|
|
1.IntroductionMany cellular functions such as differentiation, growth, motility, signal transduction, gene expression, and apoptosis are modulated by mechanical forces.1 More specifically, changing the state of force equilibrium on a cell produces global structural changes that will elicit cytoplasmic and nucleus responses. Mechanical effects influence nearly all cell types, examples of which include effects of fluid shear stress on endothelial and muscle cells,2 3 mechanical strain on vascular smooth muscle cells,4 stretching on neuromuscular junction,5 and substrate adhesivity on differentiation in epithelial cells and hepatocytes.6 7 Integrin transmembrane receptors are believed to be a class of molecules that transmit mechanical signals to (and from) the cytoskeleton through a focal adhesion complex that mechanically couples the cytoplasmic portion of the integrin to the internal actin cytoskeleton, and affect the activation and organization of the cytoskeleton.8 In this study, we investigated the mechanical response of T-lymphocytes to applied forces. T-lymphocytes are responsible for cell-mediated immunity. They recognize specific antigens on target cells (e.g., tumor cells, virus-infected cells) and antigen presenting cells (APCs) (e.g., macrophages, dendritic cells, B-lymphocytes) by the interaction of their T-cell receptors (TCRs) with the peptide-major histocompatibility complex (p-MHC) on the surface of those cells.9 T-lymphocyte activation resulting from TCR-ligand interaction has been shown to induce cytoskeleton rearrangement,10 11 12 13 increase lymphocyte adhesiveness,14 15 and internalize TCR p-MHCs following contact with an APC.16 17 18 In a recent study, the effect of applied local forces on macrophages was studied.19 Magnetic tweezers were used to apply external forces in the range of 0.5 to 5 nN to invasin-coated magnetic beads in contact with murine macrophages, and in the direction opposite to the active force exerted by the cell to engulf the bead. Forces exceeding approximately 0.5 nN induced formation of trumpet-like protrusions, resembling pseudopodia, which were attributed to the growth of the actin cortex, in the direction of the applied external force. However, it remains unknown if local mechanical forces play any roles following antigen recognition by T-lymphocytes. We have investigated the effects of mechanical forces (in the range of 200 to 250 pN) applied in directions normal and tangential to the TCR triggering using optical tweezers, and examined the lymphocyte’s morphological response. 2.Materials and Methods2.1.Cell Culture and AntibodiesProliferating lymphocytes of the human T-lymphoblast cell line HPB-ALL were used in this study. The cells were in RPMI 1640 medium (Life Technologies, Grand Island, New York) supplemented with 10 fetal bovine serum (Sigma), 1.0-mM sodium pyruvate (JRH Biosciences, Lenexa, Kansas), 2-mM L-glutamine (JRH Biosciences), 100-U/ml penicillin (JRH Biosciences), and 100-μg/ml streptomycin (JRH Biosciences) (complete medium) in a humidified atmosphere of 5 CO 2 at 37 °C. The HPB-ALL cells expressed TCRs and β1 integrin, as evidenced from previous reports.20 21 Additionally, based on flow cytometry analysis, it was verified that these cells also expressed MHC class 1 (Fig. 1). In this procedure, primary monoclonal antibodies (mAb) were added to cells, and after incubation, cells were washed and a secondary fluorochromo labeled antibody was added. Following a second incubation and washing step, the cells were analyzed using a fluorescence-activated cell sorting (FACS) device. Figure 1Flow cytometry analysis of TCR and MHC class 1 on HPB-ALL. The vertical and horizontal axes represent the number of cells and the fluorescence intensity, respectively. On each panel, the number for percent positive cells and mean fluorescence intensity (MFI) are provided. The longer horizontal line drawn on each panel is used to compute the MFI. The left end of the shorter horizontal line on each panel is used to define the threshold intensity value, above which positive cells are identified. Results are for: (a) positive control (secondary goat antimouse IgG-Alexa 488 alone); (b) negative control (1E11: mouse antihuman TSLP receptor); (c) monoclonal antibody T40/25; and (d) monoclonal antibody W6/32. 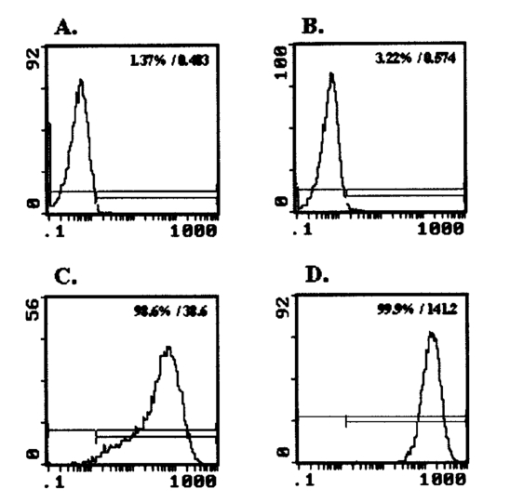 On each panel of Fig. 1, the number for percent positive cells and mean fluorescent intensity (MFI) are provided. The respective representative positive (secondary goat antimouse IgG-Alexa 488 alone), and negative (1E11, mouse antihuman TSLP receptor) control results are presented in Figs. 1(a) and 1(b). In both cases, the number of positive cells and MFI were very low. Monoclonal antibodies T40/25, specific for TCRs of the HPB-ALL cell line20 were used to bind and trigger the receptors. As demonstrated in Fig. 1(c), both the number of positive cells and MFI were increased in the presence of this antibody, indicating the expression of TCRs on HPB-ALL cells. Additionally, we used the anti-β1 integrin monoclonal antibody 33B6, and the antiMHC class 1 monoclonal antibody W6/32. As demonstrated in Fig. 1(d), both the percentage of positive cells and MFI were increased greatly in the presence of the W6/32 antibody, indicating the expression of MHC class 1 on HPB-ALL cells. Based on flow cytometry analysis, the expression of the β1 integrin on HPB-ALL cells was also verified (Fig. 2). In Fig. 2(a), the representative control results (goat antimouse IgG Alexa 488 alone) demonstrated a low percentage of positive cells and values of MFI. In the presence of the 33B6 antibody, the percentage of positive cells and MFI were greatly increased (Fig. 2(b)), indicating the expression of the β1 integrin. Figure 2Flow cytometry analysis of β1 expression on HPB-ALL. The vertical and horizontal axes represent the number of cells and the fluorescence intensity, respectively. On each panel, the number for percent positive cells and MFI are provided. The longer horizontal line drawn on each panel is used to compute the MFI. The left end of the shorter horizontal line on each panel is used to define the threshold intensity value, above which positive cells are identified. Results are for: (a) control (goat antimouse IgG Alexa 488 alone); and (b) monoclonal antibody 33B6. 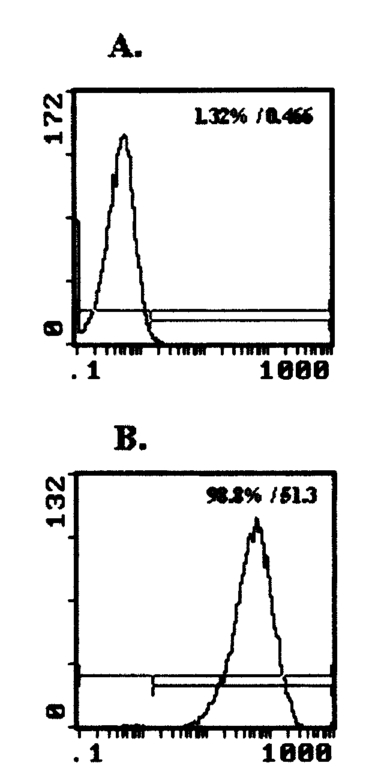 In experiments involving the optical tweezers, the T40/25 antibodies were absorbed onto the surface of 2.1- and 4.5-μm polystyrene beads by incubating the beads overnight at 4 °C in a PBS solution containing the antibody at one of three concentrations: 2.5, 25, and 50 μg/ml. The coated beads were centrifuged (1,500 rpm for 5 min), washed, and resuspended in 1.5 ml of PBS three times before use in the experiments. In experiments with 33B6 antibodies, we incubated 2.1- and 4.5-μm-diam beads with the antibody at concentrations of 40 μg/ml in PBS solution, and conducted 20 experiments (ten with each bead size). We also performed ten additional experiments with 2.1-μm beads using a lower 33B6 antibody concentration (5 μg/ml). In experiments with W6/32 antibodies, we incubated 2.1-μm-diam beads with the antibody concentration of 40 μg/ml in PBS solution, and conducted five experiments. 2.2.Optical Tweezers SystemA cw titanium-sapphire (Ti:sapph) laser (Model 3900S, Spectra Physics, Mountain View, California) pumped by a 5-W solid state, frequency-doubled Nd:YVO 4 laser (Millennia V, Spectra Physics) was used to form a single beam gradient force optical trap.22 The Ti:sapph laser was tuned to 830 nm, a wavelength at which damage to cells is minimized.23 24 25 The laser beam was expanded and focused on the sample plane of an inverted microscope (Axiovert S100 TV, Zeiss, Oberkochen, Germany) by passing through a 100× oil-immersion, 1.3 numerical aperture objective (Plan Neolfluar, Zeiss). A dichroic mirror transmitted the laser radiation to the sample but reflected only the visible light returning from the sample to a charge-coupled device (CCD) video camera (DAGE-MTI CCD 100) for image collection. Additional reviews on the optical tweezers technique and its biological applications are available in the literature.26 27 2.3.Force CalibrationForce calibration as a function of laser power was performed for 2.1- and 4.5-μm polystyrene beads. The calibration, based on the “escaping force” method,28 29 allowed determination of the maximum force exerted by the laser beam on a trapped bead against a fluid drag force. The fluid solution passed the trapped bead at a known speed generated by the motion of a piezo-electric translation stage. The force was then obtained from Stokes’ law, F=6πηrvk, with r being the particle radius, η the solution viscosity, v the fluid velocity, and k a correction factor that depended on the distance between the center of the trapped bead and the bottom of the container (5 μm in our experiments). 2.4.Experimental ProcedureLymphocytes of the HPB-ALL T-cell line were first immobilized onto a microscope cover slip previously coated with poly-L-lysine and located at the bottom of an especially designed petri dish. Individual polystyrene beads, either 2.1 or 4.5 μm in diameter, and coated with a specific antibody (T40/25) against the clonotypic epitope of the TCRs, were optically trapped and brought in contact with a single lymphocyte. The optical tweezers were used to either establish simple contact between bead and cell, or produce contact followed by application of mechanical forces at the contact site. Production of pseudopodia and other changes in cell morphology were monitored by a CCD camera. In most experiments, a bead was initially placed in contact with the lymphocyte membrane and left in place without any further manipulation while monitoring cell response for several minutes. After the cell had retracted the pseudopodia and returned to its original round shape (remaining morphologically inactive for two minutes or longer), mechanical forces of 200 to 250 pN were applied to the attached bead by the optical tweezers in a direction that was either tangential or normal to the cell membrane. These force values were in the range of reported antibody-antigen bond strength (≈100 to 250 pN30 31). Control experiments with uncoated beads were performed following the same procedure used with coated beads. 3.ResultsDepending on bead size, only a certain fraction of lymphocytes exhibited morphological changes in response to contact with the antibody-coated beads. For beads incubated in either 25 or 50 μg/ml of T40/25 antibody solution, lymphocyte response was similar. For beads incubated in the 2.5-μg/ml solution, no lymphocyte response was observed for any bead size (30 lymphocytes tested). The series of observations that we report next correspond to experiments with beads incubated in T40/25 antibody solution of 25 μg/ml. Responsive lymphocytes showed morphological changes that started between 15 s and 2 min following bead contact. In most cases, changes started between 30 s and 1 min, and lasted a few minutes. From the observable changes, a pattern of response that was affected by the application of mechanical stresses at the cell-bead contact site emerged. In most instances, the coated bead became firmly attached to the lymphocyte membrane and could not be later removed from the cell by the optical tweezers. This firm attachment highly restricted bead motion in the direction normal to the cell surface with the application of 200- 250-pN optical force. However, the bead could be effectively moved by force application in the direction tangential to the cell. This produced the effect of “cell rubbing” by the bead that exerted a lateral pull at the attached membrane and induced a tangential tensile stress to the binding site. Typical observed morphological changes are shown in Fig. 3 for a cell brought in contact with a 2.1-μm T40/25 antibody-coated bead. In Fig. 3(a), the optically trapped coated bead was simply brought in contact with a lymphocyte without any further manipulation or mechanical force application. The cell generated pseudopodia (shown by the arrow heads) at different locations, beginning one minute after contact, and oriented in various directions without any specific pattern. Some pseudopodia even appeared at locations opposite to the site of bead contact [e.g., the one formed 5 min following contact, and shown in Fig. 3(a)]. After 8 min, the lymphocyte eventually returned back to a round quiescent state. The same lymphocyte was then submitted to the application of tangential forces (depicted by the vertical arrows, FT) along the cell membrane at the site of bead contact [Fig. 3(b)]. The lymphocyte responded within 1 min with production of pseudopodia (shown by the arrow heads) in the direction of the bead, eventually pulling the bead off the optical trap and engulfing it completely 3 min after force application. Figure 3(a) An optically trapped 2.1-μm T40/25-coated polystyrene bead was brought in contact with a lymphocyte without any additional mechanical manipulation. The cell generated pseudopodia (arrow heads) at different locations and oriented in various directions without any specific pattern. Pictures correspond to moment of contact 0, 2.5, 5, and 8 min after contact. (b) Response of the same cell to the application of tangential forces along its membrane (vertical arrows, FT) at the site of bead contact. The lymphocyte responded with production of pseudopodia in the direction of the bead (arrow heads) and eventually pulled the bead off the optical trap and engulfed it completely. Images correspond to moment of force application 0, 1.5, 2, and 3 min after force application. 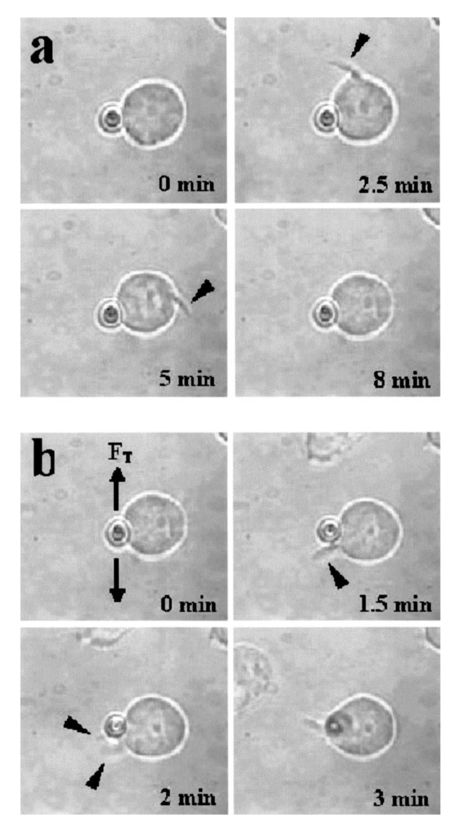 Although localized responses were observed for simple bead contact, almost twice as many responses were random and nonlocalized. In contrast, when tangential forces were applied, the localized responses were three times as many as the nonlocalized (statistics discussed later). If tangential forces were applied just a few seconds after bead contact in cells that had not been submitted to any previous bead interaction, morphological responses were similar to those of cells that were submitted to tangential forces several minutes after a previous simple bead contact. In the case of 2.1-μm beads, only between 28 and 37 of lymphocytes responded with morphological changes. Those changes corresponded to the production of pseudopodia, usually thin and not accompanied by great changes in the overall cell shape. In contrast, in the case of 4.5-μm beads, 68 to 76 of lymphocytes responded morphologically, usually with production of thicker pseudopodia followed by a significant change in overall cell shape. Although applied tangential forces had a significant effect on pseudopodia formation, little or no effect was observed on cell morphology when tensile forces were applied in a direction normal to the lymphocyte’s surface (i.e., along the TCR-ligand bonds). For the 200- 250-pN forces applied by the optical tweezers, bead motion in the normal direction was highly limited by the strength of the TCR-antibody binding and the membrane-cytoskeleton attachment. When 4.5-μm beads were used, a similar number of random and localized responses occurred for simple bead contact. However, following application of tangential forces, localized responses were almost four times as many as nonlocalized ones (statistics discussed later). In Fig. 4(a), an optically trapped 4.5-μm T40/25 antibody-coated bead was brought in contact with a lymphocyte without any force application. The lymphocyte generated a large pseudopodium (arrow head) at one side of the contact site without acting on the bead itself. The cell returned to a round resting state after 5 min with the bead position in the optical trap unaffected. In Fig. 4(b), a different T-lymphocyte was submitted to contact with a 4.5-μm T40/25 antibody-coated bead in conjunction with tangential force application at the contact site (horizontal arrows, FT) . The cell produced pseudopodia (arrow head) toward the bead, pulling it from its initial position in the trap, as seen by the change in focus of the bead image after 3 and 5 min. The cell did not engulf the bead because of its large size. Most responses to 4.5-μm beads, localized or not, involved significant change in overall cell shape. Figure 4(a) An optically trapped 4.5-μm T40/25-coated bead was brought in contact with a lymphocyte without any other mechanical manipulation. The lymphocyte generated a large pseudopodium (arrow head) at one side of the contact site without affecting the bead itself. The cell eventually returned to a round resting state with the bead remaining in position within the trap. Pictures correspond to moment of contact 0, 1.5, 3.5, and 5 min after contact. (b) Lymphocyte submitted to contact with a 4.5-μm bead in conjunction with tangential force application at the contact site (horizontal arrows, FT) . The cell produced pseudopodia (arrow head) toward the bead and pulled it from its initial position in the trap, but did not engulf it because of its large size. Images correspond to moment of force application 0, 2, 3, and 5 min after force application. 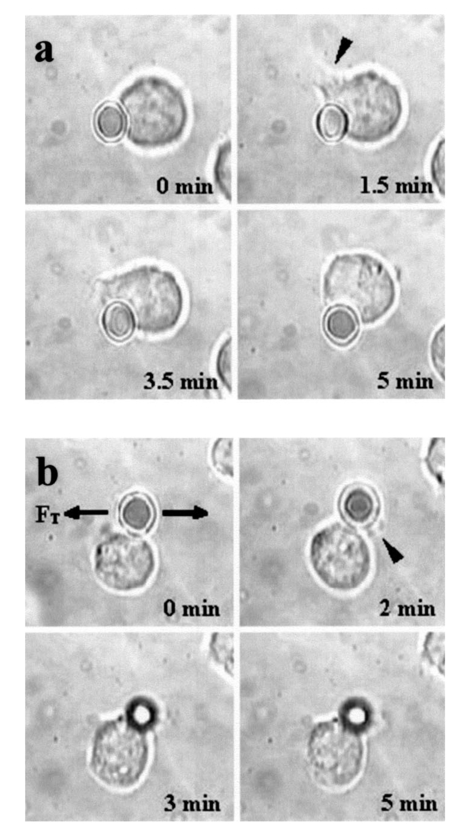 The types of lymphocyte morphological responses to 2.1- and 4.5-μm beads are quantified in terms of percentages in Figs. 5 and 6, respectively. For 2.1-μm beads, Fig. 5(a) shows that the overall lymphocyte response (sum of random and localized responses) was only 28 for simple bead contact (no force applied). The overall response increased to 37 when tangential force was applied. Taking only those lymphocytes that responded, Fig. 5(b) shows that localized responses were only 36 for simple bead contact. In contrast, when tangential force was applied, localized responses increased to 76. Engulfment of the 2.1-μm bead by the pseudopodia occurred in 46 of localized responses that followed tangential force application. Figure 5(a) Types of lymphocyte morphological response to 2.1-μm beads, given in percentages, for simple bead contact (no force applied) and tangential force application. Overall lymphocyte response (sum of random and localized responses) was 28 for simple bead contact and 37 for tangential force application. (b) Random and localized responses given in percentages of the overall response [taking from (a) only those lymphocytes that responded]. Localized response was 36 for simple bead contact and 76 for tangential force application. 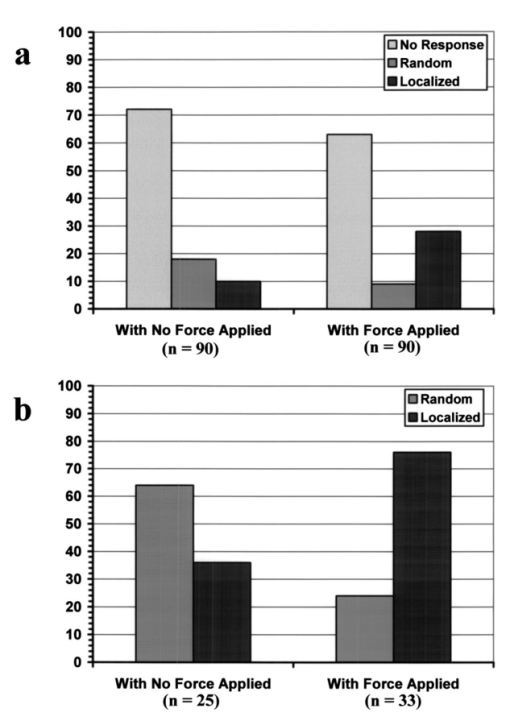 Figure 6(a) Types of lymphocyte morphological response to 4.5-μm beads, given in percentages, for simple bead contact (no force applied) and tangential force application. Overall lymphocyte response (sum of random and localized responses) was 68 for simple bead contact and 76 for tangential force application. (b) Random and localized responses given in percentages of the overall response [taking from (a) only those lymphocytes that responded]. Localized response was 53 for simple bead contact and 79 for tangential force application. 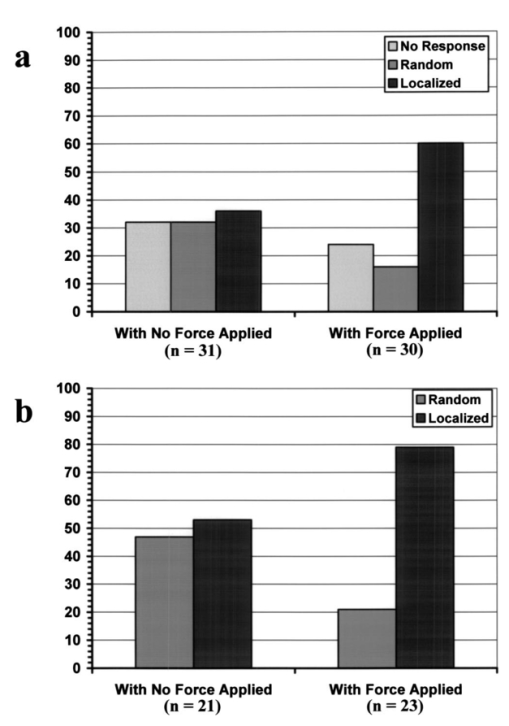 In the case of 4.5-μm beads, the overall lymphocyte response to simple bead contact was 68 [Fig. 6(a)], and increased to 76 when tangential force was applied [Fig. 6(b)]. These total-response percentage values were more than twice those for the 2.1-μm beads. Also different was the fact that 53 of responses to simple contact with the 4.5-μm beads were localized [Fig. 6(b)]. However, localized responses increased to 79 when tangential force was applied, a percentage similar to that for 2.1-μm beads. No engulfment of the 4.5-μm beads occurred due to their large size. Control uncoated beads of both 2.1- and 4.5-μm sizes attached poorly, or not at all, to the lymphocyte membrane, although these beads were incubated in assay media containing serum and could have become coated with serum proteins such as growth factors or fibronectin. Since they did not induce any changes in lymphocyte morphology, this eliminates any significant contribution of nonspecific adhesion to the observed lymphocyte response following contact with coated beads. Nevertheless, it is possible that additional factors from serum that are absorbed to the bead or remain soluble contribute to the morphological response when beads coated with antiTCR antibodies are used. Some lymphocytes were spontaneously active over the poly-L-lysine coated surface, or were activated by random contact with one or more beads in the solution, but none of these cells were used in this study. When using 40-μg/ml concentration of antiβ1 integrin monoclonal antibody 33B6, there were poor attachments of the 2.1-μm beads to the cells, and no lymphocyte response at all (i.e., no pseudopodia formation or shape changes) with either simple contact or applied force. With 4.5-μm beads, there was strong attachment to the cells, but again no lymphocyte response to either simple contact or applied force was observed. When using 5-μg/ml concentration of 33B6 concentration with 2.1-μm beads, there was poor attachment to the cells in five experiments, and strong attachment in the remaining five. In eight experiments, in response to simple bead contact, there were random pseudopodia formation and cell shape changes (but no localized response). Mechanical forces were difficult to apply because of either poor or strong attachments. No experiments with 4.5-μm beads were performed at 33B6 concentration of 5 μg/ml, since we expected even stronger attachments to cells that would prevent us from applying forces. (In our experience, whenever there was too strong of an attachment of small beads, attachment of larger beads was even stronger, precluding any bead motion with application of the forces generated by our laser trap at maximum laser power). In experiments with antiMHC class 1 monoclonal antibody W6/32, we observed no attachment to the cells, or any kind of cell response. Other beads floating in the solution and making accidental contact with other lymphocytes did not produce any response either (data not shown). Experiments were not continued. In summary, the two antibodies 33B6 and W6/32 did not have the same effect on this line of lymphocytes as did the antibodies T40/25 specific for TCRs, in terms of bead attachment and response to application of mechanical forces, production of localized pseudopodia, and promotion of endocytosis activity. 4.DiscussionMechanical forces applied to a cell surface can produce alterations in the cytoskeletal structure leading to global structural rearrangements and changes in the intracellular biochemistry and gene expression.1 Integrins are believed to function as mechanoreceptors that experience the externally applied mechanical loads and provide a gating function for signal transduction to the cytoskeleton.32 The observations in this study indicate an important effect of mechanical forces in the morphological response of T-lymphocytes to TCR-ligand interaction. In an earlier study with fibroblasts,33 it was demonstrated that these cells could sense the restraining force on fibronectin-coated beads and respond by a localized strengthening of the cytoskeleton linkages, allowing stronger force to be exerted on the integrin receptors of the cells. In the case of lymphocytes, one can speculate on how tangential forces may cause localization and reorientation of the morphological response. It was observed that tangential forces applied at the contact site were able to produce some bead displacement over the lymphocyte surface (rubbing effect), perhaps as a result of membrane slipping over the underlying cytoskeleton. T-cells are crucially dependent on the actin cytoskeleton for TCR-mediated internalization of molecules from APC.18 34 35 The induced shear stress could produce bending and mobilization of the ζ chains in the TCR complex, and perhaps other molecules that are anchored to the actin cortex, eliciting actin polymerization. Actin polymerization following TCR ligation has been reported,36 37 and association of TCR ζ chain with the actin cytoskeleton in mature T-lymphocytes has been documented.12 There is also evidence of ligands transport from an outermost ring of adhesion molecules into the central cluster of TCRs.38 The tangential force with the consequent lateral bead motion may also allow mobilization of TCRs within the lipid membrane, facilitating their interaction with signaling molecules present in the membrane glycosphingolipid-enriched microdomains (GEMs or rafts), which interface with the actin cytoskeleton.10 39 40 It has been reported that biochemical changes appear to be sensitive to both the direction as well as the magnitude of the applied force.1 We also point out that HPB-ALL cell line responses to TCR engagement include generation of intracellular signals such as diacylglycerol production and protein kinase C activation, where PKC in turn regulates calcium influx.41 42 43 The highly improved localized response observed after application of tangential forces resulted in the lymphocyte actively pulling the antibody-coated bead out of the optical trap and, for a small bead, engulfing it in almost half of the cases. TCR-mediated internalization of peptide-MHC ligand present on the surface of APCs has been reported by several investigators.16 17 18 40 Although the attachment of antibodies to a polystyrene bead may prevent internalization of TCR-antibody complexes in a similar way, some elements involved in that internalization process could contribute to bead engulfing by the lymphocyte. More specifically, it is possible that reorientation and localization of morphological response induced by mechanical forces activates a process of endocytosis. In this respect, endocytosis signals involving the CD3 epsilon subunit of the TCR complex have been reported.44 In the process of endocytosis, the lymphocyte’s ability to pull a 2.1-μm bead off the optical trap indicates that the cell can generate pulling forces in excess of 250 pN, the maximum trapping force produced by the optical tweezers in our experiments. This is an interesting measurement of force generated by stress fibers in the cell’s cytoskeleton. The use of bigger beads (4.5 μm), incubated in a solution with the same concentration of antibody against the TCRs, induced a more effective localized as well as overall response when compared to the response induced by smaller beads (2.1 μm). Several studies have shown that activation of T-lymphocytes requires engagement of a threshold number of TCRs.45 46 Mescher47 has shown that in addition to ligand density, a large contact area is important for lymphocyte activation. Using latex microspheres coated with class 1 alloantigen, he reported optimum lymphocyte stimulation with particle sizes of 4 to 5 μm, and rapidly decreasing response with decreasing particle size below 4 μm. Furthermore, it has been described that T-lymphocytes respond to contact with APCs with a cytoskeleton rearrangement focalized toward the contact site.11 27 35 36 48 In this process, induced actin polymerization leads to reorganization of actin filaments under the zone of contact with the APC. A stable cell-cell contact area facilitates a sustained signal for lymphocyte activation. It is likely, however, that a large contact area is required for proper interaction. In this study, which focused on the formation of pseudopodia at the onset of activation, localized morphological response was poor when lymphocytes were submitted to simple contact with small beads coated with antibodies specific for the TCRs. For cell-cell contact, binding and triggering of TCRs alone may not be sufficient to generate a highly localized reorganization of the underlying cytoskeleton, and other accessory molecules (CD4/CD8, CD28, CD45R, CD2, LFA-1 integrin) on the lymphocyte membrane may also need to interact with their respective ligands on the surface of a target cell or APC to achieve that high degree of localization. For example, the CD4 coreceptor may serve to boost recognition of the ligand by the TCR.49 We did not observe localized pseudopodia response and endocytotic activity when using the antiβ1 integrin antibody 33B6. This result clearly demonstrated that all classes of T-lymphocyte surface receptors (TCRs and integrins) do not give the same response, given that each of these transmembrane receptors are associated with intracellular signaling pathways that consist of kinases, scaffolding protein, and cytoskeletal components that are not shared between the two different signaling cascades. It is intriguing to speculate about the role that mechanical forces may play in the process of cell-mediated immunity. It is possible that local forces have an important role in the initial lymphocyte adhesion to antigen-bearing cells. Specifically, tangential (shear) stresses on engaged TCRs, occurring at the onset of activation as the lymphocyte crawls over and around a target cell or APC (or conversely, as an APC crawls over the lymphocyte), may redirect pseudopodia toward the site of antigen encounter and promote local adhesion by facilitating initial contact of adhesion molecules (present in the pseudopodia) with their respective ligands on the surface of the other cell. Additionally, this reorientation of pseudopodia in the direction of antigen may also promote endocytosis activity with incorporation of cell fragments. Furthermore, we point out that the APC cytoskeleton itself may play an active role in the immunological synapse, as evidenced by polarization of the dendritic cell’s cytoskeleton during interaction with T cells.50 In a broader perspective, mechanical forces could be involved in antigen-specific motility, transendothelial migration, and tissue homing to sites of inflammation. In future studies, we plan to investigate the effects of the applied force magnitude, study some of the resulting biochemical changes such as cytokines production, and carry out experiments using other antibodies and soluble ligands. AcknowledgmentsWe thank James N. Wygant, Yuko J. Miyamoto, and Jason S. Mitchell in the Department of Immunology at The University of Texas M.D. Anderson Cancer Center for their help with cells and antibodies; and Maneesh Arya and Kathryn Hicks Simpson in the Department of Bioengineering at Rice University for their assistance with the optical trap calibration. This study was supported in part by a grant from NASA (NAG2-1505), and the American Heart Association, Texas Affiliate. REFERENCES
M. E. Chicurel
,
S. Chen
, and
D. I. Ingber
,
“Cellular control lies in the balance of forces,”
Curr. Opin. Cell Biol. , 10 232
–239
(1998). Google Scholar
K. T. Nguyen
,
S. G. Eskin
,
C. Patterson
,
M. S. Runge
, and
L. V. McIntire
,
“Shear stress reduces protease activated receptor-1 expression in human endothelial cells,”
Ann. Biomed. Eng. , 29 145
–152
(2001). Google Scholar
G. N. Stamatas
,
C. W. Patrick Jr.
, and
L. V. McIntire
,
“Intracellular pH changes of human aortic smooth muscle cells in response to fluid shear stress,”
Tissue Eng. , 3 391
–403
(1997). Google Scholar
E. Wilson
,
K. Sudhir
, and
H. E. Ives
,
“Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions,”
J. Clin. Invest. , 96 2364
–2372
(1995). Google Scholar
B. M. Chen
and
A. D. Grinnell
,
“Kinetics,
Ca2+
dependence and biophysical properties of integrin-mediated mechanical modulation of neurotransmitter release from frog motor nerve terminals,”
J. Neurosci. , 17 904
–916
(1997). Google Scholar
C. D. Roskelley
,
P. Y. Desprez
, and
M. J. Bissell
,
“Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells require both physical and biochemical signal transduction,”
Proc. Natl. Acad. Sci. U.S.A. , 91 12378
–12382
(1994). Google Scholar
R. Singhvi
,
A. Kumar
,
G. P. Lopez
,
G. N. Stephanopoulos
,
D. I. C. Wang
,
G. M. Whitesides
, and
D. E. Ingber
,
“Engineering cell shape and function,”
Science , 264 696
–698
(1994). Google Scholar
D. Ingber
,
“Integrins as mechanochemical transducers,”
Curr. Opin. Cell Biol. , 3 841
–848
(1991). Google Scholar
O. Acuto
and
D. T. Cantrell
,
“Cell activation and the cytoskeleton,”
Annu. Rev. Immunol. , 18 165
–184
(1996). Google Scholar
J. Delon
,
N. Bercovici
,
R. Liblau
, and
A. Trautmann
,
“Imaging antigen recognition by naı¨ve
CD4+T
cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response,”
Eur. J. Immunol. , 28 716
–729
(1998). Google Scholar
M. Rozdzial
,
M. Malissen
, and
T. H. Finkel
,
“Tyrosine-phosphorylated T cell receptor ζ chain associates with the actin cytoskeleton upon activation of mature T-lymphocytes,”
Immunity , 3 623
–633
(1995). Google Scholar
S. Valittuti
,
M. Dessing
,
K. Aktories
,
H. Gallati
, and
A. Lanzavecchia
,
“Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy: Role of T cell actin cytoskeleton,”
J. Exp. Med. , 181 577
–584
(1995). Google Scholar
M. L. Dustin
and
T. A. Springer
,
“T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1,”
Nature (London) , 341 619
–624
(1989). Google Scholar
M. L. Dustin
and
T. A. Springer
,
“Role of lymphocyte adhesion receptors in transient interactions and cell locomotion,”
Annu. Rev. Immunol. , 9 27
–66
(1991). Google Scholar
J. F. Huang
,
Y. Yang
,
H. Sepulveda
,
W. Shi
,
I. Hwang
,
P. A. Peterson
,
M. R. Jackson
,
J. Sprent
, and
Z. Cai
,
“TCR-mediated internalization of peptide-MHC complexes acquired by T cells,”
Science , 286 952
–954
(1999). Google Scholar
I. Hwang
,
J. F. Huang
,
H. Kishimoto
,
A. Brunmark
,
P. A. Peterson
,
M. R. Jackson
,
C. D. Surh
,
Z. Cai
, and
J. Sprent
,
“T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells,”
J. Exp. Med. , 19 1137
–1148
(2000). Google Scholar
I. Hwang
and
J. Sprent
,
“Role of the action cytoskeleton in T cell absorption and internalization of ligands from APC,”
J. Immunol. , 166 5099
–5107
(2001). Google Scholar
L. Vonna
,
A. Wiedemann
,
M. Aepfelbacher
, and
E. Sackmann
,
“Local force induced conical protrusions of phagocytic cells,”
J. Cell. Sci. , 116 785
–790
(2003). Google Scholar
J. Kappler
,
R. Kubo
,
K. Haskins
,
C. Hannum
,
P. Marrack
,
M. Pigeon
,
B. W. McIntyre
,
J. Allison
, and
I. Trowbridge
,
“The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man V. Identification of constant and variable peptides,”
Cell , 35 295
–302
(1983). Google Scholar
B. W. McIntyre
,
E. L. Evans
, and
J. L. Bednarczyk
,
“Lymphocyte surface antigen L25 is a member of the integrin receptor superfamily,”
J. Biol. Chem. , 264 13745
–13750
(1989). Google Scholar
A. Ashkin
,
J. M. Dziedzic
, and
S. Chu
,
“Observation of a single-beam gradient force optical trap for dielectric particles,”
Opt. Lett. , 11 288
–290
(1986). Google Scholar
G. Leitz
,
E. Fallman
,
S. Tuck
, and
O. Axner
,
“Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence,”
Biophys. J. , 82 2224
–2231
(2002). Google Scholar
H. Liang
,
K. T. Vu
,
P. Krishnan
,
T. C. Trang
,
D. Shin
,
S. Kimel
, and
M. W. Berns
,
“Wavelength dependence of cell cloning efficiency after optical trapping,”
Biophys. J. , 70 1529
–1533
(1996). Google Scholar
K. C. Neuman
,
E. H. Chadd
,
G. F. Liou
,
K. Bergman
, and
S. M. Block
,
“Characterization of photodamage to escherichia coli in optical traps,”
Biophys. J. , 11 2162
–2164
(1997). Google Scholar
K. Svoboda
and
S. M. Block
,
“Biological applications of optical tweezers,”
Annu. Rev. Biophys. Biomol. Struct. , 23 247
–285
(1994). Google Scholar
H. Felgner
,
O. Muller
, and
M. Schliwa
,
“Calibration of light forces in optical tweezers,”
Appl. Opt. , 34 977
–982
(1995). Google Scholar
R. M. Simmons
,
J. T. Finer
,
S. Chu
, and
J. A. Spudich
,
“Quantitative measurements of force and displacement using an optical trap,”
Biophys. J. , 77 2856
–2863
(1999). Google Scholar
U. Dammer
,
M. Hegner
,
D. Anselmetti
,
P. Wagner
,
M. Dreier
,
W. Huber
, and
H. J. Gu¨ntherodt
,
“Specific antigen/antibody interactions measured by force microscopy,”
Biophys. J. , 70 2437
–2441
(1996). Google Scholar
P. Hinterdorfer
,
W. Baumgartner
,
H. J. Gruber
, and
K. Schilcher
,
“Detection and localization of individual antibody-antigen recognition events by atomic force microscopy,”
Proc. Natl. Acad. Sci. U.S.A. , 93 3477
–3481
(1996). Google Scholar
A. J. Maniotis
,
C. S. Chen
, and
D. E. Ingber
,
“Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure,”
Proc. Natl. Acad. Sci. U.S.A. , 94 849
–854
(1997). Google Scholar
D. Choquet
,
D. P. Felsenfeld
, and
M. P. Sheetz
,
“Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages,”
Cell , 88 39
–48
(1997). Google Scholar
J. A. Grasis
,
C. D. Browne
, and
C. D. Tsoukas
,
“Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal evens induced by the T cell antigen receptor,”
J. Immunol. , 170 3971
–3976
(2003). Google Scholar
I. Tskvitaria-Fuller
,
A. L. Rozelle
,
H. L. Yin
, and
C. Wulfing
,
“Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization,”
J. Immunol. , 171 2287
–2295
(2003). Google Scholar
K. E. Debell
,
A. Conti
,
M. A. Alava
,
T. Hoffman
, and
E. Bonvini
,
“Microfilament assembly modulates phospolipase C-mediated signal transduction by the TCR/CD3 in murine T helper lymphocytes,”
J. Immunol. , 149 2271
–2280
(1992). Google Scholar
M. V. Parsey
and
G. K. Lewis
,
“Actin polymerization and pseudopodium reorganization accompany anti-CD3-induced growth arrest in Jurkat T cells,”
J. Immunol. , 151 1881
–1893
(1993). Google Scholar
A. Grakoui
,
S. K. Bromley
,
C. Sumen
,
M. M. Davis
,
A. S. Shaw
,
P. M. Allen
, and
M. L. Dustin
,
“The immunological synapse: A molecular machine controlling T cell activation,”
Science , 285 221
–227
(1999). Google Scholar
T. Harder
and
K. Simons
,
“Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation,”
Eur. J. Immunol. , 29 556
–562
(1999). Google Scholar
M. H. Lee
,
D. Min
,
C. H. Sonn
,
K. N. Lee
,
K. E. Kim
,
S. G. Paik
, and
Y. S. Kim
,
“TCR internalization induced by peptide/MHC ligands requires the transmembrane domains of alphabeta chains of TCR, but not the expression of CD8 and Thy-1 molecules,”
Mol. Cells , 9 617
–624
(1999). Google Scholar
M. Biffen
,
M. Shiroo
, and
D. R. Alexander
,
“Selective coupling of the T cell antigen receptor to phosphoinositide-derived diacylglycerol production in HPB-ALL T cells correlates with CD45-regulated p59fyn activity,”
Eur. J. Immunol. , 23 2980
–2987
(1993). Google Scholar
B. B. Hashemi
,
J. P. Slattery
,
D. Holowka
, and
B. Baird
,
“Sustained T cell receptor-mediated
Ca2+
responses rely on dynamic engagement of receptors,”
J. Immunol. , 156 3660
–3670
(1996). Google Scholar
E. Shinvan
and
D. R. Alexander
,
“Protein kinase C activation inhibits TCR-mediated calcium flux but not inositol triphosphate production in HPB-ALL T cells,”
J. Immunol. , 154 1146
–1156
(1995). Google Scholar
A. Borroto
,
J. Lama
,
F. Niedergang
,
A. Dautry-Varsat
,
B. Alarcon
, and
A. Alcover
,
“The CD3 epsilon subunit of the TCR contains endocytosis signals,”
J. Immunol. , 163 25
–31
(1999). Google Scholar
S. Valitutti
,
S. Muller
,
M. Cella
,
E. Podovan
, and
A. Lanzavecchia
,
“Signal triggering of many T-cell receptors by a few peptide-MHC complexes,”
Nature (London) , 375 148
–158
(1995). Google Scholar
A. Viola
and
A. Lanzavecchia
,
“T Cell activation determined by T cell receptor number and tunable thresholds,”
Science , 273 104
–106
(1996). Google Scholar
M. F. Mescher
,
“Surface contact requirements for activation of cytotoxic T-lymphocytes,”
J. Immunol. , 149 2402
–2405
(1992). Google Scholar
E. Donnadieu
,
G. Bismuth
, and
A. Trautman
,
“Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium,”
Curr. Biol. , 4 584
–595
(1994). Google Scholar
M. F. Krummel
,
M. D. Sjaastad
,
C. Wulfing
, and
M. M. Davis
,
“Differential clustering of CD4 and CD3z during T cell recognition,”
Science , 289 1349
–1352
(2000). Google Scholar
M. M. Al-Alwan
,
G. Rowden
,
T. D. Lee
, and
K. A. West
,
“The dendritic cell cytoskeleton is critical for the formation of the immunological synapse,”
J. Immunol. , 166 1452
–1456
(2001). Google Scholar
|

