pathology. Psychophysical measurement of macular pigment requires that color perception be similar at the central and peripheral fixation sites. This is not a trivial problem since the distribution of the various types of photoreceptors varies considerably with increasing eccentricity.55 At the foveal center, there are no rod photoreceptors and no short-wavelength cones (S-cones), but there is an abundance of medium-wavelength cones (M-cones) and long-wavelength cones (L-cones). Just outside of the foveal center, the density of rods and S-cone rises, while the density of L- and M-cones drops precipitously. The S-cone peak density is within a millimeter of the foveal center, while rod cell density peaks just outside of the macula at 5 to 7 mm of eccentricity. Thus it is clear that color perception would not be expected to be the same at central and peripheral sites. HFP minimizes the contribution of S-cones and rods by providing a blue background light to bleach these shorter wavelength pigments, and the flicker rate of the testing spot is higher than their critical flicker frequencies.25 This allows the HFP task to be mediated primarily by the M-cone and L-cone systems. As long as the L to M ratio is invariant spatially, HFP should be valid. It appears that this assumption is correct for young, healthy individuals,56 but there is some evidence that with aging or macular pathology this assumption may no longer be valid.57 HFP assumes that the carotenoid optical density at the eccentric fixation reference point is zero, and all other carotenoid optical density measurements are calculated relative to the zero point in order to correct for media opacities and interindividual differences in L to M cone ratios. If this reference point is not actually zero, then all other readings will be systematically underestimated. Therefore it is important to make certain that the eccentric fixation point is far enough away from the foveal center in a region that no longer has substantial levels of carotenoids. It is known by HPLC studies that carotenoids are present a low levels throughout the peripheral retina,58 15 16 but the levels are generally low enough to be considered negligible when performing HFP unless the central concentration of carotenoids is also extremely low. Compared with objective reflectance, fluorescence, and Raman techniques, a principal disadvantage of HFP is its limitation to a nonimaging MP assessment mode. At best it is possible to map out the MP distribution point by point along some arbitrarily chosen axes, with associated measurement times that are too long for widespread clinical applications. Also, this approach is likely to miss asymmetries and central depletions of pigment distributions, as shown by reflectance and Raman techniques. Reflectance techniques are objective and have the advantage of easily generating images of MP distributions. There are some limitations of this technology, however. The apparatus may be quite expensive when attempting to obtain quantitative information about the MP levels, especially when it consists of custom-modified scanning laser ophthalmoscope systems. Also, as with HFP, it must be assumed that no other pigments anywhere in the optical path contribute significantly to relative absorbances on- and off-peak, and they are both subtractive technologies that require the carotenoid content of the periphery to be set to zero, although more recently it has been claimed that quantitative assessment of macular pigment can be performed using only 488-nm illumination.59 Comparing Raman scattering with reflectometry and autofluorescence methods, we note that besides spectral selectivity, the three methods differ dramatically in the respective light paths used for fundus excitation and reflection. Shown schematically in Fig. 10 are the ocular media and fundus tissue layers to be considered. The Raman method uses light that is Raman backscattered directly from the macular pigment layer, which is positioned in the innerermost layer of the fundus. Therefore it does not have to rely, as is the case for the other two techniques, on light propagation through deeper fundus layers or reflection at those layers or the sclera. In fact, any laser excitation light transmitted through the macular pigment layer can be considered as ineffective for a return-path Raman excitation in view of the high absorption and light scattering of the deeper fundus layers. Figure 10Schematic representation of ocular media and fundus tissue layers. ILM, inner limiting membrane; PhR, photoreceptors; RPE, retinal pigment epithelium; BM, Bruch’s membrane; CC, choriocapillaris; CS, choroidal stroma. (Adapted from Ref. 60; not to scale.) 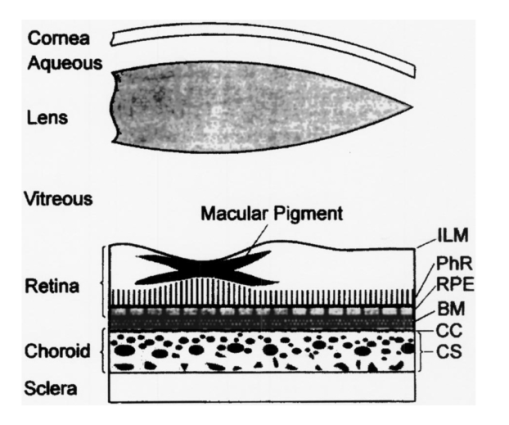 Fundus reflectometry measures macular pigment absorption indirectly by comparing highly complex fundus reflectance spectra for two different locations. The measuring light traverses all fundus layers twice and the spectral fit needed for the calculation of the pigment density relies on mathematical models of the respective in vivo optical properties of the stratified fundus layers, all assumed to be homogeneous (absorption, reflection, and scattering strengths as well as spectral profiles). This could be problematic considering that all biological tissue layers, in particular irregularly distributed choroidal vessels and melanocytes in the choroidal stroma, are principally heterogeneous optical media.37 60 Autofluorescence appears to have an advantage over reflectometry in that it uses an indirect light source (lipofuscin) in the retinal epithelial layer for a single-path measurement of macular pigments and thus avoids traversal of the deeper fundus tissue layers. However, like reflectometry, it assumes a homogeneous distribution of tissue layers. Furthermore, it assumes that the fluorophore at the fovea is the same as that as at the perifovea and that foveal-perifoveal differences in absorption by other pigments located between the macular pigment and the fluorophore (retinal blood, visual pigments, retinal pigment epithelium melanin) are negligible. If one assumes homogeneous optical properties of the tissue layers, reflectometry and autofluorescence detection methods do not require a correction for media absorption, unlike the Raman method in its current form, since the measurement at a reference location eliminates that contribution. The reference measurement also eliminates the effects of light loss by scattering, and the effects of any neutral absorber or light loss, such as from the pupil. 6.ConclusionThere has been a significant advance in recent years regarding the development of noninvasive optical MP detection technologies. Promising approaches exist to achieve a widely applicable detection technology that ideally would measure MP levels with high specificity, provide images of MP distribution, and give quantitative results. At the moment, no single existing technology can fulfill these requirements, but in view of the rapidly advancing field of biophotonics, the realization of such technology appears feasible in the near future. AcknowledgmentsThis research was supported by funds from Spectrotek, L.C., the National Eye Institute (R29-EY-11600, STTR 1 R41 EY12324-01, and STTR 2 R42 EY1234-02), and Research to Prevent Blindness, Inc. REFERENCES
R. A. Bone
,
J. T. Landrum
, and
S. L. Tarsis
,
“Preliminary identification of the human macular pigment,”
Vision Res. , 25 1531
–1535
(1985). Google Scholar
G. J. Handelman
,
D. M. Snodderly
,
A. J. Adler
,
M. D. Russett
, and
E. A. Dratz
,
“Measurement of carotenoids in human and monkey retinas,”
Methods Enzymol. , 213 220
–230
(1992). Google Scholar
D. M. Snodderly
,
“Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins,”
Am. J. Clin. Nutr. , 62 1448S
–1461S
(1995). Google Scholar
Eye Disease Case Control Study Group, “,
“Antioxidant status and neovascular age-related macular degeneration,”
Arch. Ophthalmol. (Chicago) , 111 104
–109
(1993). Google Scholar
J. M. Seddon
,
U. A. Ajani
,
R. D. Sperduto
,
R. Hiller
,
N. Blair
,
T. C. Burton
,
M. D. Farber
,
E. S. Gragoudas
,
J. Haller
,
D. T. Miller
,
L. A. Yannuzzi
, and
W. Willet
,
“Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration,”
J. Am. Med. Assoc. , 272 1413
–1420
(1994). Google Scholar
Age-Related Eye Disease Study Group. “,
“A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8,”
Arch. Ophthalmol. (Chicago) , 119 1417
–1436
(2001). Google Scholar
J. T. Landrum
,
R. A. Bone
, and
M. D. Kilburn
,
“The macular pigment: a possible role in protection from age-related macular degeneration,”
Adv. Pharmacol. (San Diego) , 38 537
–556
(1997). Google Scholar
R. A. Bone
,
J. T. Landrum
,
S. T. Mayne
,
C. M. Gomez
,
S. E. Tibor
, and
E. E. Twaroska
,
“Macular pigment in donor eyes with and without AMD: a case-control study,”
Invest. Ophthalmol. Visual Sci. , 42 235
–240
(2000). Google Scholar
D. M. Snodderly
,
J. D. Auran
, and
F. C. Delori
,
“The macular pigment, I: absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas,”
Invest. Ophthalmol. Visual Sci. , 25 660
–673
(1984). Google Scholar
R. A. Bone
,
J. T. Landrum
,
L. Fernandez
, and
S. L. Tarsis
,
“Analysis of macular pigment by HPLC: retinal distribution and age study,”
Invest. Ophthalmol. Visual Sci. , 29 843
–849
(1988). Google Scholar
R. A. Bone
,
J. T. Landrum
,
L. M. Friedes
,
C. A. Gomez
,
M. A. Kilburn
,
E. Menendez
,
I. Vidal
, and
W. Wang
,
“Distribution of lutein and zeaxanthin stereoisomers in the human retina,”
Exp. Eye Res. , 64 211
–218
(1997). Google Scholar
D. M. Snodderly
,
G. J. Handelman
, and
A. A. Adler
,
“Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys,”
Invest. Ophthalmol. Visual Sci. , 32 268
–279
(1991). Google Scholar
J. D. M. Gass
,
“The Mu¨ller cell cone, an overlooked part of the anatomy of the fovea centralis,”
Arch. Ophthalmol. (Chicago) , 117 821
–823
(1999). Google Scholar
O. G. Sommerberg
,
W. G. Siems
,
J. S. Hurst
,
J. W. Lewis
,
D. S. Kliger
, and
F. J. van Kuijk
,
“Lutein and zeaxanthin are associated with photoreceptors in the human retina,”
Curr. Eye Res. , 19 502
–505
(1999). Google Scholar
L. M. Rapp
,
S. S. Maple
, and
J. H. Choi
,
“Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina,”
Invest. Ophthalmol. Visual Sci. , 41 1200
–1209
(2000). Google Scholar
B. R. Hammond Jr.,
B. R. Wooten
, and
D. M. Snodderly
,
“Individual variations in the spatial profile of human macular pigment,”
J. Opt. Soc. Am. A , 14 1187
–1196
(1997). Google Scholar
S. Beatty
,
H.-H. Koh
,
D. Henson
, and
M. Boulton
,
“The role of oxidative stress in the pathogenesis of age-related macular degeneration,”
Surv. Ophthalmol. , 45 115
–134
(2000). Google Scholar
V. M. Reading
and
R. A. Weale
,
“Macular pigment and chromatic aberration,”
J. Am. Optom. Assoc. , 64 231
–234
(1974). Google Scholar
R. A. Bone
and
J. M. B. Sparrock
,
“Comparison of macular pigment densities in human eyes,”
Vision Res. , 11 1057
–1064
(1971). Google Scholar
J. S. Werner
and
B. R. Wooten
,
“Opponent chromatic mechanisms: relation to photopigment and hue naming,”
J. Opt. Soc. Am. , 69 422
–434
(1979). Google Scholar
B. R. Wooten
,
B. R. Hammond Jr.,
R. I. Land
, and
D. M. Snodderly
,
“A practical method for measuring macular pigment optical density,”
Invest. Ophthalmol. Visual Sci. , 40 2481
–2489
(1999). Google Scholar
J. Mellerio
,
S. Ahmadi-Lari
,
F. van Kuijk
,
D. Pauleikhoff
,
A. Bird
, and
J. Marshall
,
“A portable instrument for measuring macular pigment with central fixation,”
Curr. Eye Res. , 25 37
–47
(2002). Google Scholar
B. R. Hammond Jr.,
K. Fuld
, and
D. M. Snodderly
,
“Iris color and macular pigment optical density,”
Exp. Eye Res. , 62 293
–297
(1996). Google Scholar
B. R. Hammond Jr.,
J. Curran-Celentano
,
S. Judd
,
K. Fuld
,
N. I. Krinsky
,
B. R. Wooten
, and
D. M. Snodderly
,
“Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns,”
Vision Res. , 36 2001
–2012
(1996). Google Scholar
R. A. Bone
,
J. T. Landrum
,
Z. Dixon
,
Y. Chen
, and
C. M. Llerena
,
“Lutein and zeaxanthin in the eyes, serum and diet of human subjects,”
Exp. Eye Res. , 71 239
–245
(2000). Google Scholar
S. Beatty
,
I. J. Murray
,
D. B. Henson
,
D. Carden
,
H. Koh
, and
M. E. Boulton
,
“Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population,”
Invest. Ophthalmol. Visual Sci. , 42 439
–446
(2001). Google Scholar
T. S. Aleman
,
J. L. Duncan
,
M. L. Bieber
,
E. B. de Castro
,
D. A. Marks
,
L. M. Gardner
,
J. D. Steinberg
,
A. V. Cideciyan
,
M. G. Maguire
, and
S. G. Jacobson
,
“Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome,”
Invest. Ophthalmol. Visual Sci. , 42 1873
–1881
(2001). Google Scholar
J. T. Landrum
,
R. A. Bone
,
H. Joa
,
M. D. Kliburn
,
L. L. Moore
, and
K. E. Sprague
,
“A one year study of the macular pigment: the effect of 140 days of a lutein supplement,”
Exp. Eye Res. , 65 57
–62
(1997). Google Scholar
B. R. Hammond
,
E. J. Johnson
,
R. M. Russell
,
N. I. Krinsky
,
K. J. Yeum
,
R. B. Edwards
, and
D. M. Snodderly
,
“Dietary modification of human macular pigment density,”
Invest. Ophthalmol. Visual Sci. , 38 1798
–1801
(1997). Google Scholar
F. C. Delori
,
E. S. Gragouda
,
R. Francisco
, and
R. C. Pruett
,
“Monochromatic ophthalmoscopy and fundus photography,”
Arch. Ophthalmol. (Chicago) , 95 861
–868
(1997). Google Scholar
T. T. Berendschot
,
R. A. Goldbohm
,
W. A. Klopping
,
J. van de Kraats
,
J. van Norel
, and
D. van Norren
,
“Influence of lutein supplementation on macular pigment, assessed with two objective techniques,”
Invest. Ophthalmol. Visual Sci. , 41 3322
–3326
(2000). Google Scholar
H. Wustemeyer
,
C. Jahn
,
A. Nestler
,
T. Barth
, and
S. Wolf
,
“A new instrument for the quantification of macular pigment density: first results in patients with AMD and healthy subjects,”
Graefe's Arch. Clin. Exp. Ophthalmol. , 240 660
–671
(2002). Google Scholar
D. van Norren
and
L. F. Tiemeijer
,
“Spectral reflectance of the human eye,”
Vision Res. , 26 313
–320
(1986). Google Scholar
P. E. Kilbride
,
K. R. Alexander
,
M. Fishman
, and
G. A. Fishman
,
“Human macular pigment assessed by imaging fundus reflectometry,”
Vision Res. , 29 663
–674
(1989). Google Scholar
J. van de Kraats
,
T. T. Berendschot
, and
D. van Norren
,
“The pathways of light measured in fundus reflectometry,”
Vision Res. , 15 2229
–2247
(1996). Google Scholar
T. T. Berendschot
,
J. J. Willemse-Assink
,
M. Bastiaanse
,
P. T. de Jong
, and
D. van Norren
,
“Macular pigment and melanin in age-related maculopathy in a general population,”
Invest. Ophthalmol. Visual Sci. , 43 1928
–1932
(2002). Google Scholar
F. C. Delori
,
C. K. Dorey
,
G. Staurenghi
,
O. Arend
,
D. G. Goger
, and
J. J. Weiter
,
“In vivo fluorescence of the ocular fundus exhbits retinal pigment epithelium lipofuscin characteristics,”
Invest. Ophthalmol. Visual Sci. , 36 718
–729
(1995). Google Scholar
F. C. Delori
,
D. G. Goger
,
B. R. Hammond
,
D. M. Snodderly
, and
S. A. Burns
,
“Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry,”
J. Opt. Soc. Am. A , 18 1212
–1230
(2001). Google Scholar
J. R. Sparrow
,
C. A. Parish
,
M. Hashimoto
, and
K. Nakanishi
,
“A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture,”
Invest. Ophthalmol. Visual Sci. , 40 2988
–2995
(1999). Google Scholar
F. C. Delori
,
D. G. Goger
, and
C. K. Dorey
,
“Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects,”
Invest. Ophthalmol. Visual Sci. , 42 1855
–1866
(2001). Google Scholar
P. S. Bernstein
,
M. D. Yoshida
,
N. B. Katz
,
R. W. McClane
, and
W. Gellermann
,
“Raman detection of macular carotenoid pigments in intact human retina,”
Invest. Ophthalmol. Visual Sci. , 39 2003
–2011
(1998). Google Scholar
I. V. Ermakov
,
R. W. McClane
,
W. Gellermann
,
D. Y. Zhao
, and
P. S. Bernstein
,
“Resonant Raman detection of macular pigment levels in the human retina,”
Opt. Lett. , 26 202
–204
(2001). Google Scholar
W. Gellermann
,
I. V. Ermakov
,
M. R. Ermakova
,
R. W. McClane
,
D. Y. Zhao
, and
P. S. Bernstein
,
“In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina,”
J. Opt. Soc. Am. A , 19 1172
–1186
(2002). Google Scholar
P. S. Bernstein
,
D. Y. Zhao
,
S. W. Wintch
,
I. V. Ermakov
, and
W. Gellermann
,
“Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients,”
Ophthalmology , 109 1780
–1787
(2002). Google Scholar
I. V. Ermakov
,
M. R. Ermakova
,
W. Gellerman
, and
P. S. Bernstein
,
“Macular pigment Raman detector for clinical applications,”
J. Biomed. Opt. , 9
(1), 139
–148
(2004). Google Scholar
P. S. Bernstein
,
“New insight into the role of macular carotenoids in age-related macular degeneration: resonance Raman studies,”
Pure Appl. Chem. , 74 1419
–1425
(2002). Google Scholar
J. S. Werner
,
M. L. Bieber
, and
B. E. Schefrin
,
“Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density,”
J. Opt. Soc. Am. A , 17 1918
–1932
(2000). Google Scholar
W. Gellermann
,
I. V. Ermakov
,
R. W. McClane
, and
P. S. Bernstein
,
“Raman imaging of human macular pigments,”
Opt. Lett. , 27 833
–835
(2002). Google Scholar
S. Otake
and
C. M. Cicerone
,
“L and M cone relative numerosity and red-green opponency from foeato mid-periphery in human retina,”
J. Opt. Soc. Am. A , 17 615
–627
(2000). Google Scholar
A. E. Elsner
,
S. A. Burns
,
E. Beausencourt
, and
J. Weiter
,
“Foveal cone photopigment distribution: small alterations associated with macular pigment distribution,”
Invest. Ophthalmol. Visual Sci. , 39 2394
–2404
(1998). Google Scholar
J. T. Landrum
,
R. A. Bone
,
L. L. Moore
, and
C. M. Gomez
,
“Analysis of zeaxanthin distribution within individual human retinas,”
Methods Enzymol. , 299 457
–467
(1999). Google Scholar
D. Schweitzer
,
G. E. Lang
,
B. Beuermann
,
H. Remsch
,
M. Hammer
,
E. Thamm
,
C. W. Spraul
, and
G. K. Lang
,
“Objektive Bestimmung der optischen Dichte von Xanthophyll nach Supplementation von Lutein,”
Ophthalmologe , 99 270
–275
(2002). Google Scholar
F. C. Delori
and
K. P. Pflibsen
,
“Spectral reflectance of the human ocular fundus,”
Appl. Opt. , 28 1061
–1077
(1989). Google Scholar
|
CITATIONS
Cited by 35 scholarly publications.
Raman spectroscopy
Eye
Absorption
Retina
Luminescence
Absorbance
Tissue optics

