|
|
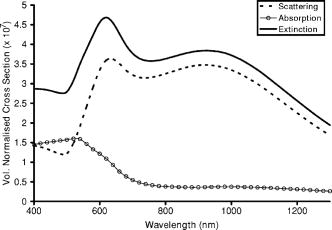
1.IntroductionOptical technologies promise high-resolution, noninvasive functional imaging of tissue with improved sensitivity, specificity, and cost effectiveness relative to current approaches. Numerous studies over the past decade have shown the viability of scattering-based optical approaches, including spectroscopy,1, 2, 3 confocal microscopy,4, 5, 6 and optical coherence tomography7, 8, 9 (OCT) in addressing some of the limitations associated with current methods of cancer detection and have generated promise in differentiating normal from diseased tissue. Confocal microscopy is a rapidly developing optical technology that can noninvasively image several hundred micrometers into tissue with micrometer resolution,10 and the capability to detect subcellular morphological changes that are potentially useful for monitoring epithelial precancers.6 OCT is another optical method that can provide both real-time and in situ imaging without the necessity for removal and processing of biological samples for biopsies and histopathological assessment. The images captured are 10 to 100 times finer in detail compared to conventional imaging techniques such as magnetic resonance imaging and ultrasound and axial resolutions as fine as have been recently demonstrated.11 Optical coherence microscopy (OCM) is another scattering-based optical method that combines the advantages of both confocal and OCT imaging, demonstrating significant promise for epithelial cancer detection.12 Subcellular changes that alter scattering signals, such as increased nuclear size and change in refractive index, are useful indicators of dysplasia.13 However, many other valuable molecular indicators of early precancers may not generate obvious intrinsic optical contrast. In these cases, targeted contrast agents are desirable. As optical imaging is still an emerging field in medical diagnostics, the application of optical contrast agents to enhance contrast of the signatures of disease is not routine. Chemical agents such as dilute acetic acid,10, 14 toluidine blue,15, 16 and Lugol’s iodine17, 18 have been shown to enhance visualization of dysplasia by augmenting the overall optical properties of dysplastic tissue relative to normal surrounding tissue.19 Acetic acid causes selective whitening of tissue and it is suggested that it alters the refractive index of the cell nucleus, causing an increased scattering effect. This effect is more pronounced in dysplastic regions with greater nuclear size and density. Toluidine blue is a dye that stains acidic components of cells such as the DNA and RNA. The higher level of nucleic acids present in enlarged nuclei of dysplastic cells enables the dye to enhance differentiation between normal and abnormal tissue. Lugol’s iodine reacts with glycogen, present in normal epithelial tissue, while dysplastic cells and carcinomas remain comparatively unstained or lightly stained, as they contain very little tissue glycogen. Other contrast agents such as indocyanine green have been widely investigated for use in enhancing imaging and detection of cancers via fluorescence.20, 21 The dye has excitation and emission peaks near 780 and , respectively, and this spectral region lies within the “water window” of the near IR (NIR), where photon absorption in biological tissue is at a minimum. This is most useful for detecting cancers in optical applications such as diffuse optical tomography.22 Particle-based technologies have also generated much interest for use to enhance contrast for optical imaging and microscopy due to its optical capabilities and ease of adding surface modifications. Lee 23 developed oil-filled encapsulating protein microspheres that can incorporate various particles such as gold and carbon to alter backscattering optical signatures for OCT. This can be used as a contrast agent and the enhancement was demonstrated by imaging a mouse liver with intravenously injected gold-shelled microspheres. Similarly, the use of intravenously injected polystyrene microspheres was shown to enhance imaging of skin and microcirculation under confocal reflectance microscopy.24 To achieve more precise and accurate diagnosis and detection of cancer, it is desirable for optical contrast agents that can target molecule specific signatures of interest. Specific targeting by therapeutic and diagnostic agents through antibody binding of tumor cell surface molecules or tumor-specific peptides has been used extensively. For example, a commercial product for the detection of prostate cancer (ProstaScint) is an antibody conjugated against prostate-specific membrane antigen also conjugated to a scintigraphic target.25 It is also possible to achieve similar targeting efficiencies with small tumor-specific peptides.26, 27 Nanoengineered technologies and molecular targeting strategies provide an exciting nexus for further development in detection, monitoring, and biosensing of biomolecules. Technologies such as nanoprobes can be used for optical sensing of biomolecular activity in single cells, while preserving vital cellular processes.28, 29 Surfaces of nanoparticles are easily modified with antibodies, peptides, and other cell-specific moieties and can be used for applications such as glucose sensing30 and immunoassays.31, 32 Hirsch devised a simple and rapid immunoassay by successfully measuring spectral changes using gold nanoshells to target very small quantities of analytes.31 Nanoparticles have also generated considerable interest for use as a target-specific contrast agent to enhance optical detection, diagnosis, and therapy of cancers.33 Recent work by Sokolov 34 demonstrated that the detection of precancerous cells using confocal reflectance imaging can be enhanced with gold nanoparticles bioconjugated with molecule-specific markers bound to their targets. Other nanoparticles, such as semiconductor quantum dots, also show similar potential for use as target-specific biological probes.35, 36 Quantum dots, such as ZnS-capped CdSe, are emissive at wavelengths ranging from the near-UV to the NIR by changing the size and composition. Their surfaces are also highly modifiable for bioconjugation of target-specific peptides, and it has been shown that it is possible to use such nanoparticles to bind to specific targets in vivo using a tumor rat model, enhancing visualization under fluorescence or confocal microscopy.35 In this paper, we consider the use of metal nanoshells as exogenous contrast agents. Metal nanoshells are composed of a dielectric core (e.g., silica) coated with an ultrathin metallic layer (e.g., gold). Gold nanoshells possess physical properties similar to gold colloid and exhibit strong scattering and absorption effects due to the strong plasmon resonance of the metallic-dielectric concentric spherical configuration.37, 38 In particular, the optical behavior of gold nanoshells in the NIR shows scattering and/or absorption cross sections often several times the particle geometric cross section. This is not seen with comparable nanoparticles such as gold colloidal nanoparticles, which show weak optical activity in the NIR spectrum region.38, 39 By varying the relative core size and shell thickness, the peak resonance of gold nanoshells can be dramatically varied across a broad range of the optical spectrum that spans the visible and the NIR spectral regions.40 The “tunability” of the optical resonance is a property unique to metal nanoshells. Figure 1 shows Mie extinctions plots with a -radius silica core. By increasing the core radius-shell thickness ratio, it is possible to shift the peak plasmon resonance of the nanoshell well into the NIR and to wavelengths greater than . Furthermore, gold nanoshells can also be tuned to show the same peak optical resonance with different size parameters.41 Under current laboratory methods, it is also possible to fabricate gold nanoshells of varying sizes with experimental observations of gold nanoshell resonances closely matching Mie theory. The fabrication method uses a combination of molecular self-assembly and colloid chemistry in aqueous solution and is well described in literature.31, 37 Fig. 1By increasing the core radius to shell thickness ratio, the peak extinction resonance can be shifted well into the NIR. For gold nanoshells with a core (silica) radius of and a decreasing gold shell thickness, the peak resonance shifts to longer wavelengths. 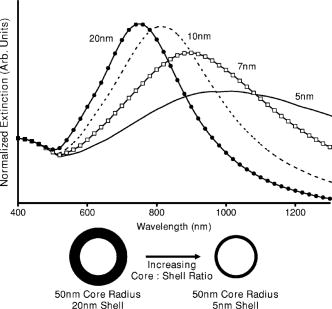 The large optical efficiencies of nanoshells in the NIR are especially useful for biological applications. Moreover, gold nanoshells can be used for bioconjugating applications as their surfaces are virtually chemically identical to gold colloid, universally used in numerous bioconjugate applications.42 By using nonbioconjugated gold nanoshells, Hirsch 43 and O’Neal 44 showed that gold nanoshells can be used as an agent for photothermal therapy of cancers. By further functionalizing gold nanoshell surfaces with target-specific antibodies, Loo showed enhanced visualization using gold nanoshells targeted to HER2-positive breast cancer cells under brightfield and darkfield microscopy.45 The targeted cancer cells can subsequently be selectively destroyed through photothermal therapy,46 paving way for an integrated imaging and therapy of cancer. Although gold nanoshells have already shown success for cancer imaging and therapy, the optical effect of adding nanoshells to tissue has not yet been elucidated. It is important to predict how gold nanoshells affect tissue reflectance for scattering-based optical methods, so that optimal parameters for its use as an optical contrast agent can be fully realized. We evaluate gold nanoshells as an optical contrast agent with computational tools. We first use analytical electromagnetic methods to calculate optical properties of various nanoshells with a wide variety of scattering and absorbing capabilities. As the optical response of nanoshells are typically described by the optical extinction,37, 38, 40 it is critical for our studies to consider the optical effect of scattering and absorption. We then employ the use of Monte Carlo methods to evaluate the effect of adding varying concentrations of gold nanoshells in tissue on photon reflectance. We also consider how the combination of scattering and absorption properties of nanoshells with different size parameters affect remitted signals. In our work, the Monte Carlo models suggest that even as the optical extinction is strongly dominated by scattering, absorption from the nanoshells cannot be neglected in predicting tissue reflectance. Furthermore, because of the strong optical behavior of nanoshells, a considerable change in reflectance was observed with only a very small concentration of nanoshells. 2.Methods2.1.Optical Properties of Gold NanoshellsThe optical response of gold nanoshells can be described by using computed solutions of Mie theory for concentric spherical shells at the boundaries between different mediums.38 For use in the Monte Carlo studies described later, Mie solutions of gold nanoshells were computed with an excitation wavelength of , within the region of optimal physiological transmissivity best suited for optical bioimaging and biosensing applications. Typically, optical resonances of gold nanoshells are calculated with air or water as the embedding medium.37, 40 However, it is important that the optical properties of gold nanoshells are calculated with , simulating the embedding medium as a physiological environment, as the Monte Carlo studies will use tissue models embedded with nanoshells. Table 1 shows the optical properties of the nanoshell with , 1.33, and 1.4. The differences in optical properties must be taken into account as it would subsequently affect the optical properties calculated and used in the Monte Carlo studies. Table 1Optical properties calculated from Mie solutions of various particles at a 830-nm excitation wavelength.
R40∕45
represents a nanoshell with a
40-nm
core radius with a
5-nm
-thick gold shell. Extinction efficiencies, scattering-to-absorption efficiency ratio (Sca/Abs ratio), and the volume-normalized scattering and absorption cross sections are shown. The units for the volume-normalized cross-sections are inverse meters. Properties of the
10-nm
-radius gold nanoparticle (R10-Au), a commonly used agent for biological interrogation, are included for comparison. The values shown are calculated with an embedding medium index of refraction,
n=1.4
, simulating a physiological medium. Also shown as an example, are optical property values of the
R75∕115
nanoshell in water
( *)
and air
( #)
with index of refraction,
n=1.33
and
n=1.0
, respectively. The differences in the values show the importance of using the medium that more accurately mimics physiological conditions. To choose different nanoshells with varying optical properties, we first assume independent scattering of the nanoshell particles for simplification. Under independent scattering, the optical cross section per unit volume of the particle becomes more appropriate than the optical efficiency for subsequent calculations of scattering and absorption coefficients47, 48 for use in the Monte Carlo studies. Core radius-to-shell thickness space-maps [see Figs. 2(a) and 2(b) ] of volume-normalized optical cross sections are used to select the nanoshells, with a range of sizes and optical properties. The space-maps were computed with an excitation wavelength of , including sizes that can be fabricated with current laboratory methods. As gold nanoshells possess not just scattering, but also absorbing properties, nanoshells with a variety different scattering and absorbing proportions are also considered for the Monte Carlo studies. A simple relation—the scattering-to-absorption efficiency ratio (Sca/Abs ratio)—can be used to compare how much more scattering a nanoshell is compared to its absorption ability [see Fig. 2(c)]. Fig. 2Computed optical properties of gold nanoshells as a function of the core radius and the shell thickness (both in nanometers) at an excitation wavelength of . These diagrams can aid in selecting specific nanoshells with desired optical properties. The graphs describe (a) volume-normalized scattering cross section , (b) volume-normalized absorption cross section , (c) scattering-to-absorption efficiency ratio (Sca/Abs), and (d) extinction efficiency. These space-maps can aid in determining the desired size and optical properties to be used in subsequent studies. 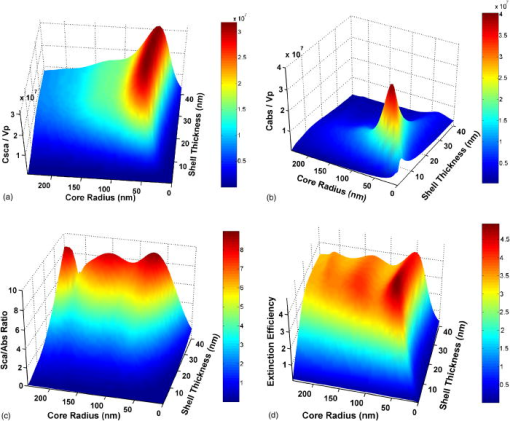 2.2.Monte Carlo ModelingMonte Carlo modeling of photon transport in multilayered tissues by Wang, 49 a computational tool written in Standard C, has been widely used to simulate random walk of photons through turbid media and is useful for investigating light propagation in tissue. The fraction of remitted and absorbed photons can be predicted from Monte Carlo computations, and the fraction changes accordingly as the optical parameters of the medium are changed. The theories behind Monte Carlo methods are well described in literature and are not be repeated here.49 Simulations were performed on two models (A and B) that will each be differentiated into two sets: a bulk tissue model (models A-1 and A-2) and a multilayered tissue model (models B-1 and B-2). The description of the two sets is provided later. The bulk tissue model was assumed to be an infinitely thick precancerous tissue layer with uniform homogeneous optical properties. The base optical properties before the addition of nanoshells of this precancerous layer was set with the absorption coefficient at , and the scattering coefficient, , both within cited literature50, 51, 52 values in the NIR. The tissue properties of this layer are then changed, simulating an increasing concentration of nanoparticles added to the tissue. A three-layered model (models B-1 and B-2) used in our studies (see Fig. 3 ) is similar to the theoretical model used by Quan and Ramanujam,53 which mimics the basic structure of epithelial and stromal tissue. The first layer represents normal surface epithelium with a thickness of , and optical parameters were kept constant at and . The second precancerous layer , originates from the basal membrane, occupying space between the stroma and the normal epithelium of the top layer. As the epithelium consists of the most superficial tissue layers, hemoglobin content is negligible. The absorption coefficients of the epithelial layers represent absorption by tissue chromophores such as lipid, water and cytochrome oxidase.54, 55, 56 Similar to model A, increasing concentrations of nanoshells were added to this representative precancerous layer and the optical properties of this second layer without any nanoshells was the same as the bulk tissue, where and . The stromal layer was modeled as a semi-infinitely thick layer with and . The following properties were kept constant for all simulations: the anisotropy factor was kept constant at 0.9 and for the tissue refractive index. Fig. 3Schematic showing the multilayered tissue model used in the Monte Carlo studies. The depths of the parameters shown are: normal surface epithelium, ; precancerous layer, ; and stromal layer (semi-infinitely thick), . The optical parameters of the layers were set as, normal surface epithelium, and , and the stromal layer optical properties of and . The concentration of nanoparticles was incremented in the precancerous layer, with base optical properties of and . 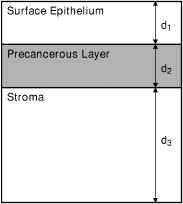 The overall scattering and absorption coefficient of the tissue is altered by the optical properties of the particles added to the models. As a basis of comparison, gold nanoparticles ( radius) commonly used for biological interrogation such as biomolecular analysis, interactions, and imaging34, 57, 58 were used in place of the gold nanoshells. To account for interparticle effects by the nanoparticles on photon scattering, a correction factor is required for volume fractions higher59, 60 than , when the diameter of the particles are much less than the wavelength to account for the reduction of bulk optical cross section as a result of non-independent scattering among subwavelength spheres. As mentioned, for these studies, we impose a limit on the density and size of nanoshells added to the precancerous layers to ensure independent scattering. Each type of nanoparticles is then added separately, so that only one type of particle is added to the models at one time. Using well established light scattering theory, the change in the optical coefficient due to particles in a medium, is related to the scattering or absorption cross section and the number of particles per unit volume48 : This can then be related to the volume fraction of nanoparticles added to the models, and also the scattering or absorption cross section normalized by the volume of the particle used ,The change in scattering or absorption coefficient can be due to the scattering or absorption of the particle added. To evaluate the overall effect adding particles with a variety of physical and optical parameters, the studies are separated into two sets for both the bulk (model A) and multilayered (model B) models. As gold nanoshells can be fabricated to be far more scattering than absorbing, for example, the (representing a core radius of and an overall radius of ) gold nanoshell shows almost 90% of the extinction as scattering, we access if such highly scattering particles can be simply considered as a scatterer while neglecting its absorption properties. Thus, the studies will be further separated into two sets, one considering only the scattering properties of the particles and neglecting the absorption. The other will take into account both the scattering and absorption properties of the particles. For the first set (models A-1 and B-1), as only the scattering effect by the nanoparticles is considered for the change of the overall scattering properties of the tissue , the tissue absorption therefore does not change and remains at the base level . The overall scattering and absorption coefficient of set 1 can be related throughIn the second set (models A-2 and B-2), the combination of both scattering and absorption by the nanoparticles are taken into consideration. The respective change in absorption coefficient can then also be calculated using a similar relation. Both the overall scattering and absorption coefficients of tissue are consequently altered by the optical response of the nanoshells. Thus the overall change in optical properties of the precancerous layer is, From this set of Monte Carlo studies, we evaluate the effect of absorption from nanoshells on tissue reflectance with absorption and/or scattering dominated gold nanoshells, and compare this to the results from set 1.3.Results and Discussion3.1.Gold NanoshellsFigures 2(a), 2(b), 2(c), 2(d) shows various optical properties of gold nanoshells compared to core radius and shell thicknesses up to an overall radius of at an excitation wavelength of . The figures show the volume normalized scattering cross section [Fig. 2(a)], volume normalized absorption cross section [Fig. 2(b)], and the scattering-to-absorption efficiency ratio [Fig. 2(c)]. Figure 2(d) shows the extinction efficiency of the nanoshells. Using these space-maps of optical properties, nanoshells of different sizes and properties (also see Table 1) were chosen and their effect on tissue diffuse reflectance investigated. The table shows that the particles with the highest scattering and absorption efficiencies do not necessarily show the highest optical cross sections per unit volume of the particle, and this becomes important and are described later. To further describe the optical versatility of gold nanoshells, Fig. 4 shows representative optical extinction profiles of some of the nanoshells ( , , and ) used in these studies showing very different responses across wavelengths. Figure 5 shows volume-normalized absorption, scattering and extinction of the nanoshell. Fig. 4Representative extinction efficiencies of three different nanoshells, , and , used in the Monte Carlo experiments. The different extinction profiles are plotted against a broad wavelength range, showing optical activity well into the NIR. Nanoshells of different sizes produce unique and different optical responses. 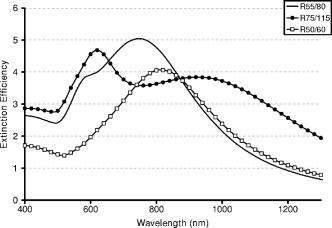 3.2.Monte Carlo StudiesThe Monte Carlo studies show that the addition of gold nanoshells to tissue causes a significant change in tissue reflectance. It is also possible to detect the change in reflectance due to a buried layer of tissue containing nanoshells. Between Monte Carlo modeling sets 1 and 2, the absorption properties of nanoshells are an important factor for predicting the tissue reflectance and cannot be neglected, even if the optical properties of the particle is dominated by scattering. The gold nanoshells were observed to scatter or absorb light far more efficiently than the gold nanoparticles. A large change in reflectance was observed with only a very small amount of nanoshells, while the gold nanoparticle caused little change in reflectance. In set 1 of the Monte Carlo studies, the diffuse reflectance generally increased as the amount of nanoshells added increased. Figures 6(a) and 6(b) shows the diffuse reflectance results from the bulk (model A-1) and multilayered (model B-1) tissue models after gold nanoshells of a variety of scattering properties and the gold nanoparticles were added to the tissue with increasing concentration. The reflectance increases after the addition of gold nanoshells, while the results from the gold colloid particle remain relatively unchanged. The photon reflectance, before adding any nanoparticles, is approximately 0.6 from model A-1 and 0.3 from model B-1. With the same concentration of particles added, the particle with the greater volume-normalized scattering cross sections shows higher reflectance. For example, the nanoshell exhibits the highest reflectance, while the shows the lowest of all the nanoshells. This corresponds to the volume-normalized scattering cross-section values as shown in Table 1. The nanoshell exhibits the highest volume-normalized scattering cross section (Abs ) and the nanoshell had the lowest value among the gold nanoshells used, with Abs . The radius gold colloid particle (R10-Au) with the lowest volume-normalized scattering cross section correspondingly shows very little change in both models A-1 and B-1. Fig. 6Diffuse reflectance from the (a) bulk (model A-1) and (b) multilayered (model B-1) models with , using different types of nanoparticles. The higher the volume-normalized scattering cross section, the more the diffuse reflectance increases with the same volume fraction of nanoshells added. The results show the studies that neglected absorption from the nanoparticles, assuming the particles as simple scatterers. 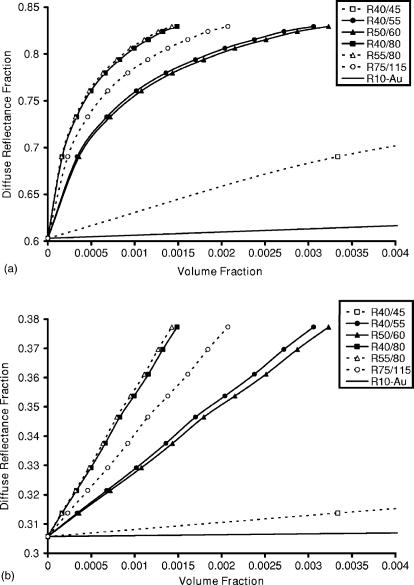 In set 2, the results, when absorption from gold nanoshells and gold colloid was considered together with the scattering, are shown in Figs. 7(a) and 7(b) . The results also show an immediate decrease in reflectance after the nanoparticles were included. When the concentration of the particles was increased, the diffuse reflectance decreased, and this occurred for all the particle configurations used in the simulations. Furthermore, the diffuse reflectance from model A-2 decreases more rapidly compared to model B-2 and eventually shows higher reflectance for the same amount of nanoshells added, as shown in the inset of Fig. 7(a). The graph shows that at a volume fraction of 0.00005, the reflectance with the gold nanoshell is approximately 0.15 in model A-2, and about 0.28 from model B-2. Compared to when no particles were added to the tissue, the reflectances are approximately 0.3 (model B-2) and 0.6 (model A-2). Further increases in concentration diminish the reflectance close to zero. The uniform change in the optical properties of the bulk model (model A-2), leads to a higher probability that photons will be absorbed in the topmost regions, whereas in model B-2, as the precancerous layer is buried away from the surface, there are greater chances of scattering when the photons first travel through the surface epithelium. Fig. 7Diffuse reflectance from the (a) bulk (model A-2) and (b) multilayered (model B-2) tissue when both scattering and absorption from the nanoparticles were considered. As the volume-normalized absorption cross section of the particle increases, the reflectance decreases. Model B-2 shows a more gradual decrease in reflectance compared to model A-2, and eventually shows higher reflectance as more particles were added, even though model A-2 showed higher reflectance before any nanoparticles were added. To describe this, the insert shown in (a) shows comparison of the diffuse reflectance fraction from the bulk (solid line) and multilayered (dotted line) models with increasing volume fractions of the nanoshell. 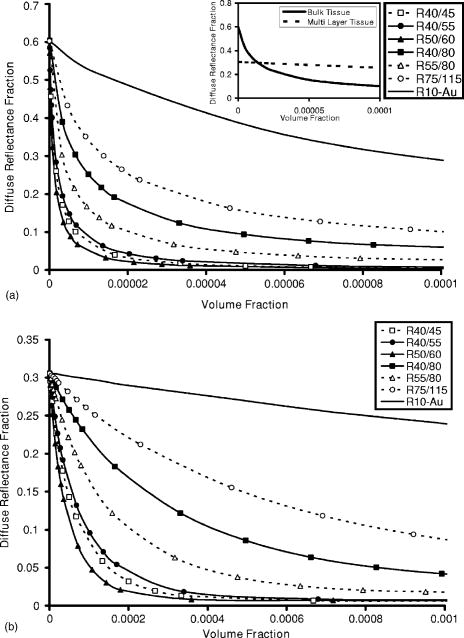 Even as it is useful to use the scattering-to-absorption ratio (Abs/Sca ratio) of the optical properties to describe how much more scattering the particle is compared to absorption, the volume-normalized absorption cross section of the particles is a more adequate description of how the optical properties of the particles influences tissue reflectance in our studies. The comparison of the scattering and absorbing properties of each particle is shown in Table 1 under Abs/Sca Ratio. For example, the nanoshell shows a scattering cross section of almost 9 times over the absorption cross section, while the scattering of the nanoshell is about 5.9% that of its absorption. Comparing this to the reflectance, the and nanoshells, however, do not show the highest and lowest reflectances, respectively. Thus, we can infer that absorption from the particles has a far greater effect on reflectance and cannot be neglected, even if the particle possess scattering cross section several times larger than its absorption. When we consider only scattering from the nanoparticles in models A-1 and B-1, it is obvious that higher scattering cross section per unit volume of the particle shows higher reflectance with the limitation of independent scattering. However, with different configurations of scattering and absorption from the gold nanoshells, the reflectance is more dependent on the volume-normalized absorption cross section than the combination of scattering and absorption. Figure 8 shows the relationship between diffuse reflectance and volume-normalized absorption cross sections of gold nanoshells. The diffuse reflectance decreases correspondingly (volume fraction, and 0.0005) as the absorption of gold nanoshells increase. This reemphasizes the importance of absorption from the particles and suggests that gold nanoshells can more efficiently lower the photon reflectance compared to gold colloid. This can be potentially useful for enhancing reflectance signatures through absorption for spectroscopic detection modalities. Furthermore, only a very small number of nanoshells are required to produce an observable change in the remitted signal. The volume-normalized absorption cross section of the nanoshell shown in Fig. 5 range from at at . As spectroscopic methods typically use a range of wavelengths, comparing these values to the R10-Au gold nanoparticle, these values are still considerably larger. Thus, when comparing normal tissue and nanoshell targeted tissue, the spectra of the diseased tissue would likely show a much reduced spectral intensity with the presence of nanoshells, which would provide an indication of the presence of targeted cells. Fig. 8The diffuse reflectance as a function of the volume normalized absorption cross section of different gold nanoshells used in the multilayered Monte Carlo studies (refer to Table 1). As the volume normalized absorption increases, the reflectance correspondingly decreases. Volume fractions of 0.0002 and 0.0005 are shown, within the constraints for independent scattering. 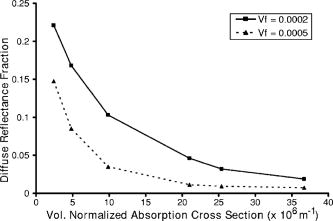 Throughout the Monte Carlo simulations, the anisotropy of the tissue was assumed to remain constant with the addition of the particles. From Mie solutions of the gold nanoshells, the values for the nanoshells can be calculated, and the overall effect on the anisotropy of the tissue is related by, where is the volume fraction of the nanoshells as described before, and is the volume fraction of the tissue not occupied by the nanoshells. With such a low-volume fraction of nanoshells, the overall change in the anisotropy of the tissue is very small and can be assumed as negligible. For instance, the nanoshell has a value of approximately at an excitation wavelength of in physiological media . At a volume fraction of , the change in the value of the tissue is less than 0.0005, not enough to significantly alter the reflectance.4.ConclusionsGold nanoshells possess unique and tunable optical signatures, in particular, strong optical responses in the NIR, desirable for biological applications. Gold nanoshells are easily fabricated for size and desired optical response under current chemistries. The absorption of gold nanoshells is critical in predicting the macroscopic behavior of light transport through turbid media embedded with nanoshells. The large scattering cross section of gold nanoshells in the NIR has already been exploited and demonstrated61 to enhance contrast for imaging in OCT. In addition, the strong absorption capabilities has been used in new imaging technologies such as photoacoustic tomography on the rat brain in vivo,62 further underlining the cross-platform potential of gold nanoshells for integrated imaging, diagnosis, and therapy of cancers. By exploring gold nanoshells with different physical parameters, we can continue to investigate the effects of changing nanoshell properties, possibly optimizing nanoshells with parameters best suited for different imaging and spectroscopic modalities. The studies reported in this paper strongly suggest that the strong optical responses of gold nanoshells can be utilized to alter signatures for scattering-based optical diagnostic methods. AcknowledgmentsWe would like to acknowledge Joe Jackson and Lee Hirsch for their invaluable advice and help on the Mie computations and gold nanoshell fabrication. Funding for this project was provided by the National Science Foundation (BES 022-1544), the National Science Foundation Center for Biological and Environmental Nanotechnology (EEC-0118007), and the Department of Defense Congressionally Directed Medical Research Program (DAMD17-03-1-0384). ReferencesH. Zeng,
M. Petek,
M. T. Zorman,
A. McWilliams,
B. Palcic, and
S. Lam,
“Integrated endoscopy system for simultaneous imaging and spectroscopy for early lung cancer detection,”
Opt. Lett., 29
(6), 587
–589
(2004). https://doi.org/10.1364/OL.29.000587 0146-9592 Google Scholar
A. Amelink,
H. J. C. M. Sterenborg,
M. P. L. Bard, and
S. A. Burgers,
“In vivo measurement of the local optical properties of tissue by use of differential path-length spectroscopy,”
Opt. Lett., 29
(10), 1087
–1089
(2004). https://doi.org/10.1364/OL.29.001087 0146-9592 Google Scholar
K. Sokolov,
L. T. Nieman,
A. Myakov, and
A. Gillenwater,
“Polarized reflectance spectroscopy for pre-cancer detection,”
Technol. Cancer Res. Treat., 3
(1), 1
–14
(2004). 1533-0346 Google Scholar
C. Liang,
K. B. Sung,
R. R. Richards-Kortum, and
M. R. Descour,
“Design of a high-numerical-aperture miniature microscope objective for an endoscopic fiber confocal reflectance microscope,”
Appl. Opt., 41
(22), 4603
–4610
(2002). 0003-6935 Google Scholar
K. B. Sung,
C. Liang,
M. Descour,
T. Collier,
M. Follen, and
R. Richards-Kortum,
“Fiber-optic confocal reflectance microscope with miniature objective for in vivo imaging of human tissue,”
IEEE Trans. Biomed. Eng., 49
(10), 1168
–1172
(2002). 0018-9294 Google Scholar
T. Collier,
A. Lacy,
R. Richards-Kortum,
A. Malpica, and
M. Follen,
“Near real-time confocal microscopy of amelanotic tissue,”
Acad. Radiol., 9
(5), 504
–512
(2002). 1076-6332 Google Scholar
T. Q. Xie,
M. L. Zeidel, and
Y. T. Pan,
“Detection of tumorigenesis in urinary bladder with optical coherence tomography: optical characterization of morphological changes,”
Opt. Express, 10 1431
–1443
(2002). 1094-4087 Google Scholar
J. G. Fujimoto,
“Optical coherence tomography for ultrahigh resolution in vivo imaging,”
Nat. Biotechnol., 21
(11), 1361
–1367
(2003). https://doi.org/10.1038/nbt892 1087-0156 Google Scholar
E. S. Matheny,
N. M. Hanna,
W. G. Jung,
Z. Chen,
P. Wilder-Simth,
R. Mina-Araghi, and
M. Brenner,
“Optical Coherence Tomography of malignancy in hamster cheek pouces,”
J. Biomed. Opt., 9
(5), 978
–981
(2004). https://doi.org/10.1117/1.1783897 1083-3668 Google Scholar
R. A. Drezek,
T. Collier,
C. K. Brookner,
A. Malpica,
R. Lotan,
R. R. Richards-Kortum, and
M. Follen,
“Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid,”
Am. J. Obstet. Gynecol., 182 1135
–1139
(2000). https://doi.org/10.1067/mob.2000.104844 0002-9378 Google Scholar
W. Drexler,
U. Morgner,
F. X. Kartner,
C. Pitris,
S. A. Boppart,
X. D. Li,
E. P. Ippen, and
J. G. Fujimoto,
“In vivo ultrahigh resolution optical coherence tomography,”
Opt. Lett., 24 1221
–1223
(1999). 0146-9592 Google Scholar
A. L. Clark,
A. Gillenwater,
R. Alizadeh-Naderi,
A. K. El-Naggar, and
R. Richards-Kortum,
“Detection and diagnosis of oral neoplasia with an optical coherence microscope,”
J. Biomed. Opt., 9
(6), 1271
–1280
(2004). 1083-3668 Google Scholar
R. Drezek,
T. Collier,
C. MacAulay,
M. Follen, and
R. Richards-Kortum,
“Light scattering from cervical cells throughout neoplastic progression: influence of nuclear size, DNA content, and chromatin texture,”
J. Biomed. Opt., 8 7
–16
(2003). https://doi.org/10.1117/1.1528950 1083-3668 Google Scholar
T. Collier,
P. Shen,
B. de Pradier,
K. B. Sung,
R. Richards-Kortum,
A. Malpica, and
M. Follen,
“Near real time confocal microscopy of amelanotic tissue: dynamics of aceto-whitening enable nuclear segmentation,”
Opt. Express, 6
(2), 40
–48
(2000). 1094-4087 Google Scholar
M. A. Onofre,
M. R. Sposto, and
C. M. Navarro,
“Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 91
(5), 535
–540
(2001). 1079-2104 Google Scholar
I. C. Martin,
C. J. Kerawala, and
M. Reed,
“The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasiam,”
Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 85
(4), 444
–446
(1998). 1079-2104 Google Scholar
A. J. Tincani,
N. Brandalise,
A. Altemani,
R. C. Scanavini,
J. B. M. Valerio,
H. T. Lage,
G. Molina, and
A. S. Martins,
“Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% Lugol dye solution in patients with head and neck cancer,”
Head Neck, 22
(2), 170
–174
(2000). 1043-3074 Google Scholar
Y. Nakanishi,
A. Ochiai,
K. Yoshimura,
H. Kato,
T. Shimoda,
H. Yamaguchi,
Y. Tachimori,
H. Watanabe, and
S. Hirohashi,
“The clinicopathologic significance of small areas unstained by Lugol’s iodine in the mucosa surrounding resected esophageal carcinoma,”
Cancer, 82
(8), 1454
–1459
(1998). 0008-543X Google Scholar
A. F. Zuluaga,
R. Drezek,
T. Collier,
R. Lotan,
M. Follen, and
R. Richards-Kortum,
“Contrast agents for confocal images of normal and cancer cells in suspension,”
J. Biomed. Opt., 7
(3), 398
–403
(2002). https://doi.org/10.1117/1.1481047 1083-3668 Google Scholar
E. M. Sevick-Muraca,
J. P. Houston, and
M. Gurfinkel,
“Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents,”
Curr. Opin. Chem. Biol., 6 642
–650
(2002). https://doi.org/10.1016/S1367-5931(02)00356-3 1367-5931 Google Scholar
A. Godavarty,
A. B. Thompson,
R. Roy,
M. Gurfinkel,
M. J. Eppstein,
C. Zhang, and
E. M. Sevick-Muraca,
“Diagnostic imaging of breast cancer using fluorescence-enhanced optical tomography,”
J. Biomed. Opt., 9
(3), 488
–496
(2004). https://doi.org/10.1117/1.1691027 1083-3668 Google Scholar
V. Ntziachristos,
A. G. Todh,
M. Schnall, and
B. Chance,
“Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement,”
Proc. Natl. Acad. Sci. U.S.A., 97
(6), 2767
–2772
(2000). https://doi.org/10.1073/pnas.040570597 0027-8424 Google Scholar
T. M. Lee,
F. J. Toublan,
S. Sitafalwalla,
A. L. Oldenburg,
K. S. Suslick, and
S. A. Boppart,
“Engineered microsphere contrast agents for optical coherence tomography,”
Opt. Lett., 28
(17), 1546
–1548
(2003). 0146-9592 Google Scholar
M. Rajadhyaksha,
S. Gonzalez, and
J. M. Zavislan,
“Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation,”
J. Biomed. Opt., 9
(2), 323
–331
(2004). https://doi.org/10.1117/1.1646175 1083-3668 Google Scholar
A. K. Gregorakis,
E. H. Holmes, and
G. P. Murphy,
“Prostate-specific membrane antigen: current and future utility,”
Semin Urol. Oncol., 16
(1), 2
–12
(1998). 1081-0943 Google Scholar
M. Kolonin,
R. Pasqualini, and
W. Arap,
“Molecular addresses in blood vessels as targets for therapy,”
Curr. Opin. Chem. Biol., 5
(3), 308
–313
(2001). 1367-5931 Google Scholar
U. Mahmood,
C. H. Tung,
A. Bogdanov Jr., and
R. Weissleder,
“Near-infrared optical imaging of protease activity for tumor detection,”
Radiology, 213
(3), 866
–870
(1999). 0033-8419 Google Scholar
P. M. Kasili,
J. M. Song, and
T. Vo-Dinh,
“Optical sensor for the detection of caspase-9 activity in a single cell,”
J. Am. Chem. Soc., 126
(9), 2799
–2806
(2004). 0002-7863 Google Scholar
J. M. Song,
P. M. Kasili, and
T. Vo-Dinh,
“Detection of cytochrome in a single cell using and optical nanobiosensor,”
Anal. Chem., 76
(9), 2591
–2594
(2004). 0003-2700 Google Scholar
K. Aslan,
J. R. Lackowicz, and
C. D. Geddes,
“Nanogold-plasmon-resonance-based glucose sensing,”
Anal. Biochem., 330
(1), 145
–155
(2004). 0003-2697 Google Scholar
L. R. Hirsch,
J. B. Jackson,
A. Lee,
N. J. Halas, and
J. L. West,
“A whole blood immunoassay using gold nanoshells,”
Anal. Chem., 75 2377
–2381
(2003). https://doi.org/10.1021/ac0262210 0003-2700 Google Scholar
J. R. Lakowicz,
J. Malicka,
J. Huang,
Z. Gryczynski, and
I. Gryczynski,
“Ultrabright fluorescein-labeled antibodies near silver metallic surfaces,”
Biopolymers, 74 467
–475
(2004). https://doi.org/10.1002/bip.20098 0006-3525 Google Scholar
I. Brigger,
C. Dubernet, and
P. Couvreur,
“Nanoparticles in cancer therapy and diagnosis,”
Adv. Drug Delivery Rev., 54 631
–651
(2002). 0169-409X Google Scholar
K. Sokolov,
M. Follen,
J. Aaron,
I. Pavlova,
A. Malpica,
R. Lotan, and
R. Richards-Kortum,
“Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles,”
Cancer Res., 63 1999
–2004
(2003). 0008-5472 Google Scholar
M. E. Akerman,
W. C. W. Chan,
P. Laakkonen,
S. N. Bhatia, and
E. Ruoslahti,
“Nanocrystal targeting in vivo,”
Proc. Natl. Acad. Sci. U.S.A., 99
(20), 12617
–12621
(2002). https://doi.org/10.1073/pnas.152463399 0027-8424 Google Scholar
W. C. W. Chan,
D. J. Maxwell,
X. Gao,
R. E. Bailey,
M. Y. Han, and
S. Nie,
“Luminescent quantum dots for multiplexed biological detection and imaging,”
Curr. Opin. Biotechnol., 13 40
–46
(2002). https://doi.org/10.1016/S0958-1669(02)00282-3 0958-1669 Google Scholar
S. J. Oldenburg,
R. D. Averitt,
S. L. Westcott, and
N. J. Halas,
“Nanoengineering of optical resonance,”
Chem. Phys. Lett., 288 243
–247
(1998). https://doi.org/10.1016/S0009-2614(98)00277-2 0009-2614 Google Scholar
R. D. Averitt,
S. L. Westcott, and
N. J. Halas,
“Linear optical properties of gold nanoshells,”
J. Opt. Soc. Am. B, 16
(10), 1824
–1832
(1999). 0740-3224 Google Scholar
U. Kreibig and
M. Vollmer, Optical Properties of Metal Clusters, Springer-Verlag, Berlin(1995). Google Scholar
S. J. Oldenburg,
J. B. Jackson,
S. L. Westcott, and
N. J. Halas,
“Infrared extinction properties of gold nanoshells,”
Appl. Phys. Lett., 75
(19), 2897
–2899
(1999). https://doi.org/10.1063/1.125183 0003-6951 Google Scholar
C. Loo,
A. Lin,
L. Hirsch,
M. Lee,
J. Barton,
N. Halas,
J. West, and
R. Drezek,
“Nanoshell-enabled photonics-based imaging and therapy of cancer,”
Technol. Cancer Res. Treat., 3
(1), 33
–40
(2004). 1533-0346 Google Scholar
W. T. Faulk and
G. Taylor,
“An immunocolloid method for the electron microscope,”
Immunochemistry, 8
(11), 1081
–1083
(1971). 0019-2791 Google Scholar
L. R. Hirsch,
R. J. Stafford,
J. A. Bankson,
S. R. Sershen,
B. Rivera,
R. E. Price,
J. D. Hazle,
N. J. Halas, and
J. L. West,
“Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance,”
Proc. Natl. Acad. Sci. U.S.A., 23 13549
–13554
(2003). 0027-8424 Google Scholar
D. P. O’Neal,
L. R. Hirsch,
N. J. Halas,
J. D. Payne,
J. L. West,
“Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles,”
Cancer Lett., 109 181
–176
(2004). 0304-3835 Google Scholar
C. Loo,
L. Hirsch,
M. Lee,
E. Chang,
J. West,
N. Halas, and
R. Drezek,
“Gold nanoshell bioconjugates for molecular imaging in living cells,”
Opt. Lett., 30
(9), 1012
–1014
(2004). 0146-9592 Google Scholar
C. Loo,
A. Lowery,
N. Halas,
J. West, and
R. Drezek,
“Immunotargeted nanoshells for integrated cancer imaging and therapy,”
Nano Lett., 5
(4), 709
–711
(2005). 1530-6984 Google Scholar
G. Zaccanti,
S. Del Bianco, and
F. Martelli,
“Measurements of optical properties of high-density media,”
Appl. Opt., 42
(19), 4023
–4030
(2003). 0003-6935 Google Scholar
H. C. van der Hulst, Light Scattering by Small Particles, Dover Press, New York(1981). Google Scholar
L. H. Wang,
S. L. Jacques, and
L. Q. Zheng,
“MCML—Monte Carlo modeling of photon transport in multi-layered tissues,”
Comput. Methods Programs Biomed., 47 131
–146
(1995). https://doi.org/10.1016/0169-2607(95)01640-F 0169-2607 Google Scholar
R. Hornung,
T. H. Pham,
K. A. Keefe,
M. W. Berns,
Y. Tadir, and
B. J. Tromberg,
“Quantitative near-infrared spectroscopy of cervical dysplasia in vivo,”
Hum. Reprod., 14
(11), 2908
–2916
(1999). https://doi.org/10.1093/humrep/14.11.2908 0268-1161 Google Scholar
A. Toricelli,
A. Pifferi,
P. Taroni,
E. Giambattistelli, and
R. Cubeddu,
“In vivo optical characterization of human tissues from by time-resolved reflectance spectroscopy,”
Phys. Med. Biol., 46 2227
–2237
(2001). https://doi.org/10.1088/0031-9155/46/8/313 0031-9155 Google Scholar
R. M. P. Doornbos,
R. Lang,
M. C. Aalders,
F. W. Cross, and
H. J. C. M. Sterenborg,
“The determination of in vivo human tissue optical properties and absolute chromophore concentrations using spatially resolved steady-state diffuse reflectance spectroscopy,”
Phys. Med. Biol., 44 967
–981
(1999). https://doi.org/10.1088/0031-9155/44/4/012 0031-9155 Google Scholar
L. Quan and
N. Ramanujam,
“Relationship between depth of a target in a turbid medium and fluorescence measured by a variable-aperture method,”
Opt. Lett., 27
(2), 104
–106
(2002). 0146-9592 Google Scholar
R. L. P. van Veen,
H. J. C. M. Sterenborg,
A. Pifferi,
A. Torricelli, and
R. Cubeddu,
“Determination of VIS-NIR absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy,”
(2004). Google Scholar
A. Akin,
“Functional imaging using near infrared light,”
209
–212
(2000). Google Scholar
B. Chance,
M. Cope,
E. Gratton,
N. Ramanujam, and
B. Tromberg,
“Phase measurement of light absorption and scatter in human tissue,”
Rev. Sci. Instrum., 69
(10), 3457
–3481
(1998). https://doi.org/10.1063/1.1149123 0034-6748 Google Scholar
N. T. K. Thanh and
Z. Rosenberg,
“Development of an aggregation-based immunoassay for anti-protein A using gold nanoparticles,”
Anal. Chem., 74 1624
–1628
(2002). https://doi.org/10.1021/ac011127p 0003-2700 Google Scholar
C. H. Teng,
K. C. Ho,
Y. S. Lin, and
Y. C. Chen,
“Gold nanoparticles as selective and concentrating probes for samples in MALDI MS analysis,”
Anal. Chem., 76 4337
–4342
(2004). 0003-2700 Google Scholar
A. Ishimaru and
Y. Kuga,
“Attenuation constant of a coherent field in a dense distribution of particles,”
J. Opt. Soc. Am., 72 1317
–1320
(1982). 0030-3941 Google Scholar
J. M. Schmitt and
G. Kumar,
“Optical scattering properties of soft tissue: a discrete particle model,”
Appl. Opt., 37
(3), 2788
–2797
(1998). 0003-6935 Google Scholar
J. K. Barton,
N. J. Halas,
J. L. West, and
R. A. Drezek,
“Nanoshells as an optical coherence tomography contrast agent,”
Proc. SPIE, 5316 99
–106
(2004). 0277-786X Google Scholar
Y. Wang,
X. Xie,
X. Wang,
G. Ku,
K. L. Gill,
D. P. O’Neal,
G. Stoica, and
L. V. Wang,
“Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain,”
Nano Lett., 4
(9), 1689
–1692
(2004). 1530-6984 Google Scholar
|