|
|
1.IntroductionSeveral years ago, the development of low-cost red diode lasers and their incorporation into pointers and illuminators ushered in an era of widespread routine use of such sources. Casual and capricious or even malicious use of these devices resulted in rapidly escalating rates of ocular laser exposures and occasional claims of eye injury and/or persistent visual problems following what would otherwise be considered noninjurious exposure levels.1, 2, 3, 4 With such large numbers of individuals receiving ocular laser exposures, questions were raised regarding the appropriate application of laser safety standards,5, 6 and government guidelines and regulations were considered and promulgated at both local and national levels. The more recent development of “blue” diode lasers (this terminology refers to diodes emitting at near-UV through short-visible wavelengths) and their expected widespread use in consumer electronics devices, raises the prospect of a similar spate of claims of visual disruption and/or eye injuries following ocular exposure incidents, and creates the likelihood that the analogous safety and regulatory issues will be revisited. In this instance, however, an interesting variation exists in the nature of the potential visual disruption. Because the majority of the incident radiation within the near-UV through blue wavelength range is absorbed by the lens of the primate eye, the visual consequence arises not just from the incident radiation transmitted directly to the retina but also (and primarily) from the blue-green fluorescence induced by lenticular absorption of the incident radiation.7, 8 The lens fluorescence, although usually unnoticed, is nevertheless present with exposure to the ambient solar environment. Weale9 has estimated that the reciprocal ratio between the luminance of a patch of sky and that of the fluorescence it induces is for the normal lens of a old human (generally unnoticeable), but increases to 0.017 for a old (generally noticeable), and to 0.121 for an old. In the latter case, the fluorescence can be an intraocular source of “veiling glare,” covering the entire field of view with intensity sufficient to impair visual function.10 With certain disease processes, including diabetes, the optical aging of the lens may be accelerated.11 The results of earlier studies on both human12 and nonhuman primate (NHP) lenses13 raised the concern that exposures to near-UV to blue wavelength laser sources at otherwise “safe” exposure levels [i.e., at or below the maximum permissible exposure (MPE) levels defined by laser safety standards5, 6] can still result in a disturbing if not debilitating fluorescence glare. However, prior to the recent development of compact, commercially viable blue-diode lasers, the question of visual interference due to lens fluorescence glare seemed an academic issue as far as impact on the general public. This report argues that the issue is no longer academic, that the possibilities for visual disruption, especially under low-light conditions, should be recognized, and that suitable preventive/protective measures should be considered. Several observations from the earlier studies by Zuclich 13, 14, 15 and van den Berg 12, 16, 17 define the scope of the current concerns. First is the finding that the wavelength range effective in inducing lens fluorescence and the consequent veiling glare spans a rather broad band from . This is illustrated by the NHP lens data seen in Table 1 , which summarizes the results of spectroradiometer/spectrophotometer measurements of fluorescence intensity as the exciting wavelength varied from . It is seen that after equating for source irradiance, both the fluorescence radiance and luminance were relatively constant over this range of exciting wavelength. Other data from the same study13 suggested that the fluorescence glare could easily reach visually debilitating levels in situ with excitation-source (in that case, a laser) intensities approximating laser safety standard MPE levels.5, 6 Table 1Fluorescence intensities measured from excised rhesus lenses.
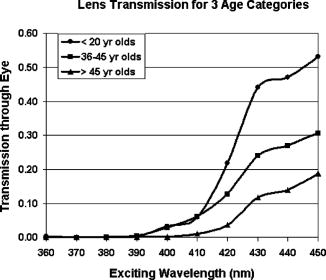
The work by van den Berg12 discussed the spatial distribution of fluorescence induced in the human lens by three wavelengths (380, 400, and ), but was limited to observations in just three human lenses. The current study extends the findings in human lenses by both covering a broader range of exciting wavelengths and by including a considerably larger pool of human lenses (24 versus 3). 2.Materials and MethodsHuman lenses were provided by participating eye banks of Tissue Banks International (see Acknowledgment section at the end of this work). Whole encapsulated lenses harvested by eye bank personnel were placed in buffered saline solution, packed in ice, and shipped to our facility. Data were collected at times ranging from postmortem, but lenses were discarded as soon as they began to show signs of deterioration (sloughing off of surface layers) at anywhere from postmortem. During experiments, the lenses were removed from the refrigerated saline and mounted upright on a vertically oriented hemicircular grooved plastic washer (analogous to the lower half of a wheel rim). The lenses remained upright with no further restraint, allowing them to be irradiated along the central axis, from the anterior side, while the lens fluorescence was detected either on or off axis from the posterior side without line-of-sight interference from the lens holder. Throughout the course of the experiment, the optical integrity of the lens was maintained by irrigation with a slow drip of saline from above. The excitation source for wavelength-dependence studies was an Oriel (Stratford, CT) mercury xenon arc lamp directed through an Oriel grating monochromator with slits set to yield a bandwidth [full width at half maximum (FWHM)]. With selected lenses, additional measurements were made with a diode laser source (Micro Laser Systems, Incorporated, Garden Grove, CA) having a maximum cw output power of at . Several instruments were used to characterize the lens fluorescence. An Ocean Optics (Dunedin, FL) USB 2000 spectrometer was used to record the fluorescence spectrum induced by each exciting wavelength (from at intervals). The radiance of the lens was measured with an International Light (Bewbury, MA) IL 1700 radiometer and the luminance with a Photo Research Incorporated (Chatsworth, CA) PR-880 automated telephotometer. Radiance and luminance measurements were made with the respective detector heads placed either along the axis of irradiation at fixed distances from the posterior surface of the lens, or along an arc at various angles to that axis but within the horizontal plane passing through the center of the lens. As the induced lens fluorescence spectrum is relatively broadband and, depending on the wavelength and bandwidth of the exciting light, partially overlaps the arc-lamp excitation envelope, both on-axis and off-axis luminance measurements were collected. This allowed delineation of the induced fluorescence from contributions due to direct transmission and/or scatter of the exciting light. The only donor information requested from the participating eye banks was age of the deceased. Donor ages ranged from 14 to 66 and, of course, was determined by chance occurrence. For purposes of characterizing the lens fluorescence properties as a function of donor age, the optical data were pooled and averaged in three age categories: youths of age (2 lenses, donor ages 14 and 16); adults of age (12 lenses, ages 36, 37, 37, 41, 45, and 45); and adults of age (10 lenses, ages 58, 59, 62, 63, and 66). 3.Results and DiscussionThe fluorescence properties measured from the eye bank lenses are qualitatively and quantitatively similar to those previously reported using NHP subjects.13 Fluorescence spectra induced by 360-, 400-, and arc-lamp excitation are displayed in Fig. 1 . Excitation wavelengths longer than the lens absorption peak result in a more red-shifted fluorescence for all ages. However, for all lenses, the red-shifted fluorescence has approximately the same luminance and radiance values for any exciting wavelength within the range from . A plot of the peak wavelength of the fluorescence spectrum as a function of the exciting wavelength (averaged across all ages) is shown in Fig. 2 . The only apparent deviation from a monotonically increasing function occurs for excitation, where the fluorescence intensity was weak and assignment of a peak wavelength for the broad spectral output was problematic. Fig. 1Fluorescence spectra induced in (a) younger or (b) older lenses by three exciting wavelengths. 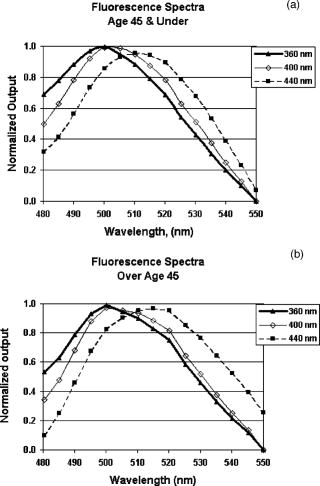 Fig. 2Variation of fluorescence peak wavelength as a function of exciting wavelength (averaged over all lenses). 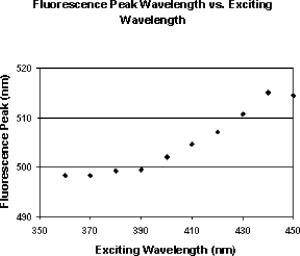 The current study includes age-dependent observations of lens luminance intensities as well as the off-axis measurements to characterize the spatial distribution of the induced fluorescence emittance. There were no analogous measurements in the earlier NHP lens fluorescence study.13 A comprehensive characterization of the spatial distribution of the fluorescence induced in the human lens by three excitation wavelengths, 380, 400, and , was published by van den Berg.12 Our radiometric measurements essentially replicate his findings. The photometric measurements reported here supplement both van den Berg’s quantum efficiency observations and the earlier photometric measurements on NHP lenses.13 The age variation of transmission of the human lens is illustrated in Fig. 3 by the spectral plots of the fraction of the incident radiation transmitted directly through the lens for the three age categories. These measurements were obtained with the radiometer detector head placed first at the position where the lens would be mounted and then directly behind the mounted lens. Thus, Fig. 3 illustrates the on-axis transmission/absorption spectra of human lenses for the three age categories. It is seen that whereas the young lenses have measurable transmission beginning at and exhibit a rapidly increasing transmission for excitation , the older lenses are virtually opaque to wavelengths and rise only to direct transmission by . Figure 4 shows the analogous (on-axis) plots of lens luminance for the same three age categories (solid lines). With excitation wavelengths , the measured luminance increases rapidly with increasing wavelength. This is due primarily to the increasing direct transmission of the source. For shorter exciting wavelengths, where the contributions of the directly transmitted radiation are minimal, all age categories show approximately the same fluorescence luminance. Fig. 4On-axis and off-axis luminance measurements plotted as a function of exciting wavelength for each age category (normalized to incident laser power). 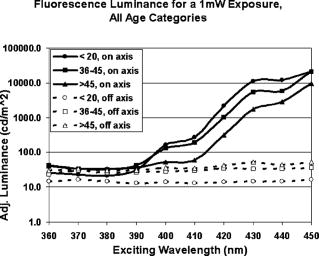 Figure 4 also depicts the off-axis luminance of the three age categories measured at from the axis of irradiation (dashed lines). In contrast to the on-axis measurements, the off-axis results show little dependence on excitation wavelength. The human lens luminance data also show a striking similarity to the NHP results reported in Table 1. This can be seen in Table 2 , where the luminance values from Table 1 are normalized versus their respective source irradiances to yield a relatively constant ratio (central column of Table 2). The analogous results from the eye-bank lenses show virtually the same trend for all age categories (right-hand columns of Table 2). The tabulated values of lens luminance averaged across all ages are approximately five times higher for the human lens than for the NHP, but interspecies comparisons are compromised by differences in experimental approach (different detecting instruments, aperture sizes, and detector head placements were used). Nevertheless, it is noted that the NHP luminance values are in much closer agreement with the values for the youngest human age group (here the differences are only a factor of ). This is likely the most valid cross-species comparison given the relatively young ages of the experimental NHP subjects (generally of age). Table 2Comparison of luminous intensities from nonhuman primate lenses and eye bank lenses. The NHP lens data are from Table 1; luminance divided by irradiance of exciting source. The eyes bank lenses are averaged across all lenses (far right), with standard error in parentheses.
Perhaps more noteworthy, although also problematic to compare on an absolute basis, is the small spread between the off-axis luminance values for the three age groups. The off-axis luminance values (as plotted in Fig. 4) have much weaker contributions from direct detection of the exciting light than do the on-axis measurements and should, therefore, more faithfully represent the luminance due solely to the emitted fluorescence for each age group. A more objective discrimination between the exciting source contributions and the induced fluorescence contributions to the measured luminance can be gleaned from the CIE chromaticity diagrams derived from the on-axis (Fig. 5 ) and off-axis (Fig. 6 ) measurements. In each case, the chromaticity coordinates are plotted for three exciting wavelengths (390, 410, and ) and for each of the three age categories. For the on-axis measurements, the color appearance reflects the dominance of the direct transmission contributions for all age groups. An exception is noted for the combination of the oldest age group and the shortest exciting wavelength , where the direct transmission is minimal. By contrast, the off-axis measurements demonstrate similar fluorescence color appearances for all ages with 390- and excitation. Contributions from direct transmission of the exciting light are evident only for wavelengths as long as (again indicative of higher transmission through the younger lenses). Fig. 5CIE chromaticity diagram, on-axis measurements for three exciting wavelengths and three age categories. 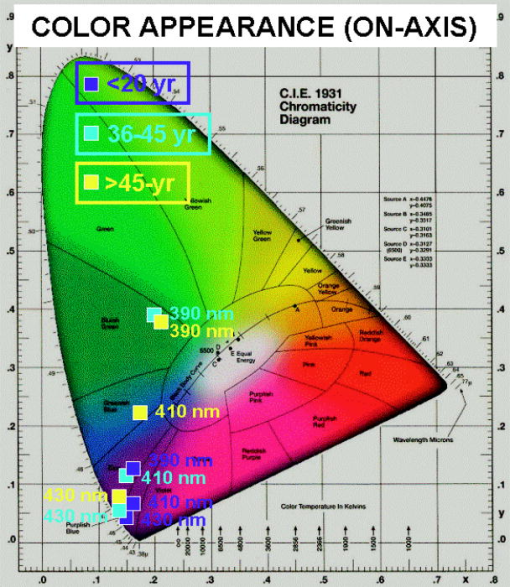 Fig. 6CIE chromaticity diagram, off-axis-measurements for three exciting wavelengths and three age categories. 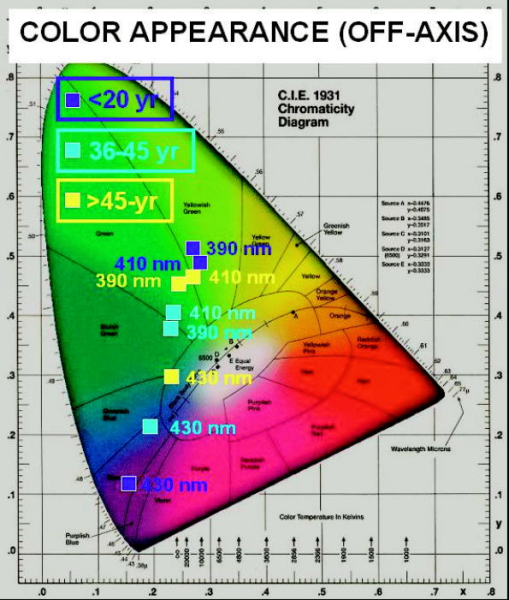 The observed similarities in luminance values and color appearance imply that the lens fluorescence and consequent glare disruption could be nearly as much of a problem for all age categories. At first thought, this is somewhat surprising, since the lens absorption increases and broadens with age and so might the induced fluorescence intensity. On the other hand, considering that the young lens is already virtually opaque (transmission ) at , any increase in absorption as the lens ages would not result in a significant incremental increase in induced fluorescence. Even at longer exciting wavelengths, where the young lens has significantly greater transmission, the age-related incremental increase in induced luminance and radiance is less than a factor of 2. Furthermore, given that the induced lens fluorescence is generated in a cylindrical (or conical) volume as the incident radiation penetrates into the lens, the younger lens, even if emitting a somewhat lower induced-fluorescence intensity, has more of its fluorescence emanating from deeper in the lens (i.e., closer to a detector positioned behind the lens). Because the emitted fluorescence has to cross through intervening lens tissue before reaching the detector, and because it is more highly absorbed and scattered by older lens tissue, the luminance as measured from behind the younger lens would be reduced by a lower factor than that measured for the older lens. For the in situ case, think of replacing the photometer or radiometer of our study with the innate detector (the retina), while maintaining the same relative geometry between the source (the induced lens fluorescence) and the detector (the retina). Then, we infer that the resultant visual interference would be reduced less in the young lens than would otherwise be expected solely on the basis of the lower absorption of the exciting light. In summary, we have examined the elicitation of a blue-green fluorescence in the human lens by near-UV through short visible wavelengths (narrow band) and determined that the fluorescence intensity and, by inference, the expected visual interference (veiling glare) is similar across all ages. From the luminance measurements on excised human lenses, we estimate that cw laser exposure at a level of (i.e., laser exposures that do not exceed safety standard MPE levels) and with any wavelength(s) within the range from can induce a lens fluorescence luminance of , sufficient to impair vision at normal indoor lighting levels and potentially debilitating at night. AcknowledgmentsThe research reported here was performed at the USAMRD and USAF laser laboratories at Brooks Air Force Base, Texas and was supported by the Joint Non-Lethal Weapons Directorate through USAF contract F41624-97-D-9000. Human donor lenses were provided by participating eye banks of Tissue Banks International. We are grateful to Jim Wagner, Executive Director, San Antonio Eye Bank, and Jim Henn, Indiana Lions Eye Bank, for directing the collections and shipment of donor lenses from their respective facilities. ReferencesJ. K. Luttrull and
J. Hallisey,
“Laser pointer-induced macular injury,”
Am. J. Ophthalmol., 127 95
–96
(1999). https://doi.org/10.1016/S0002-9394(98)00254-2 0002-9394 Google Scholar
C. H. Sell and
J. S. Bryan,
“Maculopathy from handheld diode laser pointer,”
Arch. Ophthalmol. (Chicago), 117 1557
–1558
(1999). 0003-9950 Google Scholar
C. S. Sethi,
R. H. Grey, and
C. D. Hart,
“Laser pointers revisited: a survey of 14 patients attending casualty at the Bristol Eye Hospital,”
Br. J. Ophthamol., 83 1164
–1167
(1999). 0007-1161 Google Scholar
J. A. Zuclich and
D. J. Stolarski,
“Retinal damage induced by red diode laser,”
Health Phys., 81 8
–14
(2001). 0017-9078 Google Scholar
, American National Standard for Safe Use of Lasers,
(2000) Google Scholar
International Electrotechnical Commission,
(2001). Google Scholar
G. F. Cooper and
J. G. Robson,
“The yellow colour of the lens of man and other primates,”
J. Physiol. (London), 203 411
–417
(1969). 0022-3751 Google Scholar
S. Lerman,
J. M. Megaw, and
M. N. Moran,
“Further studies on the effects of UV radiation on the human lens,”
Ophthalmic Res., 17 354
–361
(1985). 0030-3747 Google Scholar
R. A. Weale,
“Human lenticular fluorescence and transmissivity, and their effects on vision,”
Exp. Eye Res., 41 457
–473
(1985). 0014-4835 Google Scholar
D. B. Elliot,
K. C. Yang,
K. Dumbleton, and
A. P. Cullen,
“Ultraviolet-induced lenticular fluorescence: Intraocular straylight affecting visual function,”
Vision Res., 33 1827
–1833
(1993). https://doi.org/10.1016/0042-6989(93)90173-T 0042-6989 Google Scholar
M. A. Mosier,
J. R. Occhipinti, and
N. L. Burstein,
“Autofluorescence of the crystalline lens in diabetes,”
Arch. Ophthalmol. (Chicago), 104 1340
–1343
(1986). 0003-9950 Google Scholar
T. J. T. P. van den Berg,
“Quantal and visual efficiency of fluorescence in the lens of the human eye,”
Invest. Ophthalmol. Visual Sci., 34 3566
–3573
(1993). 0146-0404 Google Scholar
J. A. Zuclich,
R. D. Glickman, and
A. R. Menendez,
“In situ measurements of lens fluorescence and its interference with visual function,”
Invest. Ophthalmol. Visual Sci., 33 410
–415
(1992). 0146-0404 Google Scholar
J. A. Zuclich,
T. Shimada,
T. R. Loree,
I. Bigio,
K. Strobl, and
S. Nie,
“Rapid non-invasive optical characterization of the human lens,”
Lasers Life Sci., 6 39
–53
(1994). 0886-0467 Google Scholar
J. A. Zuclich,
“In vivo measurements of optical properties of the human lens,”
Proc. SPIE, 2134B 99
–112
(1994). 0277-786X Google Scholar
T. J. T. P. van den Berg and
J. K. IJspeert,
“Light scattering by donor lenses,”
Vision Res., 35 169
–177
(1995). https://doi.org/10.1016/0042-6989(94)00123-4 0042-6989 Google Scholar
T. J. T. P. van den Berg,
“Visual efficiency of scattering and fluorescence in the human eye lens,”
J. Biomed. Opt., 1 262
–267
(1996). 1083-3668 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||