|
|
1.Brief Review of Tissue Optical ClearingThe turbidity of most biological tissue limits biomedical applications of light-based diagnostics and therapeutics. Multiple scattering events limit the effective depth over which information about the tissue can be ascertained and the ability to localize embedded features of interest.1 Optical imaging of deep structures is difficult due to the rapid degradation of image resolution and signal strength with increased depth. Furthermore, optical scattering restricts delivery of a collimated laser beam to subsurface targets such as blood vessels, decreasing the efficacy of light-based therapeutic applications. Prior studies demonstrate that a reduction in optical scattering can improve the efficacy of light-based techniques in medical applications. 2, 3, 4, 5, 6, 7, 8, 9, 10 Nonreactive chemical agents, in particular sugars (e.g., glucose) and sugar alcohols (e.g., glycerol) have been used to temporarily increase tissue transparency. 3, 4, 5, 11, 12, 13, 14, 15 These agents have been investigated primarily in collagenous tissues with initial studies measuring induced increases in light transmittance in ocular sclera.3 In fact, agents have been shown to be most effective when applied directly to the mesenchyme of tissue systems such as the dermis of skin. Application of glycerol subdermally in vitro or by injection in vivo reduced light scattering in skin, improved detection of fluorescence signal15 from subsurface targets,5 and enhanced visualization of subsurface blood vessels.4, 11 This optical clearing effect has been studied in other tissue systems such as muscle12, 16 and gastrointestinal tract13 and was used for agent sensing, where a reduction in tissue scattering occurred with increased blood glucose levels.2 Common properties of these chemical agents suggest that refractive index matching and dehydration are possible mechanisms for tissue optical clearing. Light scattering in dermis is predominantly from ubiquitous collagen fibers.17 Indeed, all studies cited used agents with indices18, 19 of refraction within the range reported for collagen17, 20 (from 1.35 to 1.55). Dehydration is also believed to play a role as all reported agents are hyperosmotic with respect to biological tissue. However, a mechanistic description of tissue optical clearing using refractive index and osmolarity remains to be substantiated experimentally. In fact, there is mounting evidence that these parameters are insufficient to describe tissue optical clearing 15, 16, 21, 22 and have been shown to have no correlation with chemical agent optical clearing potential.16, 22 Tissue optical clearing with glycerol shows a strong inverse dependence on the degree of covalent cross-linking present,21 and in vitro studies of collagen gel opacity correlated with sugar and sugar alcohol concentrations23 suggesting the importance of agent-collagen molecular interactions. Molecular interactions of sugars and sugar alcohols and their destabilizing and inhibitory effects on collagen structure and fibrillogenesis have been extensively studied within the biochemistry community. Such studies may provide insight into a molecular mechanism of tissue optical clearing and a means of rational selection and design of effective chemical agents. Evidence for a molecular mechanism of tissue optical clearing is presented as well as a review of recent work demonstrating destabilizing and inhibitory effects of sugars and sugar-alcohols on collagen structures and their self-assembly. 2.Clearing Agent Activity on Collagen Reduces Tissue ScatteringNonlinear optical microscopy (NLOM) is a laser scanning technique that can render thin images from within intact, living tissues.18, 24 Endogenous two-photon fluorescence excitation and second-harmonic generation (SHG) have been characterized for collagen; SHG can provide a unique spectral signature for collagen-specific imaging.18, 19, 25 SHG in fibrillar collagen has been used to enhance NLOM image contrast and constituent specific segmentation in skin,21 cornea,19 and articular cartilage25 without using exogenous stains or dyes. A necessary condition for SHG is that the constituent molecules lack an inversion center. Fibrillar collagens satisfy this condition; its secondary structure is an alpha helix. For type I collagen, two identical and one chains form a triple helix. Despite 30 yr of research, the fine structure and assembly mechanisms of collagen structures remain topics of intense study and inquiry. 26, 27, 28, 29 Nevertheless, high-order, macroscopic collagen structures exhibit long-range molecular order, providing a nonlinear medium with characteristic lengths of the order of near-IR wavelengths. Disruption of long-range molecular order extinguishes SHG, making it sensitive to perturbations of collagen structure and useful for optically monitoring denaturation.30, 31, 32 NLOM was used previously to image collagen with SHG in tissues during optical clearing and subsequent rehydration after applying glycerol and saline, respectively.21 Tissue systems investigated were fibroblast-seeded collagen tissue constructs and rodent skin, both untreated and fixed. For fibroblast-seeded collagen tissues, application of glycerol resulted in loss of SHG from collagen and concomitant increase in gel transparency. Loss of SHG was indicative of collagen destabilization by glycerol, an effect demonstrated with electron33 and polarization light microscopy21 in glycerinated rodent tail tendon. Application of glycerol to rodent skin did not extinguish SHG signal, but fibrous collagen morphology unraveled to a matted appearance concomitant with increased tissue transparency. Subsequent rehydration with saline in fibroblast-seeded collagen tissues restored SHG signal, fibrous collagen morphology, and gel turbidity. Similarly, rehydration of rodent skin with saline resulted in reformation of fibrous collagen morphology and return in tissue turbidity. Differences in collagen structure between fibroblast-seeded collagen tissue and rodent skin following glycerol application, as observed by NLOM, were hypothesized to be due to the presence of native covalent cross-links. Indeed, the introduction of an abundance of covalent bonds through formalin fixation defeated glycerol induced optical clearing and structural changes to collagen as measured by NLOM. 3.Destabilizing Effect of Glycerol on Collagen in Rodent Tail TendonThe destabilization of native collagen structures using nonreactive chemical agents suggests the primary bonding forces for these high order protein assemblies are noncovalent in nature. Type I collagen is the predominant structural component in most biological tissues and shows increased thermal stability against denaturation in solution with sugar alcohols.34, 35, 36 The earliest evidence of native collagen dissociation using glycerol was reported over 20 yr ago using transmission electron microscopy (TEM) and x-ray diffraction (XRD) techniques to measure collagen fiber ultrastructure in rodent tail tendon following one of six exposures: (1) water, (2) phosphate buffer, (3) glutaraldehyde, (4) glutaraldehyde followed by glycerol, (5) glycerol, or (6) glycerol followed by phosphate buffer.33 Consistent with observations21 using NLOM, only glycerol treatment induced swelling of interfibrillar space and dissociation of collagen fibrils into microfibrils (and loss of characteristic banding in some regions), as observed using TEM and molecular disorder as measured with XRD. Ultrastructurally, glutaraldehyde fixation (exposure 4) nullified, whereas rehydration with phosphate buffer (exposure 6) reversed the dissociative effects of glycerol.33 These ultrastructural and NLOM studies show that (1) bonding forces for high-order collagen structures are primarily noncovalent in nature; (2) glycerol interrupts these bonding forces resulting in disassembly; and (3) on rehydration, these bonding forces are restored resulting in reassembly of high-order collagen structures. 4.Molecular Interactions of Agents with Collagen In VitroStudies using synthetic collagen-like peptides show that stable formation of secondary, tertiary and, consequently, higher order structures depend on specific binding sites.37 Stability of secondary and tertiary structures was shown to be enhanced by inductive (electronegativity) effects provided by hydroxylated proline residues.38 For quaternary and higher order structures, attractive forces mediated by hydrophilic environments39 are preeminent, and sugars and sugar alcohols have been used effectively to elucidate collagen interactions and self-assembly mechanisms. 4.1.Hydrogen Bonding in FibrillogenesisThe dynamics of collagen fibril formation has been described as nucleation followed by growth during which higher order structures (fibrils) develop.23 Noncovalent forces driving collagen fibrillogenesis could include, for example, hydrogen bonding, van der Waals forces, and steric interactions. Sugars and sugar alcohols have been used to modulate and characterize these forces 23, 40, 41, 42, 43, 44 in conjunction with measurement techniques utilizing osmotic pressure to study macromolecular interactions.45 Typically, these techniques apply force through osmotic pressure via polymer solution and measure intermolecular distance by XRD. Force-distance measurements have been performed using concentrated collagen thin films immersed in solutions of polyethylene glycol 40, 41, 42, 43, 45 (PEG). PEG does not penetrate these thin films, thus exerting osmotic pressure on the collagen fibers in a manner directly related to its concentration. Osmotic removal of water decreases the molecular (interaxial) separation of triple helices; this osmotic force is balanced by repulsion. Decreasing PEG concentration was shown to correspond with decreasing osmotic pressure and increasing interaxial distances until attractive forces dominated. At this point, decreasing applied osmotic pressure did not result in further increases in interaxial distances due to intermolecular interaction forces. These attractive forces are responsible for molecular recognition and drive collagen fibrillogenesis. Collagen attractive forces may be characterized with the addition of sugars and sugar alcohols. These agents screen collagen attractive forces resulting in force-distance measurement curves that reflect intermolecular repulsion (i.e., interaxial distance continued to increase with decreasing osmotic pressure). Collagen attractive forces were characterized by taking the difference between curves, with and without agents, as a function of interaxial distance. 40, 41, 42, 43 Force-distance measurements of collagen led to two significant findings in determining the mechanism for fibrillogenesis: (1) the repulsive force increases exponentially with a decrease in interaxial spacing (1.5 to ), and (2) both interhelical spacing and net repulsive force decrease with an increase in temperature.42 These findings, along with the discovery that force-decay lengths and force magnitudes were insensitive to ionic strength at high osmotic stress, suggest that either “hydration forces” between polar surfaces or the “hydrophobic effect” of nonpolar moieties is the dominant force in fibrillogenesis. Although the temperature sensitivity of attraction is qualitatively consistent with both hydration forces and hydrophobicity, the magnitude of this sensitivity suggests a hydrophilic mechanism.43 A mechanism for fibrillogenesis was further defined when considering the effects of pH on collagen fiber formation. Collagen fibrillogenesis was strongly favored at physiologic pH . However, when pH was lowered to 6, there was substantial weakening of the attraction between collagen helices—consistent with titration of specific hydrophilic residues.43 pH effects suggest involvement of histidine residues which have the lowest value of the three basic amino acids making it the most sensitive to reduction of pH from 7.4 to 6. At neutral pH, histidine is neutral and forms hydrogen bonds as a proton acceptor. Reducing pH protonates histidine, whereby it becomes a hydrogen bond donor and disrupts the interactions between helices. The reaction of collagen to pH, in conjunction with force-distance measurements, support hydrophilic interactions as the main driving force for fibrillogenesis. 21, 40, 42, 43 4.2.Collagen Solubility (Inhibition of Fibrillogenesis)Addition of sugars and sugar alcohols was shown to enhance collagen solubility in buffered saline by masking hydration mediated attractive forces between triple helices and inhibiting fibrillogenesis. 23, 36, 40, 41, 42, 43, 44 These chemical agents slow and limit fibrillogenesis by disrupting hydrogen bond facilitated water bridges between collagen triple helices. The efficiency of fibrillogenesis in the presence of glucose, fructose, and sucrose (see Fig. 1 ) as well as ethylene glycol, glycerol, and sorbitol (see Fig. 2 ) has been studied previously and showed an inverse dependence on agent chain length.23, 40 Ethylene glycol was the shortest (two-carbon chain) agent tested and displayed little effect on collagen fibrillogenesis.40 Inhibitory effects of glycerol on collagen self-assembly are well documented.23, 40, 44 Sorbitol is twice as long as glycerol (six and three carbons, respectively) and was shown to have twice the collagen solubility.40 Glucose and fructose are six-carbon sugars and was shown to have greatest collagen solubility when compared to sugar alcohols40 (ethylene glycol, glycerol, and sorbitol). Sucrose, a disaccharide of glucose and fructose, showed similar inhibitory effects on collagen fibrillogenesis as with the monosaccharides.23 Fig. 2Chemical structure representations of sugar alcohols ethylene glycol, glycerol, and sorbitol and 1,2- and 1,3-propane diol. 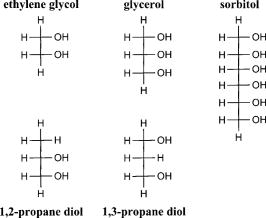 Insight into the difference in collagen solubility between ethylene glycol and glycerol can be gained by comparison studies of 1,2- and 1,3-propane diol, which both have similar dielectric properties, e.g., their indices of refraction are 1.43 and 1.44, respectively. If electrostatic, van der Waals, or hydrophobic interactions were the major attractive forces in fibrillogenesis, these two agents should have similar collagen solubilities. In fact, 1,3-propane diol had times greater collagen solubility (comparable with glycerol) than 1,2-propane diol which showed comparable collagen solubility with ethylene glycol.40 Collagen solubility of propane diols, ethylene glycol, glycerol, and sorbitol suggests a stereochemical effect.40, 43 For propane diols, adjacent or terminal placement of hydroxyl groups on the three-carbon backbone has dramatic effects on collagen solubility. This is reflected in sugar alcohols where ethylene glycol (glycerol) and 1,2- (1,3-)propane diol have similar distances between hydroxyl end groups. Placement of hydroxyl end groups on 1,3-propane diol and glycerol may correspond to the spacing of hydrophilic interaction sites of collagen tertiary structures. The fact that sorbitol has twice the collagen solubility as glycerol is supportive, but studies with sugar alcohols of other chain lengths would further refine this hypothesis. 5.Rational Selection and Design of Tissue Optical Clearing AgentsEvidence of a molecular mechanism for tissue optical clearing was presented, providing insight for rational selection and design of exogenous, nonreactive clearing agents. For glycerol, it has been established that the induced increase in tissue transparency results from the destabilization of high-order collagen structures.21, 33 This destabilization effect was shown to be reversible structurally33 and mechanically.46 Glycerol also has been shown to have a stabilization effect on collagen tertiary structure against thermal denaturation.34, 36 This apparent dichotomy may be explained by attractive forces involved in the collagen protein structure hierarchy. Synthetic collagen-like peptides have been used to demonstrate that self-assembly of collagen I secondary and tertiary structures is driven by specific hydrophobic and electrostatic binding sites37 and that stability of these structures is enhanced by inductive (electronegativity) effects conferred by hydroxyproline residues.38 For higher order structures, osmotic stress and x-ray scattering measurements using sugars and sugar alcohols demonstrated that hydrogen bonding is the primary bonding force between collagen triple helices. 40, 41, 42, 43 Screening of hydrogen bonding forces by these chemical agents was shown to destabilize/disassociate high-order collagen structures and results in increased tissue transparency.21, 23, 33 Comparison studies of collagen solubility in clearing agents suggest a testing protocol to determine dose and evaluate potential optical clearing efficacy in vitro prior to testing in vivo. Clearing agent interactions with collagen are nonreactive. In addition to tissue optical properties, however, clearing agent interactions with collagen, the primary structural protein in most tissues, suggest other properties may be affected as well, for example, tissue mechanical properties. Mechanical testing (stress-strain measurements) of collagenous tissues reveals a bimodal behavior.47 These measurements are typically acquired relative to a reference state, which is attained after preconditioning tissue to low stress. For low strain, collagenous tissues show compliance, accommodating stretch by collagen fiber reorientation and undulation straightening. Tissue stiffening follows when these degrees of freedom are exhausted and stretch is accommodated by matrix proteins, in particular, collagen. The compliant and stiff response of tissue can be associated with noncovalent and covalent bonding forces, respectively.47 The effects of glycerol on the mechanical properties of epicardium and their reversibility have been characterized using stress-strain measurements.46 Bovine epicardium was used as a model 2-D collagenous tissue. Following glycerol application, “cleared” epicardium exhibited bimodal mechanical responses with a reduced compliant region. This reduction in the compliant region was consistent with glycerol screening of noncovalent interactions. On rehydration with buffered saline, epicardial mechanical response was completely restored to pre-glycerol-treated levels.46 These results were encouraging in that the mechanical integrity of treated collagenous tissue was not permanently compromised by optical clearing but, in fact, was completely reversible. Agent-induced destabilization of collagen structures leading to reduced optical scattering in tissue was shown to be reversible, but hindered by covalent cross-linking.21, 33 This suggests the use of clearing agents adjuvant with clinical light-based diagnostics and therapeutics may be limited by factors such as patient age. In addition, current challenges to tissue optical clearing include epidermal penetration by clearing agents. Effective, topically applied clearing agents require properties that facilitate breach of the lipid barrier yet retain hydrophilic properties to destabilize collagen structures. Synergistic approaches have been investigated, including topical applications of miscible solutions of transepidermal carrier and clearing agents.9, 10, 14 Solutions of dimethyl sulfoxide and glycerol applied topically to gastric tissue was shown to enhance light transmission when compared to administering glycerol alone.14 The clinical utility of topical lipophilic polypropylene glycol and polyethylene-glycol-based polymer mixtures has been demonstrated in laser removal of tattoos, showing reduced skin injury and increased therapeutic efficacy when compared with using laser treatment alone.9 These results are promising and warrant further exploration of topical clearing regimens. Studies characterizing the effects of clearing agents on tissue properties and function continue; initial results suggest modulation of tissue physical properties are temporary and completely reversible.3, 4, 46 Observed agent effects on microvasculature and blood flow11, 48 suggest biological response may be elicited, which may impact tissue function. Comparative studies with accepted treatment protocols are required to further define efficacy enhancement for light-based therapeutics and to characterize potential side effects. AcknowledgmentsWe thank Drs. J. Stuart Nelson, Bernard Choi, Paul B. Wells, and Jay D. Humphrey for critical review of this manuscript and insightful discussions. This work was supported by a Faculty Early Career Development (CAREER) Award from the National Science Foundation and a Whitaker Foundation Special Opportunity Award. ReferencesD. A. Benaron,
W. -F. Cheong, and
D. K. Stevenson,
“Tissue optics,”
Science, 276 2002
–2003
(1997). https://doi.org/10.1126/science.276.5321.2002 0036-8075 Google Scholar
J. S. Maier,
S. A. Walker,
S. Fantini,
M. A. Franceschi, and
E. Gratton,
“Possible correlation between blood glucose concentration and the reduced scattering coefficient of tissues in the near infrared,”
Opt. Lett., 19
(24), 2062
–2064
(1994). 0146-9592 Google Scholar
V. V. Tuchin,
I. L. Maksimova,
D. A. Zimnyakov,
I. L. Kon,
A. H. Mavlutov, and
A. A. Mishin,
“Light propagation in tissues with controlled optical properties,”
J. Biomed. Opt., 2
(4), 401
–417
(1997). https://doi.org/10.1117/1.429841 1083-3668 Google Scholar
G. Vargas,
E. K. Chan,
J. K. Barton, H. G. Rylander III, A. J. Welch,
“Use of an agent to reduce scattering in skin,”
Lasers Surg. Med., 24 133
–141
(1999). https://doi.org/10.1002/(SICI)1096-9101(1999)24:2<133::AID-LSM9>3.0.CO;2-X 0196-8092 Google Scholar
G. Vargas,
K. F. Chan,
S. L. Thomsen, and
A. J. Welch,
“Use of osmotically active agents to alter optical properties of tissue: effects on the detected fluorescence signal measured through skin,”
Lasers Surg. Med., 29 213
–220
(2001). https://doi.org/10.1002/lsm.1110 0196-8092 Google Scholar
R. K. Wang,
X. Xu,
V. V. Tuchin, and
J. B. Elder,
“Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents,”
J. Opt. Soc. Am. B, 18
(7), 948
–953
(2001). 0740-3224 Google Scholar
R. K. Wang,
“Signal degradation by multiple scattering in optical coherence tomography of dense tissue: a Monte Carlo study towards optical clearing of biotissues,”
Phys. Med. Biol., 47
(13), 2281
–2299
(2002). https://doi.org/10.1088/0031-9155/47/13/307 0031-9155 Google Scholar
R. K. Wang,
X. Xu,
Y. He, and
J. B. Elder,
“Investigation of optical clearing of gastric tissue immersed with hyperosmotic agents,”
IEEE J. Sel. Top. Quantum Electron., 9
(2), 234
–242
(2003). 1077-260X Google Scholar
M. H. Khan,
S. Chess,
B. Choi,
K. M. Kelly, and
J. S. Nelson,
“Can topically applied optical clearing agents increase the epidermal damage threshold and enhance therapeutic efficacy?,”
Lasers Surg. Med., 35 93
–95
(2004). 0196-8092 Google Scholar
M. H. Khan,
B. Choi,
S. Chess,
K. M. Kelly,
J. McCullough, and
J. S. Nelson,
“Optical clearing of in vivo human skin: implications for light-based diagnostic imaging and therapeutics,”
Lasers Surg. Med., 34 83
–85
(2004). https://doi.org/10.1002/lsm.20014 0196-8092 Google Scholar
E. I. Galanzha,
V. V. Tuchin,
A. V. Solovieva,
T. V. Stepanova,
Q. Luo, and
H. Cheng,
“Skin backreflectance and microvascular system functioning at the action of osmotic agents,”
J. Phys. D, 36 1739
–1746
(2003). 0022-3727 Google Scholar
X. Xu and
R. K. Wang,
“The role of water desorption on optical clearing of biotissue: studied with near infrared reflectance spectroscopy,”
Med. Phys., 30
(6), 1246
–1253
(2003). https://doi.org/10.1118/1.1576228 0094-2405 Google Scholar
Y. He and
R. K. Wang,
“Dynamic optical clearing effect of tissue impregnated with hyperosmotic agents and studied with optical coherence tomography,”
J. Biomed. Opt., 9
(1), 200
–206
(2004). https://doi.org/10.1117/1.1629682 1083-3668 Google Scholar
X. Xu and
R. K. Wang,
“Synergistic effect of hyperosmotic agents of dimethyl sulfoxide and glycerol on optical clearing of gastric tissue studied with near infrared spectroscopy,”
Phys. Med. Biol., 49 457
–468
(2004). https://doi.org/10.1088/0031-9155/49/3/008 0031-9155 Google Scholar
R. Cicchi,
F. S. Pavone,
D. Massi, and
D. D. Sampson,
“Contrast and depth enhancement in two-photon microscopy of human skin ex vivo by use of optical clearing agents,”
Opt. Express, 13
(7), 2337
–2344
(2005). https://doi.org/10.1364/OPEX.13.002337 1094-4087 Google Scholar
S. Plotniknov,
V. Juneja,
A. B. Isaacson,
W. A. Mohler, and
P. J. Campagnola,
“Optical clearing for improved contrast in second harmonic generation imaging of skeletal muscle,”
Biophys. J., 90
(1), 328
–339
(2006). 0006-3495 Google Scholar
I. S. Saidi,
S. L. Jacques, and
F. K. Tittle,
“Mie and Rayleigh modeling of visible-light scattering in neonatal skin,”
Appl. Opt., 34
(31), 7410
–7418
(1995). 0003-6935 Google Scholar
P. J. Campagnola and
L. M. Loew,
“Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms,”
Nat. Biotechnol., 21
(11), 1356
–1360
(2003). https://doi.org/10.1038/nbt894 1087-0156 Google Scholar
A. T. Yeh,
N. Nassif,
A. Zoumi, and
B. J. Tromberg,
“Selective corneal imaging using combined second harmonic generation and two-photon excited fluorescence,”
Opt. Lett., 27
(23), 2082
–2084
(2002). 0146-9592 Google Scholar
M. Gisselberg,
J. I. Clark,
S. Vaezy, and
T. B. Osgood,
“A quantitative evaluation of Fourier components in transparent and opaque calf cornea,”
Am. J. Anat., 191 408
–418
(1991). 0002-9106 Google Scholar
A. T. Yeh,
B. Choi,
J. S. Nelson, and
B. J. Tromberg,
“Reversible dissociation of collagen in tissues,”
J. Invest. Dermatol., 121
(6), 1332
–1335
(2003). https://doi.org/10.1046/j.1523-1747.2003.12634.x 0022-202X Google Scholar
B. Choi,
L. Tsu,
E. Chen,
T. S. Ishak,
S. M. Iskandar,
S. Chess, and
J. S. Nelson,
“Determination of chemical agent optical clearing potential using in vitro human skin,”
Lasers Surg. Med., 36 72
–75
(2005). https://doi.org/10.1002/lsm.20116 0196-8092 Google Scholar
T. Hayashi and
Y. Nagai,
“Factors affecting the interactions of collagen molecules as observed by in vitro fibril formation,”
J. Biochem. (Tokyo), 72
(3), 749
–758
(1972). 0021-924X Google Scholar
W. R. Zipfel,
R. M. Williams, and
W. W. Webb,
“Nonlinear magic: multiphoton microscopy in the biosciences,”
Nat. Biotechnol., 21
(11), 1369
–1377
(2003). https://doi.org/10.1038/nbt899 1087-0156 Google Scholar
A. T. Yeh,
M. J. Hammer-Wilson,
D. C. Van Sickle,
H. P. Benton,
A. Zoumi,
B. J. Tromberg, and
G. M. Peavy,
“Nonlinear optical microscopy of articular cartilage,”
Osteoarthritis Cartilage, 13 345
–352
(2005). 1063-4584 Google Scholar
D. J. S. Hulmes,
“Building collagen molecules, fibrils, and suprafibrillar structures,”
J. Struct. Biol., 137 2
–10
(2002). https://doi.org/10.1006/jsbi.2002.4450 1047-8477 Google Scholar
J. Engel and
D. J. Prockop,
“The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper,”
Annu. Rev. Biophys. Biophys. Chem., 20 137
–152
(1991). 0883-9182 Google Scholar
I. V. Yannas,
“Collagen and gelatin in the solid state,”
J. Macromol. Sci. Rev. Macromol. Chem., C7
(1), 49
–104
(1972). 0022-2356 Google Scholar
M. E. Nimni,
“Collagen: structure, function, and metabolism in normal and fibrotic tissues,”
Semin Arthritis Rheum., 13
(1), 1
–85
(1983). 0049-0172 Google Scholar
T. Theodossiou,
G. S. Rapti,
V. Hovhannisyan,
E. Georgiou,
K. Politopoulos, and
D. Yova,
“Thermally induced irreversible conformational changes in collagen probed by optical second harmonic generation and laser-induced fluorescence,”
Lasers Med. Sci., 17 34
–41
(2002) Google Scholar
A. T. Yeh,
B. Kao,
W. G. Jung,
Z. Chen,
J. S. Nelson, and
B. J. Tromberg,
“Imaging wound healing using optical coherence tomography and multiphoton microscopy in an in vitro skin-equivalent tissue model,”
J. Biomed. Opt., 9
(2), 248
–253
(2004). https://doi.org/10.1117/1.1648646 1083-3668 Google Scholar
S. -J. Lin,
C. -Y. Hsiao,
Y. Sun,
L. Wen,
W. -C. Lin,
G. -J. Jan,
S. -H. Jee, and
C. -Y. Dong,
“Monitoring the thermally induced structural transitions of collagen by use of second-harmonic generation microscopy,”
Opt. Lett., 30
(6), 622
–624
(2005). https://doi.org/10.1364/OL.30.000622 0146-9592 Google Scholar
L. Leonardi,
A. Ruggeri,
N. Roveri,
A. Bigi, and
E. Reale,
“Light microscopy, electron microscopy, and x-ray diffraction analysis of glycerinated collagen fibers,”
J. Ultrastruct. Res., 85 228
–237
(1983). 0022-5320 Google Scholar
A. E. Russell,
“Effect of alcohols and neutral salt on the thermal stability of soluble and precipitated acid-soluble collagen,”
Biochem. J., 131 335
–342
(1973). 0264-6021 Google Scholar
C. A. Miles and
T. V. Burjanadze,
“Thermal stability of collagen fibers in ethylene glycol,”
Biophys. J., 80 1480
–1486
(2001). 0006-3495 Google Scholar
G. C. Na,
“Interaction of calf skin collagen with glycerol: linked function analysis,”
Biochemistry, 25 967
–973
(1986). 0006-2960 Google Scholar
D. J. Prockop and
A. Fertala,
“Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides,”
J. Biol. Chem., 273
(25), 15598
–15604
(1998). 0021-9258 Google Scholar
S. K. Holmgren,
K. M. Taylor,
L. E. Bretscher, and
R. T. Raines,
“Code for collagen’s stability deciphered,”
Nature (London), 392 666
–667
(1998). 0028-0836 Google Scholar
J. Israelachvili and
H. Wennerstrom,
“Role of hydration and water structure in biological and colloidal interactions,”
Nature (London), 379 219
–225
(1996). https://doi.org/10.1038/379219a0 0028-0836 Google Scholar
N. Kuznetsova,
S. L. Chi, and
S. Leikin,
“Sugars and polyols inhibit fibrillogenesis of type I collagen by disrupting hydrogen-bonded water bridges between the helices,”
Biochemistry, 37 11888
–11895
(1998). 0006-2960 Google Scholar
N. Kuznetsova,
D. C. Rau,
V. A. Parsegian, and
S. Leikin,
“Solvent hydrogen-bond network in protein self-assembly: solvation of collagen triple helices in nonaqueous solvents,”
Biophys. J., 72 353
–362
(1997). 0006-3495 Google Scholar
S. Leikin,
D. C. Rau, and
V. A. Parsegian,
“Direct measurement of forces between self-assembled proteins: temperature-dependent exponential forces between collagen triple helices,”
Proc. Natl. Acad. Sci. U.S.A., 91 276
–280
(1994). 0027-8424 Google Scholar
S. Leikin,
D. C. Rau, and
V. A. Parsegian,
“Temperature-favoured assembly of collagen is driven by hydrophilic not hydrophobic interactions,”
Nature Struct. Molec. Biol., 2
(3), 205
–210
(1995) Google Scholar
G. C. Na,
L. J. Butz,
D. G. Bailey, and
R. J. Carroll,
“In vitro collagen fibril assembly in glycerol solution: evidence for a helical cooperative mechanism involving microfibrils,”
Biochemistry, 25 958
–966
(1986). 0006-2960 Google Scholar
V. A. Parsegian,
R. P. Rand,
N. L. Fuller, and
D. C. Rau,
“Osmotic stress for the direct measurement of intermolecular forces,”
Methods Enzymol., 127 400
–416
(1986). 0076-6879 Google Scholar
P. B. Wells,
A. T. Yeh, and
J. D. Humphrey,
“Influence of glycerol on the mechanical behavior and thermal damage susceptibility of collagenous tissues,”
IEEE Trans. Biomed. Eng., Google Scholar
J. D. Humphrey, Cardiovascular Solid Mechanics: Cells, Tissues, and Organs,
(2002) Google Scholar
G. Vargas,
A. Readinger,
S. S. Dozier, and
A. J. Welch,
“Morphological changes in blood vessels produced by hyperosmotic agents and measured by optical coherence tomography,”
Photochem. Photobiol., 77
(5), 541
–549
(2003). https://doi.org/10.1562/0031-8655(2003)077<0541:MCIBVP>2.0.CO;2 0031-8655 Google Scholar
|