|
|
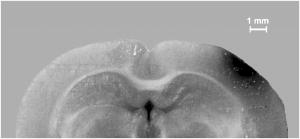
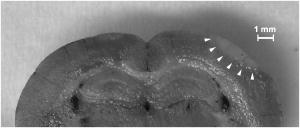
1.IntroductionBrain edema is induced in association with brain damage and diseases such as head injuries, cerebrovascular diseases, encephalitides, and brain tumors, and it causes an increase in the volume of brain tissue due to an increase in its water content.1, 2 This results in an increase in intracranial pressure (ICP) and hence a decrease in cerebral blood flow, and these increase the risk of death and cessation of brain function. Brain edema is classified into vasogenic edema, cytotoxic edema, and interstitial edema.2 Vasogenic edema is caused by increased permeability of brain capillaries. Cytotoxic edema results from failure of the adenosine-triphosphate-dependent sodium pump within cells. Interstitial edema is caused by obstructive hydrocephalus. Vasogenic edema is the most common form of brain edema occurring in brain traumas and tumors, and we therefore focused on vasogenic edema in this study. In animals, vasogenic edema can be induced by cold injury, stab wound, insertion of a pellet, inflation of a balloon, and implantation of neoplasmas.1 In this study, we examined a cold injury model since it is highly reproducible, widely used, and can be made without craniotomy. We speculated that optical properties of brain tissue change due to formation of edema since water content of brain tissue increases. To verify this speculation, we performed transcranial measurement of diffuse reflectance from cold-injured brains. In this study, light was used to detect change in the scattering coefficient of the tissue, and light was also used to monitor local blood volume change, which was expected in such a trauma model. To assess the distribution of brain edema, Evans blue (EB) was intravenously administrated. Since EB molecules bind to serum albumin, which is included in vasogenic edema fluid, the extent of edema can be assessed on the basis of the density of EB-labeled albumin in brain tissue. EB molecules absorb visible light efficiently, and the intensity distribution of diffuse light reflectance therefore reflects distribution of edema in the brain. In this paper, we present the results of diffuse reflectance measurement and discuss how the reflectance intensity correlates with edema induced in brain tissue. 2.Materials and Methods2.1.Animal PreparationWe examined cold-injured brains in rats,3 a standard animal model of brain edema. The experiments were performed in accordance with the guidelines for Laboratory Animal Facilities and Care Regulation of the National Defense Medical College, Saitama, Japan. Sprague-Dawley male rats (Japan SLC, Inc.) weighing were each anesthetized with of pentobarbital sodium and placed in a stereotaxic apparatus (Narishige Co., Ltd.). After exposing the parietal bone with a scalp incision, a -diam liquid nitrogen-cooled copper probe was applied to the right parietal bone for at ; the coordinate corresponds to the left-to-right direction with the origin on the midline (Fig. 1 ). Fifty ml/kg of lactated Ringer’s solution was intraperitoneally injected into each rat for accelerating formation of edema, and the incised scalp was closed with sutures. After this procedure, the rats were returned to their cages with water and food pellets. For an additional transfusion, each rat was anesthetized with of pentobarbital sodium and injected with the same amount of lactated Ringer’s solution after the injury. After the second transfusion, the rats were returned to their cases again. Fig. 1Positions of the source and detector fibers used for measurement of diffuse light reflectance on the skull in a rat. The shadowed region indicates the domain for monitoring. A -diam liquid-nitrogen-cooled copper probe was applied to the hatched circular area; its center corresponded to a position of . Measurement of diffuse reflectance was performed every in the range of . The distance between the scanning line for the detection fiber and the bregma was . 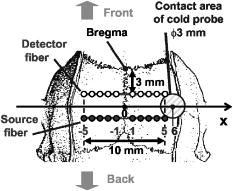 2.2.Monitoring of Diffuse Light ReflectanceIn measurements of diffuse light reflectance, near-IR light is widely used because of its deep optical penetration in tissue. In this study, however, we intended to monitor not only change in the absorption coefficient but also change in the scattering coefficient, and we therefore used visible light (633 and ) whose scattering coefficient of brain tissue is about 1.5 times larger than those in the near-IR spectral region.4 In the visible spectral region, the scattering coefficient gradually increases with decrease in wavelength; the difference in the scattering coefficients at 633 and is within 10%.4 On the other hand, the absorption coefficient of blood at is about 10 times larger than that at . Thus, intensity of diffuse reflectance at is more greatly affected by a change in the absorption coefficient due to blood volume change than by a change in the scattering coefficient; at , the change in light scattering in brain tissue was investigated. By measuring diffuse light reflectances at , the influence of change in blood flow on diffuse reflectance can be assessed. For the measurements, brain tissue was transcranially irradiated with , HeNe laser (NEC, Inc.) and the , second harmonics of an laser (Spectra-Physics, Inc.); both lights were simultaneously transmitted through an optical fiber with a core diameter. Diffuse light reflectance from the brain tissue was collected with another optical fiber with the same core diameter, and the collected diffuse light was delivered to a polychromator (Hamamatsu Photonics, Inc.) and intensity of the light was measured. The ends of two fibers were in contact with the skull and separated by (Fig. 2 ). For this configuration, we performed Monte-Carlo simulation to estimate detectable depth in the brain using optical properties reported in the literature.4, 5 The simulation showed that the measurement depth was approximately in the cerebral cortex (Fig. 2). The thickness of the cerebral cortex in the rats was determined to be . Fig. 2Diagram of the experimental setup for diffuse light monitoring (left) and optical paths of diffuse light reflectances at in a cross-sectional view by Monte-Carlo simulation (right). HeNe laser light was transcranially introduced into the brain tissue through a source fiber. Diffuse light reflectance was delivered to a polychromator through a detector fiber. The source and detector fibers were in core diameter and they were separated by . The maximum optical penetration depth was calculated to be about in the cerebral cortex. The thickness of the cerebral cortex in the rats was determined to be . 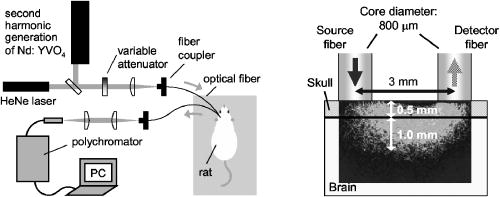 The measurement area of diffuse reflectance was limited by the size of parietal bone. The width of parietal bone in the rats used in this study was about . Therefore, measurements were performed every in the range of , as shown Fig. 1. The distance between the scanning line for the detection fiber and the bregma was . For each rat, measurements were performed before and at after induction of cold injury. After the second measurement, some rats were sacrificed for histological analysis of brain tissues that were stained with hematoxylin-eosin (HE) or India ink. The other rats were intravenously each injected with of 2% EB solution at after the injury,6, 7 and then the third measurement of diffuse light reflectance was performed at after dye administration to assess the distribution of edema by observing diffuse light absorbed by EB in the brain. The interval of between the second and the third measurements was shorter than the elapsed time after making cold injury, and the difference between the distributions of edema at the time of the second measurement and at the time of the third measurement should therefore be small. These rats were also sacrificed for histological analysis. 2.3.Statistical AnalysisResults are expressed as means ± standard error of the mean. A paired test was used to verify change in the detected light intensities at after making cold injury. Difference in the intensities was considered to be significant at a probability level of less than 0.01 and very significant at a probability level of less than 0.001 . 2.4.Histological StudiesThe area of necrotic tissue due to cold injury was examined for HE-stained brain tissue, while the region of edematous tissue was examined for EB-stained brain tissue. The distribution of blood flow was assessed by India ink perfusion. For these analyses, rats were deeply each anesthetized with of pentobarbital sodium and perfused intracardially with of saline, followed by 10% formalin. The brain of each rat was removed from the skull and placed in 10% formalin for . The brain was placed in phosphate buffer solution prior to cutting of coronal sections. The brain was embedded in paraffin and sliced on a microtome for HE staining. HE-stained tissues were observed by an optical microscope (Nikon Corporation). EB-stained brain tissues were not embedded in paraffin because EB stain is too light to be observed in thin sections of tissue. A coronal section was cut with a trimming blade for pathological examination, and images of EB-stained brain were obtained by using a digital camera (Fuji Photo Film Co., Ltd.). For India ink perfusion, rats were deeply each anesthetized with of pentobarbital sodium and intracardially perfused with of saline, followed by of saline containing 20% India ink. The mean diameter of India ink particles was about . By the perfusion, particles in the ink adhered to endothelia of blood vessels. Since particles cannot be leaked out of the blood vessels, vessels with blood flow are selectively stained.8, 9 The brain of each rat was removed from the skull, and images of the brains in dorsal aspect were acquired by using a digital camera. After fixation of the brain in 10% formalin, a coronal section including the center of cold injury was obtained using a trimming blade for pathological examination. Images of the coronal section were also acquired by using a digital camera. Pentobarbital sodium not only has an anesthetic effect but also reduces brain edema. However, the latter effect is slow-acting and, in addition, continual administration of pentobarbital sodium is needed for treatment of brain edema.10 In this study, the time interval between the second measurement and the perfusion fixation of EB-stained brain tissue was about , and administration of pentobarbital sodium was discontinuous. Therefore, the difference between the state of edema at the time of the second measurement and that at the time of perfusion fixation of EB-stained brain tissue is thought to be small. 3.Results3.1.Diffuse Light Reflectance from the Brain Stained with EBIntravenously injected EB may be distributed both in the blood vessels and in the brain tissue; the latter is caused by the increased permeability of blood vessels due to edema. Owing to the former, i.e., EB distributed in the blood vessels, reflectance intensity decreased not only in the injured hemisphere but also in the contralateral hemisphere regardless of wavelength (Fig. 3 ). On the other hand, EB leaked from the vessels was probably localized around the cold injury. Therefore, the ratio of diffuse reflectance intensity in the injured hemisphere to that in the contralateral hemisphere may indicate the distribution of EB leaked from the vessels and hence the distribution of edema (Fig. 4 ). In the measurement at , the ratios at were about 60%, while the ratios at were about 30% [Fig. 4(a)]. In the measurement at , the ratio at was higher than that at , and the ratio was decreased with distance from the midline [Fig. 4(b)] in the region of . Fig. 3Graphs showing intensities of diffuse light reflectance at (a) 633 and (b) as a function of position on the skull in rats. The shadowed region indicates the domain of cold injury. Before and at after injury, measurements were performed for 28 rats. After EB injection, the measurement was performed for 9 rats. Data are expressed as means ± standard error of the mean. 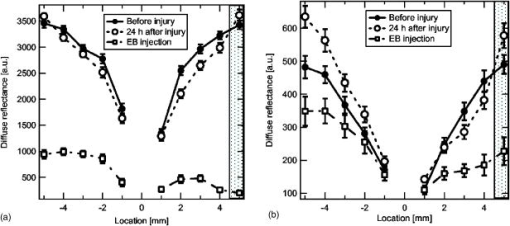 Fig. 4Graphs showing ratio of diffuse light reflectance intensity in the injured hemisphere to that in the contralateral hemisphere as a function of distance from the midline at (a) 633 and (b) . Before and at after injury, measurements were performed for 28 rats. Measurements after EB injection were performed for 9 rats. Data are expressed as means ± standard error of the mean. 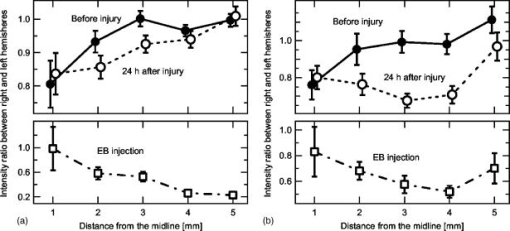 3.2.Diffuse Light Reflectance from the Brain without EB StainingMeasurement of diffuse light reflectance was performed for 28 rats. Intensities of diffuse light reflectance that were measured before and at after injury at 633 and are shown in Figs. 3(a) and 3(b), respectively, as a function of position on the skull. Figure 5 illustrates the results of statistical analysis based on the data shown in Fig. 3. Fig. 5Bar graphs showing diffuse light reflectances from the brains of rats at (a) and (b) 633 and (c) and (d) . (a) and (c) show intensities before and at after the injury in the injured hemisphere. (b) and (d) illustrate intensities at symmetrical points about the midline at after injury. Each bar represents the means ± standard error of the mean. Single asterisk shows a probability level of less than 0.01 , and double asterisks shows a probability level of less than 0.001 . 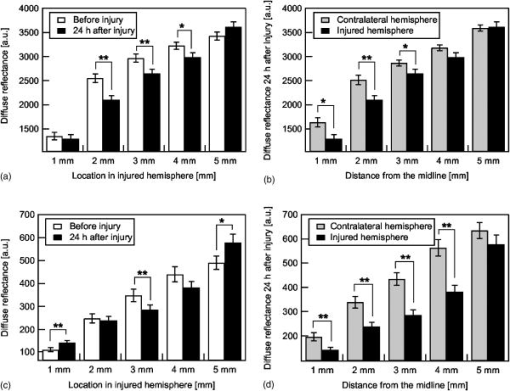 In the measurement at , change in reflectance intensity at after injury was induced chiefly in the injured hemisphere [Fig. 3(a)]. At in the injured hemisphere, significant decreases in reflectance intensity were observed ( at , at ) [Fig. 5(a)]. At after injury, reflectance intensities at in the injured hemisphere were significantly lower than those at in the contralateral hemisphere ( at , at ) [Fig. 5(b)]. In the measurement at , there were both regions where reflectance intensity was increased and decreased in the injured hemisphere. In the contralateral hemisphere, on the other hand, increased reflectance intensity was observed at all locations of measurement [Fig. 3(b)]; at , increasing rate of reflectance intensities was almost constant at about 20%. At in the injured hemisphere, reflectance intensity was significantly decreased , while at , reflectance intensity was significantly increased ( at , at ) [Fig. 5(c)]. At after injury, reflectance intensities at all locations in the contralateral hemisphere were significantly higher than those in the injured hemisphere ( at ) except at . 3.3.Histological StudiesHistological analysis of the brain tissues revealed spatial characteristics of cold injuries. In the HE-stained tissues, necrosis was found in the region corresponding to and at depths of less than (Fig. 6 ). In the case of HE staining, it was found that tissue shrank during the procedure of paraffin embedding; the width of the brain section shrank from about 17 to about (about 30% shrinkage in length). Taking into account tissue shrinkage, the area of necrosis before paraffin embedding was estimated to be from , indicating that the area of necrosis corresponded to the region where a cold probe was applied. While spongiform brain tissue was observed by a optical microscopic observation of HE-stained tissue in the injured hemisphere, such denaturation was not found in the contralateral hemisphere. Figure 7 shows an EB-stained brain that was placed in 10% formalin for after perfusion fixation and cut in coronal sections without embedding. Intense EB staining was observed in the region corresponding to with a maximum depth of about , around which incidental dye staining was observed. Figure 8 illustrates the coronal aspect of a rat brain that was fixed in 10% formalin for after India ink perfusion. In the area corresponding to with a maximum depth of about , tissue was not stained by India ink, indicating that blood volume was reduced in this region. We found that both the area intensely stained with EB and the area not stained with India ink coincided with the area of necrosis shown in the HE-stained tissue when shrinkage of approximately 30% was taken into account for the HE stained tissue. No lesions were observed in the control hemispheres. Fig. 6Coronal section of a rat brain stained with HE. The tissue shrank in the procedure of paraffin embedding; the width of the brain section shrank from about 17 to about (about 30% shrinkage). 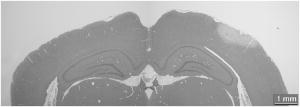 4.DiscussionIn this study, intensities of diffuse light reflectance from cold-injured brains were measured. Under all of the conditions of measurements, intensities of diffuse light reflectance were greatly decreased near the midline, resulting from the light absorption by blood in the superior sagittal sinus. This is not directly concerned with the discussion in this study. As already stated, we speculated that edema causes a decrease in the scattering coefficient of brain tissue, and this study was designed to detect this change by measuring diffuse light reflectance from the brain. However, cold injury may induce change not only in the scattering coefficient but also in the absorption coefficient of brain tissue since blood flow may be changed due to the effect of injury; both changes affect diffuse light reflectance. Therefore, in addition to light, we used light to estimate the change in blood volume in the measurement of diffuse light reflectance. Intensity distributions of the diffuse light reflectance for the brain stained with EB and for the brain without EB staining are discussed in the following two sections. 4.1.Diffuse Light Reflectance from the Brain Stained with EBAfter EB injection, intensity of diffuse light reflectance decreased drastically both at 633 and (Fig. 3). The reason for the larger decrease in the intensity at is the higher absorption coefficient of EB at 633 than at . At , the ratios of reflectance intensity were about 60% at and about 30% at [Fig. 4(a)]. The results correspond to the density distribution of EB in the coronal aspect of brain tissue (Fig. 7); the tissue was intensely stained with EB in the region of , around which the tissue was lightly stained. At , the ratio of reflectance intensity decreased with approach to the site of cold injury in the region of [Fig. 4(b)]. However, at a point in the cold injury, i.e., at , the ratio of reflectance intensity was increased. This is attributable to a considerable reduction of blood volume in the injured area; Fig. 8 shows that perfusion was reduced or blocked in the area of injury,11 i.e., . The reason why increase in the reflectance intensity was not observed in the area of injury at [Fig. 5(a)] is that the absorption coefficient of blood is much lower at 633 than at . These findings indicate that distribution of EB and hence the distribution of edema can be assessed transcranially by measuring diffuse light reflectance. Decrease in the blood flow due to cold injury can also be monitored by measurement at . 4.2.Diffuse Light Reflectance from the Brain without EB StainingIn the contralateral hemisphere, intensities of diffuse light reflectance measured at were increased over the whole area at after injury, while those measured at showed little change (Fig. 3). In the contralateral hemisphere, the absorption coefficient of brain tissue might be decreased because of a decrease in cerebral blood flow. Milhorat investigated the change in cerebral blood flow due to cold injury by the use of a radioactive tracer.12 They made cold injury by almost the same method as ours and observed a 10 to 20% decrease in cerebral blood flow in a cerebral cortex of the contralateral hemisphere. We measured cerebral blood flow in a cerebral cortex of the contralateral hemisphere using laser Doppler flowmetry (Perimed Co.) and observed a 10% decrease in cerebral blood flow at after injury. Increase in diffuse reflectance was clearly detected by measurement at because of a much larger absorption coefficient of blood at 532 than at . In the injured hemisphere, reflectance intensity was found to be increased in the area of injury at both wavelengths, this being attributable to the decrease in blood flow due to injury. As already described, decreased blood flow in the area of injury was also detected by measurements of diffuse reflectance at after EB injection. In the periphery of the area of injury, reflectance intensities were decreased at both wavelengths. At , reflectance intensities were significantly decreased at and in the injured hemisphere, where brain tissue was lightly stained with EB [Figs. 5(a) and 7], indicating that tissue in this region was edematous to a certain extent. In this region, necrosis was not found based on the histological analysis of the HE-stained tissue. These findings suggest that the reflectance intensities around the area of cold injury were decreased due to formation of edema. Aitken reported that diffuse light reflectance from sliced brain tissue was decreased with increase in cell volume due to osmotic pressure.13 They pointed out that osmotically induced cell swelling may reduce light scattering because of the decrease in the concentration of macromolecules in the cytoplasm. This is consistent with our results, since brain edema causes increase in the water content of brain tissue and hence increase in the tissue volume. At , reflectance intensity at after injury was significantly lower than that before injury only at [Fig. 5(c)]. Decrease in reflectance intensity due to a decreased scattering coefficient may be balanced by a decreased absorption coefficient caused by decreased in blood flow at this wavelength, because of a large absorption coefficient of blood. Our results demonstrated that cold injury caused site-dependent changes in both the absorption and scattering characteristics of brain tissue. In this study, however, the effect of morphological change of brain tissue due to cold injury was not investigated. In future works, the effects of morphological change and optimization of the wavelengths should be investigated. 5.ConclusionsFor cold-injured brains in rats, diffuse light reflectance was measured as a function of position on the skull. At after injury, reflectance intensity at was significantly decreased in the region around the area of injury. The results of histological analysis showed that edema was formed in this region and that there was no decrease in blood flow. These findings suggest that the scattering coefficient of the brain tissue was decreased due to edema in this region. For tissue in the area of injury, reflectance intensity at after injury was significantly increased at , while no significant change in the reflectance intensity was observed at , indicating that blood flow was reduced or blocked in the area of injury. These results demonstrate that change in the optical properties for a cold-injured brain can be detected by measurement of diffuse light reflectance, suggesting the possibility of transcranial diagnosis of brain edema. ReferencesI. Klatzo,
“Neuropathological aspects of brain edema,”
J. Neuropathol. Exp. Neurol., 26 1
–14
(1967). 0022-3069 Google Scholar
R. A. Fishman,
“Brain edema,”
N. Engl. J. Med., 293 706
–711
(1975). 0028-4793 Google Scholar
J. C. Lee and
L. Bakay,
“Ultrastructural changes in the edematous central nervous system. II. Cold-induced edema,”
Arch. Neurol., 14 36
–49
(1966). 0003-9942 Google Scholar
P. van der Zee,
M. Essepreis, and
D. T. Delpy,
“Optical properties of brain tissue,”
Proc. SPIE, 1888 454
–465
(1993). 0277-786X Google Scholar
M. Firbank,
M. Hiraoka,
M. Essenpreis, and
D. T. Delpy,
“Measurement of the optical properties of the skull in the wavelength range ,”
Phys. Med. Biol., 38 503
–510
(1993). https://doi.org/10.1088/0031-9155/38/4/002 0031-9155 Google Scholar
P. H. Chan,
S. Longar, and
R. A. Fishman,
“Phospholipid degradation and edema development in cold-injured rat brain,”
Brain Res., 277 329
–337
(1983). 0006-8993 Google Scholar
P. H. Chan,
S. Longar, and
R. A. Fishman,
“Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema,”
Ann. Neurol., 21 540
–547
(1987). 0364-5134 Google Scholar
H. Segawa and
R. H. Patterson,
“Role of platelets in vasogenic brain edema. I. Significance of thrombus formation in the damaged vessels,”
Arch. Neurol., 38 265
–270
(1981). 0003-9942 Google Scholar
M. C. Curras-Collazo,
U. B. Patel, and
M. O. Hussein,
“Reduced susceptibility of magnocellular neuroendocrine nuclei of the rat hypothalamus to transient focal ischemia produced by middle cerebral artery occlusion,”
Exp. Neurol., 178 268
–279
(2002). 0014-4886 Google Scholar
M. Gaab,
F. Herrmann,
J. Kerscher,
K. Rausch,
J. Lochner, and
K. W. Pflughaupt,
“Comparison of the effects of dexamethasone, barbiturate, and THAM on experimental brain oedema,”
Acta Neurochir. Suppl. (Wien), 28 493
–497
(1979). 0065-1419 Google Scholar
S. B. Hays,
“Pathogenesis and pathologic process,”
Cold Injury, Ground Type, 235
–257
(1958) Google Scholar
T. H. Milhorat,
W. D. Johnson, and
D. L. Dow-Edwards,
“Relationship between oedema, blood pressure, and blood flow following local brain injury,”
Neurol. Res., 11 29
–32
(1989). 0160-6412 Google Scholar
P. G. Aitken,
D. Fayuk,
G. G. Somjen, and
D. A. Turner,
“Use of intrinsic optical signals to monitor physiological changes in brain tissue slices,”
Methods, 18 91
–103
(1999). 1046-2023 Google Scholar
|