|
|
1.IntroductionMany groups1 2 3 have demonstrated the clinical utility of fluorescence spectroscopy, and industry is avidly pursuing this technology for clinical implementation. Our group is studying fluorescence spectroscopy for the detection of cervical cancer, hoping to decrease the costs of screening and detection in the developed world, as well as lessen the need for infrastructure in the developing world. Fluorescence spectroscopy has demonstrated a sensitivity and specificity of 86 and 74 in the diagnostic colposcopy clinic and 75 and 804 5 6 7 in the screening setting, showing its potential for improved diagnosis. However, additional validation studies are required to demonstrate the reliability of its diagnostic capability. It has been shown that factors including smoking, race,8 and menstrual cycle9 do not account for differences in fluorescence intensity within and between patients, while age, menopausal status,8 and histologic diagnosis10 do cause changes in fluorescence intensity and lineshape. Frequently, in vivo fluorescence spectroscopy measurements are made using a fiber optic probe which is placed in gentle contact with the tissue. The amount of pressure exerted by the operator can vary. The effect of varying pressure of the fiber optic probe on the cervix must be evaluated to determine whether it accounts for any of the intra-patient and inter-patient variations in fluorescence spectroscopy measurements. Although the effect of probe pressure on intensity readings was found to be insignificant in Raman spectroscopy on the gastrointestinal tract,11 it may play a role in cervical fluorescence spectroscopy. A possible scenario is that increased probe pressure will force blood away from the site measured, decrease hemoglobin content, and therefore blood absorption of incident light, thus increasing the fluorescence intensity of this site. This increase in intensity due to probe pressure would cause unnecessary and problematic variability. If probe pressure is a significant variable, then pressure will need to be measured and taken into consideration in algorithm development. If the variability due to pressure is insignificant, however, compared to that due to tissue abnormality, menopausal state, and columnar/squamous tissue type, then the variability will not pose problems to diagnosis. In this study, we explore the impact of probe pressure as a variable affecting fluorescence spectroscopic measurements in cervical tissue. 2.Materials and Methods2.1.InstrumentationThe spectroscopic system used to measure fluorescence excitation emission matrices (EEMs) has been described in detail previously.12 Briefly, the system measures fluorescence emission spectra at 19 excitation wavelengths, ranging from 300 to 480 nm in 10-nm increments with a spectral resolution of 5 nm. The system incorporates a fiber optic probe, a xenon arc lamp coupled to a monochromator to provide excitation light, and a polychromator and thermoelectrically cooled charge-coupled device (CCD) camera to record fluorescence intensity as a function of emission wavelength. The fiber optic probe consists of 25 illumination fibers and 12 collections fibers (200 μm diameter with 0.2 numerical aperture) arranged at random. They are placed on a 15-mm-thick quartz mixing element (2 mm diameter, 0.2 numerical aperture). The outer diameter of the probe is 5 mm. At the distal tip of the probe, the fibers are placed in contact with a short piece of quartz-core optical fiber, which serves as an optical shield. The shield is placed in contact with the sample surface and ensures that the area, which is illuminated, is the same as that from which fluorescence is collected. 2.2.ProbeA spring-loaded device (Fig. 1) was mounted at the proximal end of the fiber optic probe to deliver consistent pressure on the measurement site. The device consists of an inner sleeve that attaches directly onto the probe, a handle that slides over the inner sleeve, a coiled spring to deliver the translational force from handle to the tip of the probe, and a fixture on the probe. The inner sleeve has a pressure scale that is calibrated at three pressure levels, as described in the next section. As the operator slides the handle over the inner sleeve, a proportional level of force is exerted onto the fixture through a coiled spring (Small Parts Inc. CS-51), which is then delivered to the measurement site at the tip of the probe. 2.3.Pre-Clinical Methods: Probe Pressure MeasurementPrior to the clinical study, a quantitative standard for light, medium, and firm levels of probe pressure was calibrated based on measurements from each provider (three nurse practitioners and one physician). A commercial weight scale (Soehnle 8041) with 2-g resolution was placed vertically in order to acquire pressure measurements from a setup that simulates the cervix. A slice of chicken breast was glued on the weight scale to reproduce the elasticity of cervical tissue. The scale was adjusted to a height comparable to that of the cervix during spectroscopic measurements. Each provider placed the fiber optic probe on the chicken breast specimen using light, medium, and firm levels of pressure, as normally performed in clinic. The readings from the weight scale at each pressure level were recorded. Five sets of measurements were acquired from each provider. Provider A had made thousands of measurements over 12 years. Providers B and D had made 100 measurements over 10 years. Provider C was new to the system. The average force exerted at each pressure level with standard deviation bars is shown in Fig. 2(a), with A, B, C, and D representing different providers. The three pressure levels used in the study were to be set at these average values and markings were set at the spring compression values corresponding to these settings. A decision was made to calibrate to Provider A after looking at the data. Prior to using the pressure device, once calibrated, some patients complained of pain. After the calibration, no patients complained of pain. This experience was like that of all previously performed studies; that is, no patients ever complained of the probe being painful. Thus, the investigators reassured that provider A, who had measured thousands of measurements over twelve years, had the most consistent method of measurement. Figure 2(a) Average force values measured by four different providers (A,B,C,D) when asked to press with low pressure, medium pressure, and hard pressure on a piece of chicken breast tissue using the fiber optic probe without the pressure calibration sleeve. (b) Average force values measured by the same four providers, using the pressure calibration sleeve to deliver three calibrated levels of pressure. (c) Average force values measured by same four providers using the same pressure device and an arm rest. 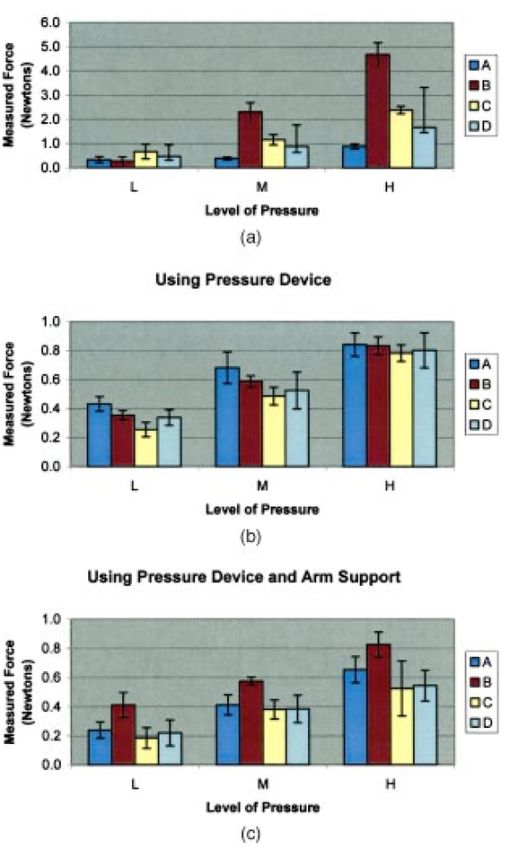 The effectiveness of the device in ensuring a constant reading for each pressure level was then tested. Providers pressed on the chicken breast using the probe with pressure device, pushing the gauge to the appropriate pressure marking level for each measurement. This was repeated five times successively for each provider. Figure 2(b) shows the average of the five measurements for each provider (A,B,C,D) with standard deviation bars. An arm support was also tested for effectiveness in helping providers maintain a constant pressure level. The arm support, however, was shown to lessen the abilities of providers to consistently apply the correct level of pressure and was therefore not utilized in clinical measurements, as shown in Fig. 2(c). The providers underwent a training session using the probe with pressure device over several sessions in clinic with SC. 2.4.Clinical MeasurementsThe study protocol was reviewed and approved by the Institutional Review Boards at the University of Texas M.D. Anderson Cancer Center and the University of Texas at Austin. Patients 18 years and older, not pregnant, and referred to the colposcopy clinic with an abnormal Pap smear were eligible. All patients signed an informed consent and underwent a history, complete physical exam, Pap smear, cultures, pan-colposcopy, and colposcopically-directed biopsies. Patients were compensated $50.00 for participation in the study as they were asked to stay in clinic for additional time; data collection for three EEMs required approximately 2 min. Fluorescence spectra were measured from up to three sites per patient: one squamous normal site, one abnormal site, and if colposcopically visible, one columnar normal site. At each site, spectroscopic measurements were obtained using the three pre-calibrated pressure levels (light, medium, firm) of probe pressure. At each site, the probe was applied for 2 min, with a 30-sec interval between pressure measurements. The spectroscopic device with excitation wavelengths ranging from 300 to 480 nm in 10-nm intervals is a non-significant risk device by FDA standards. However, in order to minimize UV exposure due to repeat measurements and to reduce total measurement time, fluorescence excitation wavelengths between 320 and 470 nm at 30-nm intervals were used; emission wavelengths spanned 330 to 700 nm in increments of 5 nm. 2.5.Device for Clinical Pilot StudyBefore use, the fiber optic probe was disinfected with Metricide (Metrex Research Corp.) for 20 min. The probe was then rinsed with water and dried with sterile gauze. Acetic acid, which enhances the difference in fluorescence between normal and dysplastic tissue,13 was applied to the cervical epithelium prior to the placement of the probe. The disinfected probe was guided into the vagina and its tip positioned flush with the cervical epithelium. When noticeable bleeding was observed, the blood was removed from the measurement site and the probe tip using an alcohol swab immediately before the optical measurement. Fluorescence excitation-emission matrices were obtained from one to three sites of the cervix for 20 patients. For histopathologic diagnosis, biopsies were taken from each of the sites that underwent spectroscopic measurement. As a negative control, a background EEM was obtained with the probe immersed in a nonfluorescent bottle filled with distilled water at the beginning of each day. Prior to each patient’s measurement, a fluorescence EEM was taken with the probe placed on the surface of a quartz cuvette containing a solution of Rhodamine 610 (Exciton, Dayton, OH) dissolved in ethylene glycol (2 mg/mL). This measurement is used as a standard to calibrate the device for each patient measurement. To correct for the non-uniform spectral response of the detection system, the spectra of two calibrated sources were measured at the beginning of the study; in the visible spectrum, a National Institute of Standards and Technology traceable calibrated tungsten ribbon filament lamp was used and in the ultraviolet spectrum, a deuterium lamp was used (550C and 45D, Optronic Laboratories Inc, Orlando, FL). Correction factors were derived from these spectra. EEMs with dark current subtracted were then corrected for the non-uniform spectral response of the detection system. Variations in the intensity of the fluorescence excitation light source at different excitation wavelengths were corrected. This correction was made with the excitation light intensity measured from calibrated photodiode (818-UV, Newport Research Corp.) placed at the probe tip. 2.6.Data AnalysisFluorescence intensity data were pre-processed at the University of Texas at Austin spectroscopy laboratory. Statistical analyses were performed with MATLAB (Version 6.5, The MathWorks) at UT-MDACC, UT Austin, and Rice University. Patient sites with incomplete data were removed. Two different statistical analyses were performed to investigate the effect of probe pressure on the spectroscopic measurement. In the first analysis, average peak intensities of the fluorescence emission spectra at different levels of probe pressure were calculated. Previous studies show that fluorescence measurement is affected by the diagnosis of the measurement site (normal vs. dysplastic),10 tissue type (columnar vs. squamous),4 5 6 and menopausal status (pre- vs. post- menopausal)8 of the patient. In order to isolate the effect of probe pressure, normal and abnormal patients were separated when average peak intensities were calculated. Pre-menopausal and post-menopausal patients were also investigated separately. Two permutation analyses were performed using non-normalized and normalized spectral data. To determine if there was a significant effect on mean intensity at any excitation/emission wavelength pair, a nonparametric statistical analysis on non-normalized data was first performed. The null hypothesis at a given excitation/emission pair is that probe pressure does not cause a systematic change in fluorescence intensity. Hence, if within each patient and within each site, the three pressures were switched randomly, the comparison between the pressure groups of the randomized data would remain the same as in the original data. Note that any effect from patient or site within patient is preserved as only the pressure variable is randomized. To test this null hypothesis, we used the one-way analysis of variance (ANOVA) F-statistic with pressure as the factor (explanatory variable) and fluorescence intensity as the response variable.14 The F-statistic was computed for the original data and for randomized data (where the three pressure levels within patient and site are randomly reassigned to the intensities). We generated 15,000 different randomized data sets. A P-value is computed as the proportion of randomized F values which are larger than the real F-statistic. This was done at each of 325 excitation/emission pairs, so we expect about 5 of 325 or 16 P-values below the 0.05 even if there is no effect from pressure at any excitation/emission wavelength pair. To correct for this multiple comparisons problem, we employed the method of Westfall and Young.15 A new set of 15,000 randomized F-statistic values was designated as the “correction” set. These were converted to P-values using the original set of 15,000 randomized F-statistic values exactly as was done with the original data (this latter operation consumes the most computer time). The Westfall-Young procedure was then applied to the P-values of the correction set. The procedure was then repeated using the normalized data. The results of each analysis are presented separately. To summarize, the “Original Data” is a set 325 excitation/emission pairs. The “15,000 pairs data set”=“Randomized Data Set” is the Original Data Set randomized with different pressure labels. The “Randomized Data Set” is used to generate two analyses: the “Reference Set” that yields the F statistic and the P values and the “Correction Set” that yields the data set for the multiple corrections test. This type of analysis allows the creation of a “distribution” of the data, getting around the problem of finding the appropriate test for the data as it is distributed. This is a very difficult problem with large and cumbersome data sets such as these. While the first steps in analysis are to look at the raw data, check the distribution, and then choose the appropriate statistical comparisons based on the distribution, those steps are applied with more difficulty to data such as these. While examination of the raw data is of interest, it is difficult to check the distribution of the EEMs. Creating a distribution with a meaningful subset of the data allows a robust exploration of the data. Additionally, with data sets of this size, many relationships among pairs could be significant based on the size of the data set alone. The multiple corrections test allows us to exclude these pairs and focus on those that are not due to chance alone. 3.ResultsFluorescence EEMs were measured from a total of 41 sites in 20 patients in the study. The age and menopausal status of each patient as well as the diagnostic classification of each biopsy are listed in Table 1. Measurements from eight sites in three patients (patients 1, 2, and 9) were not used for further analysis because of poor spectroscopic measurements. Data were available for analysis, therefore, from a total of 33 sites in 17 patients. Table 1
The patients ranged in age from 19 to 70 years, were pre-, peri-, and post-menopausal, and all were good candidates for cervical biopsy in that the squamo-columnar junction, squamous epithelium, and columnar epithelium could be identified. Not all patients have all sites visible. Most patients do not have columnar epithelium accessible for biopsy. Sites were measured from the squamous epithelium, columnar epithelium, and transformation zone. Table 2 lists the sites measured and tissue types obtained from colposcopically directed and histologically verified biopsies. Since this was a pilot study, some providers had only a few measurements; one provider made two measurements in one patient while another provider measured 19 sites in 9 patients. Table 2
Figure 3(a) displays spectra measured from a squamous normal site at all three pressures at 320-nm excitation from patient 13, site 2. Note that light, medium, and firm pressures are not even in the count order. While the fluorescence spectra differ in overall intensity, they have similar fluorescence lineshape. In order to further investigate the changes in spectral shape, fluorescence spectra were normalized to their maximum intensity, as shown in Fig. 3(b). Differences can be observed at emission wavelengths between 420 to 450 nm. This wavelength range corresponds to the Soret absorption band due to hemoglobin.16 Figure 3Typical fluorescence emission spectra at 320-nm excitation measured at three different pressures (light, medium, and firm). Data are shown (a) before and (b) after normalizing the peak emission intensity to unity. 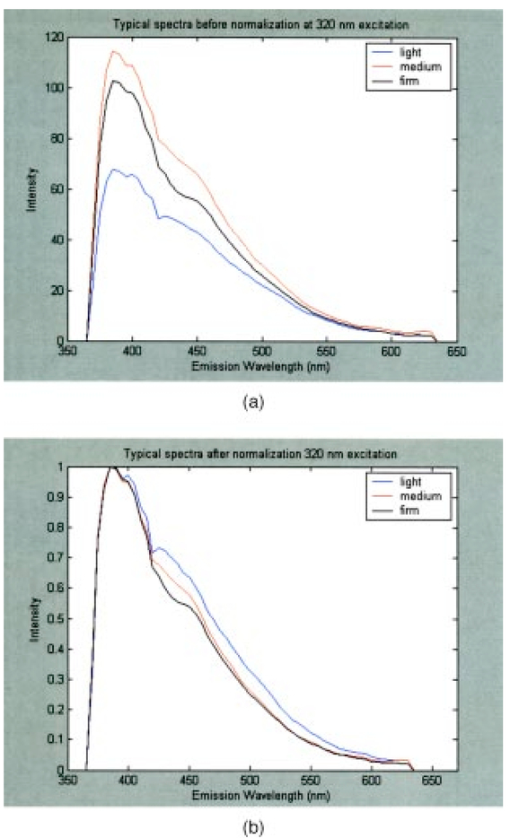 Figure 4 shows mean peak intensity of the 22 squamous normal and 7 squamous abnormal sites at all three pressures at 320-nm excitation [Fig. 4(a)], 380-nm excitation [Fig. 4(b)], and 470-nm excitation [Fig. 4(c)]. Error bars indicate plus and minus one standard error. For these analyses, biopsies showing inflammation were included with normal tissue, and biopsies showing human papillomavirus (HPV) and cervical intraepithelial neoplasia (CIN) were classified as abnormal. On average, the mean fluorescence intensity of abnormal tissue is lower than that of normal tissue, as previously demonstrated in many clinical studies.4 17 18 19 This is not a statistically significant difference. This suggests that pressure variability may not interfere with the diagnostic capability of spectroscopy, but that finding will need to be confirmed in a larger study. Figure 4Mean peak intensity at all three pressure (light, medium, and firm) levels for 22 squamous normal sites and 7 squamous abnormal sites at (a) 320-nm excitation, (b) 380-nm excitation, and (c) 470-nm excitation. 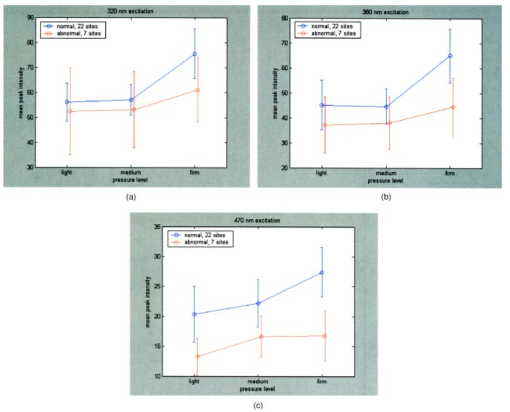 Figure 5 shows the mean fluorescence intensity at each pressure for the 17 squamous sites in pre-menopausal and 12 squamous sites in post-menopausal patients, at the same three excitation wavelengths: 320 nm [Fig. 5(a)], 380 nm [Fig. 5(b)], and 470 nm [Fig. 5(c)]. Peri-menopausal patients were grouped with post-menopausal patients in this analysis. On average, the fluorescence intensity of post-menopausal patients is higher than that of pre-menopausal patients, similar to results measured in other clinical studies.20 Again, this finding is not statistically significant. Figure 5Mean peak intensity at all three pressure (light, medium, and firm) levels from 17 squamous sites in pre-menopausal women and 12 squamous sites in post-menopausal women at (a) 320-nm excitation, (b) 380-nm excitation, and (c) 470-nm excitation. 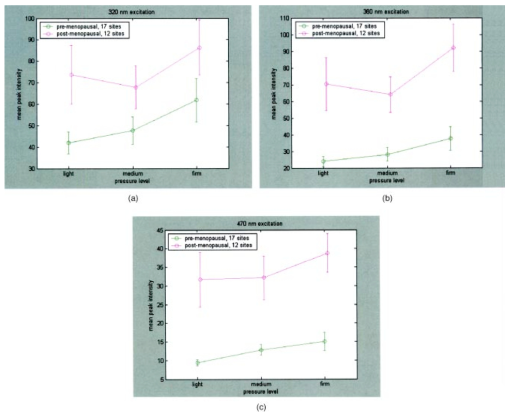 Figure 6 shows the average peak fluorescence intensity of the squamous normal sites measured by each of the four providers at 320-nm excitation [Fig. 6(a)], 380-nm excitation [Fig. 6(b)], and 470-nm excitation [Fig 6(c)]. There is a great deal of variability among the providers, though some of this variability may be due to the differences between number of sites measured by each provider and the types of sites each measured. Some providers’ measurement readings were influenced more by pressure than those of other providers. Figure 6Mean peak intensity at all three pressure (light, medium, and firm) levels for 13 squamous sites measured by provider A, 1 squamous site measured by provider B, 13 squamous sites measured by provider C, and 2 squamous sites measured by provider D at (a) 320-nm excitation, (b) 380-nm excitation, and (c) 470-nm excitation. 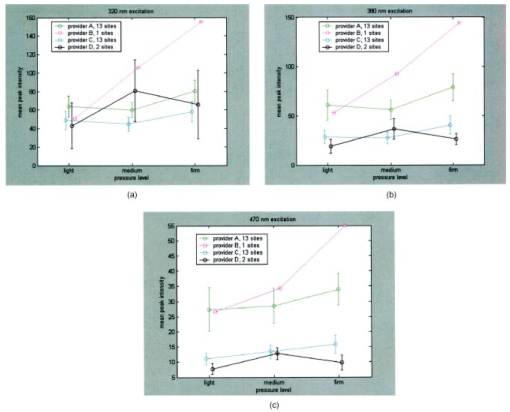 Data from the four non-squamous sites were also analyzed to investigate changes in intensity due to tissue type as shown in Fig. 7 at 320-nm excitation [Fig. 7(a)], 380-nm excitation [Fig. 7(b)], and 470-nm excitation [Fig. 7(c)]. As observed previously,4 5 6 columnar tissue and tissue from the transformation zone have a lower fluorescence intensity than squamous tissue. Trends in columnar tissue and tissue from the transformation zone due to pressure were difficult to assess due to the small number of sites measured in these groups. These findings are also not statistically significant. Figure 7Mean peak intensity at all three pressure (light, medium, and firm) levels for squamous normal, columnar normal, and transformation zone tissue (a) 320-nm excitation, (b) 380-nm excitation, and (c) 470-nm excitation. 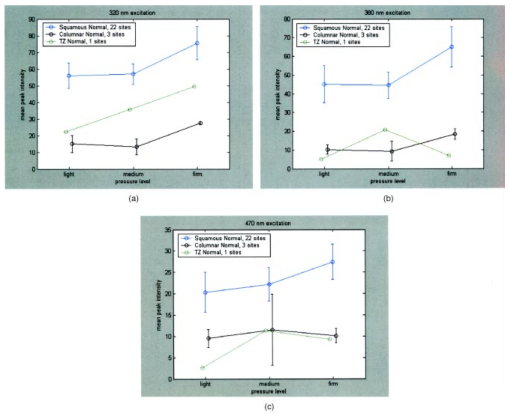 Using the non-normalized spectral data, the nonparametric statistical analysis indicated statistical significance at the 0.05 level for 107 of the 325 excitation/emission wavelength pairs. Figure 8 shows a plot of the mean emission spectra for each of the three pressure values; individually significant excitation/emission wavelength pairs are indicated by a dot at the bottom of the plot. Note that the six emission spectra are plotted side by side. Most of the significant differences are found in the second, third, and fourth emission spectra corresponding to excitation wavelengths of 350, 380, and 410 nm. The effect is a somewhat higher intensity for the measurements at high pressure. After applying the Westfall-Young correction for multiple comparisons, data at only one excitation/emission wavelength pair was found to be significant, with a corrected P-value of 0.024. This occurred at excitation of 320 nm and emission of 625 nm, which is the last emission wavelength in that emission spectrum. The intensity is very low at this point, and the majority of the values were measured at 0. This particular measurement is of little practical diagnostic utility. Figure 8Average fluorescence emission spectra from all sites at the three pressures at 320- (left), 350-, 380-, 410-, 440-, and 470-nm excitation (right), plotted as a function of emission wavelength. Dots at the bottom of the figure indicate those excitation-emission wavelength combinations at which differences in the fluorescence intensity were statistically significant in the nonparametric permutation analysis before the correction for multiple comparisons. 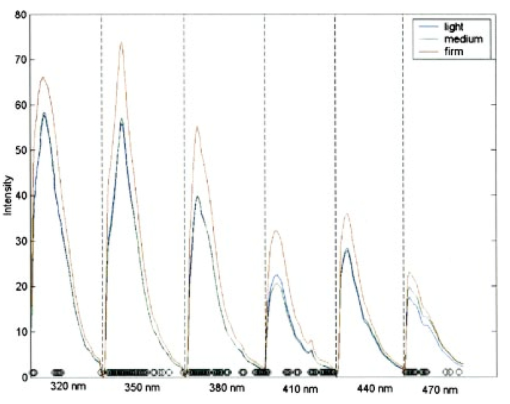 A further nonparametric statistical analysis was performed on normalized fluorescence emission spectra. Each EEM was normalized by dividing by the average of the intensities at 15 excitation/emission wavelength pairs that corresponded to the 15 highest intensity values in the average EEM of all the samples. These 15 values included 5 values from the emission spectrum at 320-nm excitation and 10 values from the emission spectrum at 350-nm excitation. Furthermore, the value of this average was kept as a variable in the analysis (called the “Normalizing Value”). When the identical randomization method was applied to the normalized data, 22 excitation/emission wavelengths showed statistical significance for an effect due to pressure. Additionally, the normalizing value was significant (p=0.03). Recall that we expect about 16 significant variables out of 326 by chance alone even if there is no effect from the pressure on any of the variables. When the Westfall-Young correction for multiple comparisons was applied, none of the p-values remained significant after correction. The method of using normalization to remove extraneous sources of variation in a diagnostic algorithm has been proven useful in previous studies.4 5 6 4.DiscussionThe effect of changing probe pressure on measurements might have provided an obstacle to the use of fluorescence spectroscopy in the development and implementation of these optical technologies, had small changes in probe pressure produced significant variations in the intensity and/or the line shape of fluorescence. In this pilot study, we see some evidence that higher pressures do result in overall higher fluorescence intensity on the average, but our nonparametric statistical analysis did not show a clearly significant effect. While there are some differences amongst providers in applying pressure, the overall variability does not appear to be significant enough to affect the diagnostic capacity of fluorescence spectroscopy. The statistical analysis based on the normalized data strongly suggests that any effects from pressure variation are mitigated by normalization of the spectra. This method has been shown to reduce other sources of variation in the diagnostic algorithm. On average, abnormal tissue had lower fluorescence intensity than normal tissue, perhaps due to angiogenesis in the abnormal tissue and a decrease in stromal collagen fluorescence.10 It is possible that at higher pressures, less hemoglobin would be present in the stroma. As we develop probes capable of separating epithelial and stromal signal, it will be possible to sort out these effects. In addition, post-menopausal patients had higher fluorescence intensities than premenopausal patients, on average. This may be due to the thinner epithelial layer of post-menopausal patients and increased stromal collagen fluorescence.20 A major limitation of this pilot study is sample size. A larger sample size would allow a more thorough investigation of potential dependencies on pressure and provider. Between measurements of tissues, we allowed 30 sec for the tissue to recover from the previous measurement. This timing was based on the visual observation that no impression from the probe remained on the surface of the cervix. There may, however, have been some residual changes in elasticity that could not be seen with the eye, but could be measured with the probe. Future studies should take this into consideration. The type of statistical analysis we used, while sophisticated, did not take into account some of the advances made by other investigators in cervical algorithms.21 22 23 Another limitation of the study is that it applies to the probe that we used to make the measurements and may not be generalizable to other types of probes. Details of our probe can be found in Ref. 24. What can be assumed is that these pressures, which we believe are reproducible and resemble those used in these types of studies, probably do not alter blood flow sufficiently to be of concern. Again, a limitation of this pilot study is that it measured only mean pressure. Both the order of pressure and variability of pressure need to be tested in further studies. Furthermore, larger numbers of measurements at medium pressure need to be conducted in both normal and abnormal types of tissue to ensure the reliability of these findings. In addition, future studies should focus on repeated measurements taken at one pressure at each site to show that an identical spectrum can be obtained at that site. Repeated measures taken as routine measurements from larger clinical trials will be of interest. Also, a study considering the application of the different pressures in different orders would remove the potential confounding of order of measurement with pressure value. Future studies should confirm that pressure is not an important source of variability in the measurement of fluorescence spectroscopy in tissue. This being the case, pressure sensitive probes, which are harder to sterilize, would be unnecessary and the issues that pressure presents would not need to be included in development of any diagnostic algorithms. Currently, these devices are designated as low-risk devices by the Food and Drug Administration,25 and we are hoping to keep their design and construction as low-tech and cost-effective as possible. This would simplify the use of diagnostic fluorescence spectroscopy in developing countries, where the potential for decreased mortality is the highest. AcknowledgmentsSupport is gratefully acknowledged from NCI, Program Project Grant PO1 CA 82710-04. We would also like to thank providers Judy Sandella, Alma Sbach, and Karen Rabel. REFERENCES
I. J. Bigio
,
T. R. Loree
, and
J. Mourant
,
“Spectroscopic diagnosis of bladder cancer with elastic light scattering,”
Lasers Surg. Med. , 16 350
–357
(1995). Google Scholar
R. R. Alfano
,
G. C. Tang
,
A. Pradham
,
W. Lam
,
D. S. J. Choy
, and
E. Opher
,
“Fluorescence spectra from cancerous and normal human breast and lung tissues,”
IEEE J. Quantum Electron. , 23
(10), 1806
–1811
(1987). Google Scholar
I. Georgakoudi
,
B. C. Jacobson
,
M. G. Muller
,
E. E. Sheets
,
K. Badizadegan
, and
D. L. Carr-Locke
,
“NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes,”
Cancer Res. , 62 682
–687
(2002). Google Scholar
N. Ramanujam
,
M. F. Mitchell
,
A. Mahadevan
,
S. Warren
,
S. Thomsen
,
E. Silva
et al.;,
“In vivo diagnosis of cervical entraepithelial neoplasia using 337-nm-excited laser-induced fluorescence,”
Proc. Natl. Acad. Sci. U.S.A. , 91 10193
–10197
(1994). Google Scholar
N. Ramanujam
,
M. F. Mitchell
,
A. Mahadevan
,
S. Thomsen
,
A. Malpica
,
T. Wright
et al.;,
“Spectroscopic diagnosis of cervical intraepithelial neoplasia (CIN) in vivo using laser-induced fluorescence spectra at multiple excitation wavelengths,”
Lasers Surg. Med. , 19 63
–74
(1996). Google Scholar
N. Ramaujam
,
M. F. Mitchell
,
A. Mahadevan-Jansen
,
S. L. Thomsen
,
G. Staerkel
,
A. Malpica
et al.;,
“Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths,”
Photochem. Photobiol. , 64
(4), 720
–735
(1996). Google Scholar
C. Brookner
,
U. Utzinger
,
G. Staerkel
,
R. Richards-Kortum
, and
M. F. Mitchell
,
“Cervical fluorescence of normal women,”
Lasers Surg. Med. , 24 29
–37
(1999). Google Scholar
C. Brookner
,
U. Utzinger
,
M. Follen
,
R. Richards-Kortum
, and
N. Atkinson
,
“Effects of biographical variables on cervical fluorescence,”
J. Biomed. Opt. , 8
(3), 479
–483
(2003). Google Scholar
S. K. Chang
,
M. Y. Dawood
,
G. Staerkel
,
U. Utzinger
,
R. R. Richards-Kortum
, and
M. Follen
,
“Fluorescence spectroscopy for cervical pre-cancer detection: is there variance across the menstrual cycle?,”
J. Biomed. Opt. , 7
(4), 595
–602
(2002). Google Scholar
R. Drezek
,
C. Brookner
,
I. Pavlova
,
I. Boiko
,
A. Malpica
,
R. Lotan
,
M. Follen
, and
R. Richards-Kortum
,
“Autofluorescence microscopy of fresh cervical tissue sections reveals alterations in tissue biochemistry with dysplasia,”
Photochem. Photobiol. , 73 636
–641
(2001). Google Scholar
M. G. Shim
,
L. M. Song
,
N. E. Marcon
, and
B. C. Wilson
,
“In vivo near-infrared Raman spectroscopy: demonstration of feasibility during clinical gastrointestinal endoscopy,”
Photochem. Photobiol. , 72
(1), 146
–150
(2000). Google Scholar
D. L. Heintzelman
,
U. Utzinger
,
H. Fuchs
,
A. Zuluaga
,
K. Gossage
,
A. M. Gillenwater
et al.;,
“Optical excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy,”
Photochem. Photobiol. , 72 103
–113
(2000). Google Scholar
A. Agarwal
,
U. Utzinger
,
C. Brookner
,
C. Pitris
,
M. F. Mitchell
, and
R. Richards-Kortum
,
“Fluorescence spectroscopy of the cervix: influence of acetic acid, cervical mucus and vaginal medications,”
Lasers Surg. Med. , 25 237
–249
(1999). Google Scholar
W. G. Zijlstra
,
A. Buursma
, and
W. P. Meeuswen-van der Roest
,
“Absorption spectra of human fetal and adult oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, and methehemoglobin,”
Clin. Chem. , 37
(9), 1633
–1638
(1991). Google Scholar
R. Drezek
,
K. Sokolov
,
U. Utzinger
,
I. Boiko
,
A. Malpica
,
M. Follen
, and
R. Richards-Kortum
,
“Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements and implications,”
J. Biomed. Opt. , 6 385
–396
(2001). Google Scholar
A. Mahadevan
,
M. F. Mitchell
,
E. Silva
,
S. Thomsen
, and
R. Richards-Kortum
,
“Study of fluorescence properties of normal and neoplastic human cervical tissue,”
Lasers Surg. Med. , 13 647
–655
(1993). Google Scholar
R. Richards-Kortum
,
M. F. Mitchell
,
N. Ramanujam
,
A. Mahadevan
, and
S. Thomsen
,
“In vivo fluorescence spectroscopy: potential for non-invasive, automated diagnosis of cervical intraepithelial neoplasia and use of a surrogate endpoint biomarker,”
J. Cell. Biochem. , 19 111
–119
(1994). Google Scholar
C. K. Brookner
,
M. Follen
,
I. Boiko
,
J. Galvan
,
S. Thomsen
,
A. Malpica
et al.;,
“Autofluorescence patterns in short-term cultures of normal cervical tissue,”
Photochem. Photobiol. , 71
(6), 730
–736
(2000). Google Scholar
R. J. Nordstrom
,
L. Burke
,
J. M. Niloff
, and
J. F. Myrtle
,
“Identification of cervical intraepithelial neoplasia (CIN) using UV-excited fluorescence and diffuse-reflectance tissue spectroscopy,”
Lasers Surg. Med. , 29 118
–127
(2001). Google Scholar
H. Weingandt
,
H. Stepp
,
R. Baumgartner
,
J. Diebold
,
W. Xiang
, and
P. Hillemanns
,
“Autofluorescence spectroscopy for the diagnosis of cervical intraepithelial neoplasia,”
British J. Obstet. Gynecol. , 109 947
–951
(2002). Google Scholar
I. Georgakoudi
,
E. E. Sheets
,
M. G. Muller
,
V. Backman
,
C. P. Crum
,
K. Badizadegan
,
R. R. Dasari
, and
M. S. Feld
,
“Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo,”
Amer J. Obstet. Gynecol. , 186 374
–382
(2002). Google Scholar
U. Utzinger
and
R. Richards-Kortum
,
“Fiber optic probes for biomedical optical spectroscopy,”
J. Biomed. Opt. , 8 121
–147
(2003). Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||