|
|
1.IntroductionObstruction of coronary flow (myocardial ischemia) produces several changes in the affected area of the heart, such as a decrease in oxygenation, dissipation of mitochondrial membrane potential , and a decrease in oxidative phosphorylation. Optical reflectance/absorbance and fluorescence methods have been used, with various degrees of success, to assess these changes. For example, in isolated mitochondria, changes in can be accurately evaluated using lipophilic fluorescent cationic dyes, such as safranine, cyanine, and rhodamine derivatives that accumulate in the mitochondrial matrix in a -dependent manner.1, 2 The fluorescence intensity of rhodamine derivatives is quenched when the dyes are accumulated by the mitochondria.2 Fluorescent cationic dyes have been also used, although less effectively, in isolated cells where interpretation of the fluorescence and absorbance changes was more complicated due to several factors including the dyes toxic effects and their retention in the cytosol.1, 3 Several reports indicate that measuring of in situ is also attainable, and fluorescence measurements of visible-range dyes, safranin and N,N”-dimethylaminostyrylmethyl-pyridiniumiodide (DASPMI) were successfully used for monitoring on the surface of perfused rat hearts.4, 5, 6 However, analysis of spectral changes in this range was not straightforward because of the interference with endogenous hemoproteins.4 Use of near-infrared optical probes that have emission and fluorescence maxima away from the absorption of natural chromophores should allow easier interpretation of the spectral changes in intact hearts. An additional advantage of using NIR optical probes is that not only epicardial, but also deeper myocardial tissue can be probed (in the centimeter range). However, light scattering and absorption (inner filter effect) can be significant, depending on the geometry of the light path. Rhodamine 800 (rhod800), also known as MitoFluor Far Red 680, is a cationic fluorescent dye that accumulates in the mitochondria of isolated hepatocytes due to the existing .7 It is a near-infrared probe: in ethanol, rhod800 absorption maximum is at and fluorescence maximum is at (Molecular Probes technical information). Advantages of rhod800 over other rhodamine derivatives are: (1) long-wavelength excitation and fluorescence are able to penetrate tissues at the centimeter range, and (2) there is no emission and absorption interference with natural chromophores, since the most abundant natural chromophores [NAD(P)H, flavoproteins, cytochromes, oxy- and deoxy-myoglobins] have the strongest absorption and fluorescence bands between 340 and . Rhod800 fluorescent properties have been studied in isolated liver mitochondria, hepatocytes,7 and whole blood,8 and it has been used for flow cytometry.9 Sakanoue demonstrated that rhod800 absorbance difference (at 730 minus or 730 minus ) and fluorescence intensity at were proportional to the mitochondrial in isolated mitochondria.7 These characteristics make rhod800 a good candidate for studies of mitochondrial energetics in intact hearts. Previously, we successfully used this dye to estimate in isolated rat liver mitochondria by measuring distribution of rhod800 in the mitochondrial suspensions in the absence and presence of modulators.10 The present investigation had several objectives: (1) to investigate binding and fluorescing properties of rhod800 in isolated rat liver mitochondria, hepatocytes, cardiomyocytes, and Langendorff-perfused hearts, under normal conditions and after addition of mitochondrial uncouplers, (2) to measure fluorescence of rhod800 from deeper layers of the myocardium and monitor changes in the fluorescence upon redistribution of the dye upon loading and washout, (3) to investigate the effect of different light pass geometries on absorbance and fluorescence of rhod800 in Langendorff-perfused rat hearts, and (4) to evaluate the significance of inner filter effect in different settings. 2.Materials and MethodsThe investigation conforms with the “Guide to the Care and Use of Experimental Animals” published by the Canadian Council on Animal Care ( edition, Ottawa, Ontario, 1993). 2.1.MaterialsBovine serum albumin (BSA), dimethylsulfoxide (DMSO), 2,4-dinitrophenol (DNP), ethylene glycol bis-( -aminoethyl ether) N,N,N’N’-tetraacetic acid (EGTA), DL-glutamic acid, K-gluconate, carbonyl cyanide 4-trifluoromethoxyphenyl hydrazone (FCCP), N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), Mg-ATP, sodium pyruvate, and succinic acid were purchased from Sigma (St. Louis, Missouri, U.S.A.). Rhodamine 800 (Molecular Probes, Eugene, Oregon, U.S.A.) was dissolved in DMSO , aliquoted and stored at until further use when diluted to the required concentration in aqueous buffer. Collagenase type 3 was purchased from Worthington Biochemical Corporation, Lakewood, New Jersey, U.S.A. 2.2.BuffersMitochondrial incubation buffer A (in mM): 20 HEPES-Na (pH 7.2), , 100 K-gluconate, and 1 EGTA-Na. Hepatocyte incubation buffer B (in mM): 30 HEPES-Na (pH 7.4), , , , , , , and 5.5 glucose. Cardiomyocyte incubation buffer C (in mM): 25 HEPES-Na (pH 7.4), , , , , 15 glucose, 2 carnitine, 5 taurine, 2 creatine, 15 butadione monooxime, and BSA . Cardiomyocyte high-potassium buffer D (in mM): 20 HEPES-Na, , 1 EGTA, , 11 glucose. Krebs-Henseleit buffer (KHB, for isolated heart perfusion) (in mM): , , , , , 0.5 EDTA, and 11 glucose. 2.3.Isolation of Rat Liver MitochondriaMale Sprague-Dawley rats were anesthetized with pentobarbital solution intraperitoneally. The liver was taken immediately following the heart excision from the same animal. Liver mitochondria were isolated as described elsewhere.11 Mitochondrial preparations were suspended in the medium containing 20-mM HEPES-Na (pH 7.2), and Na-EGTA to bring the concentration to of protein/ml and stored on ice. Mitochondrial protein was determined by bicinchoninic acid assay using Sigma kit BCA-1. Mitochondrial oxygen consumption was measured using a Clark-type oxygen electrode (Yellow Springs Instruments, Ohio, U.S.A.) at in the mitochondrial incubation buffer A. Glutamate-Na malate-Na or succinate-Na were used as oxidizable substrates. Following stabilization of state 2 respiration, MgADP was added to stimulate respiration (state 3), which returned to the level close to state 2 (state 4) upon completion of ADP phosphorylation. FCCP was added at the end of the assay to obtain uncoupled respiration rate. The respiration rates were expressed in nmol and the respiratory control index (RCI) as a ratio of state 3 to state 2 respiration rate. RCI was 6.9 with glutamate malate and with succinate. 2.4.Isolation of Rat Hepatocytes and CardiomyocytesHepatocytes were isolated according to a procedure described elsewhere,12 based on a modified collagenase perfusion method.13 Isolated hepatocytes were resuspended in hepatocyte incubation buffer B. Cell viability of the hepatocyte preparations checked by the Evans Blue exclusion test was over 60%. Cardiomyocytes were isolated by the collagenase perfusion method14 and resuspended in buffer C. Cell viability counts were performed using a hemocytometer and a light microscope. Approximately 70% of isolated cardiomyocytes were rod-shaped, and were therefore considered viable. All measurements were performed immediately after cell isolation. 2.5.Measurements of Rhod800 Binding in Isolated Rat Liver Mitochondria, Hepatocytes, and CardiomyocytesRhod800 binding in mitochondria was determined as follows: mitochondria were incubated in of aerated buffer A plus with rhod800 for in the presence of MgATP and oxidizable substrates( succinate-Na) at . FCCP was added simultaneously with rhod800. The reaction mixture, in open scintillation vials (25-mm diameter), was continuously shaken to provide adequate aeration. Following 5-min incubation, the mixture was rapidly cooled and centrifuged for at . Rhod800 was extracted from the mitochondrial pellet with 95% ethanol and its content was then determined from the absorbance at in the suspension supernatant and mitochondrial extract (Beckman DU 650 spectrophotometer, Beckman Instruments Inc., Missisauga, Ontario, Canada) . Isolated cells were incubated on a slowly shaking water bath at . Hepatocytes were incubated in the hepatocyte incubation buffer B. To collapse sarcolemmal membrane potential, cardiomyocytes were diluted 1:11 in of buffer D in the presence or absence of FCCP . Rhod800 was added to a concentration of and allowed to equilibrate for . Samples were then centrifuged for at . Rhod800 content in the supernatant was determined spectrophotometerically. Rhod800 bound to the cells was extracted with of 95% ethanol. After , the cells were centrifuged, absorbance of the extract was measured at and used to determine the amount of dye. Ethanol extraction of the dye was close to 100%. To calculate the ratio of bound to free rhod800 (B/F) in cell preparations corrected for cell viability, , we assumed that nonviable cells were uncoupled, then where is viability (%), and and are experimentally determined ratios for coupled and uncoupled preparations, respectively. From Eq. 12.6.Measurements of Rhod800 Fluorescence in Rat Liver Mitochondria, Hepatocytes, and CardiomyocytesFor all measurement, mitochondria were incubated in buffer A plus ATP, glutamate, and succinate, hepatocytes in buffer B, and cardiomyocytes in buffer C plus pyruvate or buffer D. Preparations were diluted 1:10 in a cuvette containing high-potassium buffers with a continuously stirring magnetic bar, to a final concentration of protein: mitochondria , hepatocytes and cardiomyocytes to . Fluorescence was measured by exciting the sample in the respective incubation buffer in a disposable plastic cuvette using a 670-nm laser diode ( , NVG Inc., Hazlehurst, Georgia, U.S.A.); emitted fluorescence (at approximately ) was detected at 90° and transmitted through a fiber optic cable to a spectrometer (Control Development, South Bend, Indiana, U.S.A). To reduce scattered laser light intensity, a 695-nm high-pass filter (CRG 695) was introduced before the detector. The fluorescence spectrum of rhod800 was taken before the addition of the sample. Additional dye was added to ensure the rhod800 concentration was maintained after addition of the sample. Spectra were taken every for . After , FCCP was added to a final concentration of to reduce to near zero. Measurements were taken after each chemical addition. 2.7.Heart PerfusionRat hearts were removed quickly and perfused in a Langendorff mode with KHB aerated with a mixture of 95% and 5% at 36°C. Following the placement of a left ventricular apical drain, a latex balloon was inserted through the mitral valve into the left ventricular cavity and filled with . The balloon was connected to a Statham P23Db pressure transducer and to a Digi-Med Model-210 heart performance analyser (Micro-Med, Louisville, Kentucky, U.S.A.) to monitor heart rate (HR), left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and perfusion pressure (PP). Pressure-rate-product (PRP), calculated as developed pressure (LVSP minus LVEDP) multiplied by HR, was used as an index of mechanical work. The coronary flow rate was monitored using an ultrasonic blood flow meter (Transonic Systems Inc., Ithaca, New York, U.S.A.), and PP was measured continuously through the catheter connecting the aortic line and the second pressure transducer. 2.8.Myocardial Absorbance MeasurementsReflectance and absorbance spectra in perfused rat hearts, in the range , were acquired using a probe connected to a bifurcated fiber-optic cable. One end of the cable was connected to a source of white light (Fiber Optic Illuminator, model 77501, Oriel Instruments, Stratford, Connecticut, U.S.A.), while a second end terminated at the detector. The individual fibers of the cable were combined into a common probe tip that was in a direct contact with a left ventricle. Nongated spectra were acquired every . 2.9.Measurements of Rhod800 Fluorescence and Apparent Absorbance at the Laser Wavelength in Intact HeartsFluorescence measurements were performed by exciting rhod800 in the hearts with a laser diode (690 or , , NVG Inc., Georgia, U.S.A.). Signal acquisition was performed in two settings: at 90° (orthogonal mode) and 360° (reflection mode), respective to the laser beam direction. In the first setting, a 690-nm laser was in contact with the anterior heart wall and the signal was acquired at 90° to the laser beam, at the surface of the left ventricle. The collected signal consisted of scattered excitation and emitted fluorescence light from the deeper layers of the hearts. Nongated spectra were acquired in the range every ; acquisition time was . Apparent absorbance by rhod800 loaded in the hearts was estimated from the changes in the laser signal registered by the detector over the time-course of dye loading, using the equation where is intensity of the laser signal detected before the dye loading and is intensity of the scattered laser light at any given moment during dye loading (55 or ).In the second setting, a front face illumination-acquisition mode, fibers transmitting excitation light from a 670-nm laser and fibers receiving reflected and emitted light were combined in a single probe tip that was in a direct contact with a left ventricle. To reduce reflected light intensity, a 695-nm high-pass filter was introduced in the receiving fibers before the detector. The acquired signal consisted of a greatly reduced reflected laser light and emitted fluorescence from the epicardial layer of the heart. Nongated spectra were acquired in the range every ; acquisition time was . All spectral data were processed using Grams/32 Version 4.11 computer program (Galactic Industries Corp., U.S.A.). 3.Results3.1.Rhod800 Binding and Fluorescence in Isolated Rat Mitochondria and CellsIn mitochondrial suspensions, almost all rhod800 (greater than 99%) was taken up by the mitochondria (Table 1 ). This uptake was -dependent: the average ratio of bound-to-free rhod800 (B/F) was over 40 in the energized mitochondria. After addition of FCCP, almost all of the dye was released into the supernatant (Table 1). The amount of rhod800 bound specifically to the viable cells (containing energized mitochondria) was much lower: B/F decreased dramatically in isolated cells in comparison to the isolated mitochondria (Table 1). In cell preparations, B/F, corrected for cell viability, was approximately 2 (67% of the total dye bound) for the cardiomyocytes and 3 (75% bound) for the hepatocytes (Table 1). Table 1Effects of FCCP on rhod800 binding and fluorescence in liver mitochondria, cardiomyocytes, and hepatocytes.1
2Bound/Free ratio of rhod800 in isolated mitochondria and cells was determined as described in “Materials and Methods” section. 3 B∕Fcorr is the ratio of bound to free rhod800, corrected for cell viability, using the following relationship: B∕Fcorr=(B∕Fx−B∕F0∙(100−X))∕X (see “Materials and Methods”). 4The change in fluorescence ratio ΔIF∕IF0=IF0∕IF0−IF∕IF0=1−IF∕IF0 , where IF0 is the initial fluorescence of 1μM rhod800, was determined for coupled and uncoupled cell/mitochondria in the presence and absence of a mitochondrial uncoupler, carbonyl cyanide 4-trifluoromethoxyphenyl hydrazone (FCCP, 1μM ). Fluorescence of rhod800 in the incubation buffer decreased after addition of mitochondria and increased after addition of FCCP [Fig. 1a ]. Similar fluorescence responses were observed in cell preparations; however, the magnitude of the changes was lower [Fig. 1b]. The fluorescence peak was normalized to the initial fluorescence and calculated (Table 1). In the mitochondrial preparations, was almost 80% in the energized vs. 13% in the de-energized mitochondria (Table 1). In the hepatocytes and cardiomyocytes, the differences between in coupled and FCCP-treated (uncoupled) cells were in the 10% range (Table 1). Again, this corresponds to a smaller fraction of rhod800 released into the buffer from the FCCP-treated cells than from the FCCP-treated mitochondria. Fig. 1Fluorescence of rhod800 in mitochondria and cardiomyocyte suspension. (a) Spectra of rhod800 fluorescence in the incubation buffer before (solid), after (dot) addition of mitochondrial suspension, and after addition of FCCP (dash-dot). (b) Spectra of rhod800 fluorescence in the incubation buffer before (solid), after (dot) addition of isolated cardiomyocytes, and after addition of FCCP (dash-dot). A spike under was an artifact. See details in Sec. 2. 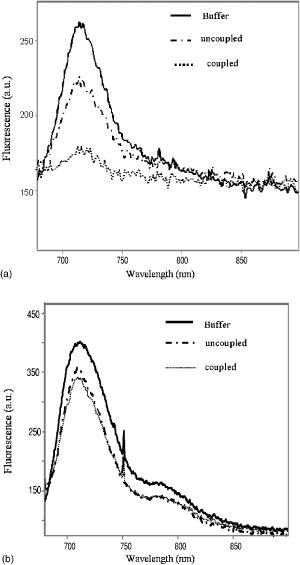 De-energization of mitochondria by FCCP extruded the dye from the matrix into the supernatant, where fluorescence quenching is lower. However, nearly all rhod800 was bound to mitochondrial membranes and proteins. We estimated the fraction of free matrix dye as 0.15 and 0.75% of the total mitochondria dye (assuming matrix volume of ) in uncoupled and coupled mitochondria respectively. This implies a very high partition coefficient of rhod800 between water and hydrophobic milieu such as membranes, perhaps due to the high hydrophobicity of the dye. Indeed, we estimated that rhod800 partition coefficient was approximately 5-fold higher than that of TMRM (Kupriyanov, unpublished observation). This property of rhod800 may explain relatively small changes in the binding and fluorescence in response to uncoupling of cardiomyocytes and hepatocytes (see Table 1). The dye binding to the plasma membrane, endo/sarcoplasmic reticulum and polyanions such as DNA/RNA greatly increased the fraction of passively bound dye, which increased -independent binding and fluorescence thereby reducing relative magnitude of the -dependent response. Therefore quantitative estimation with rhod800 in cell suspensions is more difficult than in mitochondria. 3.2.Changes in the Myocardial Absorbance Spectra Caused by Rhod800Perfusion of isolated rat hearts with KHB containing rhod800 changed the myocardial absorbance spectra resulting in the appearance of a prominent peak at and an additional smaller peak at [Fig. 2a ]. The peaks were well separated from the peaks of endogenous chromophores in the visible range, such as oxy- and deoxymyoglobin, and cytochrome c oxidase. The dye concentration of was selected to bring its free cytosol concentration, after 60-min perfusion, to approximately , which is expected at the resting membrane potential of . Changes in the myocardial absorbance at were almost linear during 60-min perfusion with rhod800: [Fig. 2b]. Thirty-min washout resulted in only partial ( , ) dye washout, as determined from the changes in myocardial absorbance at . Fig. 2Myocardial absorbance of a rat heart perfused with rhod800. Front-face white light illumination and acquisition mode was employed. (a) Representative cardiac absorbance spectra before (dash) and after (solid) 60-min loading with rhod800. (b) Kinetics of myocardial absorbance at during rhod800 loading and washout. 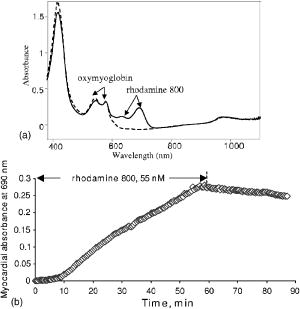 3.3.Deep Tissue Fluorescence and Apparent Absorption Versus Surface Fluorescence of Rhod800 in Rat HeartsTo measure rhod800 fluorescence in rat hearts, a laser diode was used for the dye excitation. In the first setting, we did not use a cut-off filter and the signal collected at 90° to the direction of the laser beam consisted of two peaks: a scattered laser light peak (at ), and a fluorescence peak [Fig. 3a ]. This setup allowed us to obtain spectral information from the deeper layers of the hearts. The position of the fluorescence peak shifted from 715 to during loading [Fig. 3a]. The intensity of the scattered excitation light passed through the myocardium and registered orthogonally to the laser beam direction was used to calculate changes in the apparent absorbance at 690-nm (excitation wavelength) due to the rhod800 accumulation, according to Eq. 3. This parameter provided a relative measure of the dye concentration in the hearts. Actual dye content was determined by ethanol-extraction of rhod800 from the hearts at the end of the experiments (Table 2 ). At rhod800 in perfusate, average tissue concentration reached (Table 2), which at gives an effective path-length at of . In this setting, the intensity of the fluorescence peak reached a maximum at approximately after perfusion with rhod800 and decreased on further loading [Fig. 3b]. However, apparent absorbance at 690-nm increased linearly during the same time [Fig. 3c], indicating that the observed decrease in the fluorescence signal was most probably a result of an inner filter effect.15 Because of the significant increase in apparent absorbance during rhod800 loading, we concluded that the observed inner filter effect was due to absorption of the excitation (primary effect) and emission (secondary effect) light by the accumulated rhod800.16 Washout of rhod800 resulted in a linear decrease in apparent absorbance by accompanied by an increase in fluorescence, probably as a result of an increase in the intensity of excitation light inside the tissue and excitation volume. However, at a given absorbance, fluorescence was higher during washout than during loading [Fig. 3d]. This effect may have been caused by the dye re-distribution at the washout stage (release from the mitochondria?) and a change of quantum efficiency taking place in the mitochondria or cytosol of perfused rat hearts. It has been reported that cholesterol could affect self-quenching of the fluorescence of lipid-conjugated rhodamines.17 In the heart tissue, different types of membrane are present: cholesterol-containing (cytoplasmic) and cholesterol-free (mitochondrial), as well as different polyanions (DNA and RNA). Re-distribution of rhod800 between these structures may have resulted in the fluorescence increase. Fig. 3Detection of rhod800 deep tissue fluorescence from a rat heart. A 690-nm laser diode placed against a left ventricle anterior heart wall was used for the dye excitation. The signal was acquired at 90° to the laser beam at the surface of the left ventricle. (a) Fluorescence emitted from a representative intact rat heart before (solid), after 20-min (dot), 40-min (dash), and 60-min (dash-dot) infusion of rhod800. (b) Time course of a fluorescence signal at . (c) Time course of apparent absorbance at . (d) Fluorescence signal intensity plotted against apparent absorbance at . 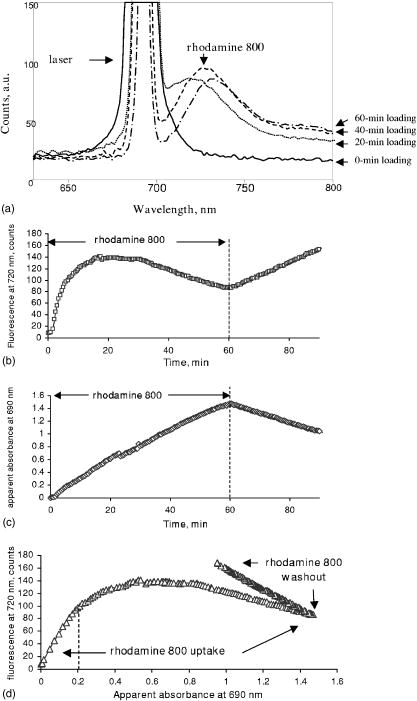 To reduce the inner filter effect and dye quenching, the hearts were perfused with rhod800, which eliminated the fluorescence decline phase (not shown). Apparent absorbance at linearly increased to approximately half of that observed during loading with rhod800 (Table 2); thus, further confirming that this parameter was proportional to the amount of dye in the myocardium. In agreement with the apparent absorbance data, amount of rhod800 extracted from the hearts perfused with rhod800 was nearly half of that extracted from the hearts perfused with rhod800 (Table 2). In the second setting, to eliminate the inner filter effect, a fluorescence signal was collected from the surface of the heart at 360° to the direction of a 670-nm laser beam. Fluorescence intensity was relatively weak in comparison to the reflected laser light intensity; thus, to improve the signal-to-noise ratio for the fluorescence signal, increase acquisition time and avoid detector saturation with the laser signal, a 695-nm high-pass filter was introduced before the detector. Detected signal consisted mostly of a fluorescence peak at and a greatly reduced reflected laser signal at [Fig. 4a ]. Fluorescence increased nearly linearly during 60-min loading with rhod800: [Fig. 4b]. Dissipation of with DNP at the loading stage did not significantly affect the fluorescence kinetics: the fluorescence peak reached counts in the hearts treated with DNP and ( , N.S.) in the absence of DNP. After the loading stopped, the fluorescence reached the plateau [Fig. 4b]. Treatment of the hearts with DNP at the washout stage resulted in a small (approximately 5%), but statistically significant increase in the heart’s fluorescence [Fig. 4c]. In comparison to the isolated cells, the scarcely detectable response to DNP uncoupling in the intact hearts was probably due to further increased background binding of the dye to the vascular and extracellular matrix elements. To test the effect of dissipation of the sarcolemmal membrane potential, the hearts were perfused with a high potassium buffer , which resulted in the sarcolemmal membrane depolarization by approximately and caused cardiac arrest. No changes were observed in the heart’s fluorescence during high potassium perfusion [Fig. 4c]. Fig. 4Detection of rhod800 surface fluorescence from a rat heart. Fibers transmitting excitation light from a 670-nm laser and fibers receiving reflected and emitted light were combined in a single probe tip that was in a direct contact with a left ventricle. (a) Fluorescence emitted from a representative intact rat heart before (solid), after 30 (dot), and 60-min (dash) of infusion of rhod800. (b) Average time course of a fluorescence signal at . (c) Average apparent absorbance at of the hearts treated with DNP ( final concentration, ) or perfused with high-potassium KHB ( , ) at the indicated periods of time. 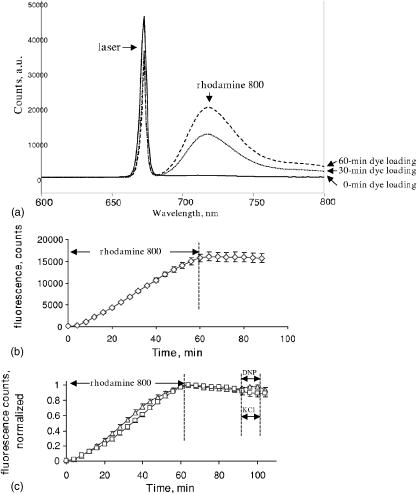 Table 2Effect of the dye loading conditions on rhod800 accumulation in rat hearts.1
2Apparent absorbance at 690nm was calculated as A=logIex0∕Iex , where Iex0 is intensity of 690-nm laser signal collected at the surface of the left ventricle at 90° to the laser at time 0 (no rhod800 in the heart), and Iex is intensity of the laser signal collected at any given time during perfusion with rhod800. 3.4.Cardiac FunctionFollowing an approximately 10-min stabilization period, the hearts were perfused at a constant flow of to provide the desired concentration of the dye/drugs during infusion. Baseline parameters were: (no pacing); , , and mean . These parameters did not change significantly during perfusion with KHB for 1 hour. Sixty-min infusion of rhod800 resulted in a moderate increase in LVEDP to , LVSP to , and PP to . The HR declined to approximately 230 beats per minute, however, the change in HR was not significant. Infusion of DNP simultaneously with rhod800 resulted in a decline in pressure-rate-product to of the baseline due to a well-known uncoupling effect of DNP on the oxidative phosphorylation. 4.DiscussionThe intensity of a dye fluorescence is linearly dependent on its concentration if the sample absorbance (A) is relatively low .15 This was the case for the measurements of rhod800 fluorescence in suspensions of isolated mitochondria and cells where the dye concentration was ( at path length). In the experiments with isolated hearts, apparent absorbance measured at 90° to the laser beam, reached approximately 0.7 and 1.7 after perfusion with 27.5 and rhod800, respectively (Table 2). This high absorbance resulted in a nonlinear relationship between fluorescence intensity and absorption [a measure of an average dye concentration, Fig. 3d]. This, in turn, produced a well-known inner filter effect (absorption of excitation and emission light by the dye) and a self-quenching effect that are characteristic of high absorbance.15 An obvious solution, to decrease the dye loading time, was not acceptable since the dye distribution was far from the equilibrium. Even 60-min perfusion did not bring the distribution to the full equilibrium [Fig. 3c]. We explored a different approach by measuring reflected fluorescence (at 360°), which reduced absorbance significantly (to less than 0.2) due to a decrease in the effective path length and hence the “interrogation” volume ( in depth). In this case, the relationship between fluorescence and the time of loading (proportional to the amount of dye loaded) did not significantly deviate from linearity [Fig. 4b] and no quenching was observed. Surface fluorescence did not change after rhod800 loading stopped [Fig. 4b]. Therefore even small changes in the fluorescence induced by DNP treatment were detectable [Fig. 4c]. Measuring myocardial absorbance at (reflected light normalized to the reference) and apparent absorbance (calculated using a scattered laser signal detected at the heart’s surface at 90° to the laser beam) provided a means to study kinetics of rhod800 uptake by the cardiac tissue. Rhod800 was retained in the cardiac tissue and only a small fraction of the dye washed out. This formed the basis for the direct determination of the rhod800 tissue content by an ethanol extraction assay. Ethanol extraction data confirmed that absorbance values were proportional to the dye content in the cardiac tissue and therefore can be used to adequately estimate dye supply (i.e., heart perfusion). This approach can potentially be used to evaluate myocardial perfusion in bigger hearts (e.g., swine hearts where local blockage of the flow can be easily modeled). Previously, a visible range rhodamine derivative, TMRM, was used for histological mapping of hypoperfused areas in the biopsies from rat hearts.18 Accumulation of rhod800 in cardiac mitochondria in perfused rat hearts resulted in a slight inhibition of cardiac mechanical function. We observed a steady, almost linear increase in diastolic and perfusion pressure in isolated rat hearts (indicators of the heart’s stiffness) after 20-min perfusion (not shown). Sakanoue reported that inhibition of state 3, 4, and the uncoupled state of succinate-dependent respiration rate in liver mitochondria by rhod800 was negligible at .7 However, in isolated beating hearts respiration is dependent on NADH dehydrogenase, which is sensitive to inhibition by rhodamines.19 Rhod800 may have additional toxic effects in mitochondria. For example, -ATPase is inhibited by a rhod800 parent compound, rhodamine 123.19 Notably, a mitochondrial dye successfully used in isolated living cells, MitoTracker (Molecular probes), caused an instantaneous death of the experimental animals.18 Therefore use of rhod800 (or any other lipophilic rhodamine derivatives) in vivo deserves a cautious warning. In conclusion, although rhod800 binding and fluorescence are -dependent in isolated mitochondria and cells, excessive hydrophobicity of rhod800 precluded its use as a probe for mitochondrial membrane potential in intact hearts. Even application of a visible range dye with lower hydrophobicity, TMRM failed to detect changes on the surface of intact rat hearts.2 Thus, the design of a probe with optical properties similar to that of rhod800 and much lower hydrophobicity would be useful for the evaluation of mitochondrial coupling in intact tissues. AcknowledgmentsThis research was supported, in part, by an operating grant MT 42626 from the Canadian Institutes for Health Research (CIHR) to V.K., an operating grant from the Heart and Stroke Research Foundation of Manitoba to V.K. and O.J., and by Women in Engineering and Science (WES) undergraduate program to L.H. ReferencesJ. C. Smith,
“Potential-sensitive molecular probes in membranes of bioenergetic relevance,”
Biochim. Biophys. Acta, 1016 1
–28
(1990). 0006-3002 Google Scholar
R. C. Scaduto Jr., L. W. Grotyohann,
“Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives,”
Biophys. J., 76 469
–477
(1999). 0006-3495 Google Scholar
B. Ehrenberg,
V. Montana,
M. D. Wei,
J. P. Wuskell, and
L. M. Loew,
“Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes,”
Biophys. J., 53 7857
–7894
(1988). 0006-3495 Google Scholar
R. A. Kauppinen and
I. E. Hassinen,
“Monitoring of mitochondrial membrane potential in isolated perfused rat heart,”
Am. J. Physiol., 247 H508
–H516
(1984). 0002-9513 Google Scholar
P. Veit,
J. Fuchs, and
G. Zimmer,
“Uncoupler- and hypoxia-induced damage in the working rat heart and its treatment. I. Observations with uncouplers of oxidative phosphorylation,”
Basic Res. Cardiol., 80 107
–1015
(1985). 0300-8428 Google Scholar
J. Fuchs,
G. Zimmer, and
J. Bereiter-Hahn,
“A multiparameter analysis of the perfused rat heart: responses to ischemia, uncouplers and drugs,”
Cell Biochem. Funct., 5 245
–253
(1987). 0263-6484 Google Scholar
J. Sakanoue,
K. Ichikawa,
Y. Nomura, and
M. Tamura,
“Rhodamine 800 as a probe of energization of cells and tissues in the near-infra-red region: a study with rat liver mitochondria and hepatocytes,”
J. Biochem. (Tokyo), 121 29
–37
(1997). 0021-924X Google Scholar
O. O. Abugo,
R. Nair, and
J. R. Lakowicz,
“Fluorescence properties of rhodamine 800 in whole blood and plasma,”
Anal. Biochem., 279 142
–150
(2000). https://doi.org/10.1006/abio.2000.4486 0003-2697 Google Scholar
R. Hakem,
A. Hakem,
G. S. Duncan,
J. T. Henderson,
M. Woo,
M. Soengas,
A. Ella,
J. L. de la Pompa,
D. Kagi,
W. Khoo,
J. Potter,
R. Yoshida,
S. A. Kaufman,
S. W. Lowe,
J. M. Penninger, and
T. W. Mak,
“Differential requirement for caspase 9 in apoptotic pathways in vivo,”
Cell, 94 339
–352
(1998). 0092-8674 Google Scholar
O. Jilkina,
B. Kuzio,
G. J. Grover,
C. D. Folmes, H. -J. Kong, V. V. Kupriyanov,
“Sarcolemmal and mitochondrial effects of a opener, P-1075, in ‘polarized’ and ‘depolarized’ Langendorff-perfused rat hearts,”
Biochim. Biophys. Acta, 1618 39
–50
(2003). 0006-3002 Google Scholar
L. A. Sordahl,
C. Johnson,
Z. R. Blailock, and
A. Schwartz,
“The Mitochondrion,”
Methods in Pharmacol., 1 247
–286
(1971) Google Scholar
P. O. Seglen,
“Preparation of isolated rat liver cells,”
Methods Cell Biol., 13 29
–83
(1976). 0091-679X Google Scholar
M. N. Berry and
D. S. Friend,
“High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study,”
J. Cell Biol., 43 506
–5020
(1969). 0021-9525 Google Scholar
H. Viko,
J. B. Osnes,
A. E. Sjetnan, and
T. Skomedal,
“Improved isolation of cardiomyocytes by trypsination in addition to collagenase treatment,”
Pharmacol. Toxicol., 76 68
–71
(1995). 0901-9928 Google Scholar
A. G. Szabo,
“Fluorescence principles and measurement,”
Spectrometry & Spectrophotometry, 33
–67
(2000) Google Scholar
S. A. French,
P. R. Territo, and
R. S. Balaban,
“Correction for inner filter effects in turbid samples: fluorescence assays of mitochondrial NADH,”
Am. J. Physiol., 275 C900
–909
(1998). 0002-9513 Google Scholar
R. I. MacDonald,
“Characteristics of self-quenching of the fluorescence of lipid-conjugated rhodamine in membrane,”
J. Biol. Chem., 265 13533
–13539
(1990). 0021-9258 Google Scholar
F. Brasch,
M. Neckel,
R. Volkmann,
G. Schmidt,
G. Hellige, and
F. Vetterlein,
“Mapping of capillary flow, cellular redox state, and resting membrane potential in hypoperfused rat myocardium,”
Am. J. Physiol., 277 H2050
–H2064
(1999). 0002-9513 Google Scholar
J. S. Modica-Napolitano and
J. R. Aprille,
“Basis for the selective cytotoxicity of rhodamine 123,”
Cancer Res., 47 4361
–4365
(1987). 0008-5472 Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

