|
|
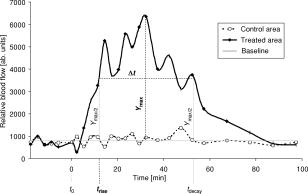
1.IntroductionCutaneous microcirculation of blood plays an important role in thermoregulation, skin metabolism, and percutaneous penetration. Topically applied products, e.g., corticosteroids and nicotinates, can induce significant changes in cutaneous blood flow. Such changes in microcirculation can be detected using several techniques.1 Of all these methods, laser Doppler flowmetry is the most widely used technique as it enables cutaneous microcirculation to be measured noninvasively. This measurement is based on the Doppler effect, which describes the shift in the frequency of light scattered at moving red blood cells.2 The integral over the power spectrum of the detected Doppler frequency signal is proportional to the concentration of the red blood cells [Eq. 1], and the integral of the product of the power spectrum and the frequency shift is proportional to the product of and the mean velocity of the red blood cells3 [Eq. 2]: The product indicates the flow, whereas the term is the flow spectrum. Equation 2 is valid only for integration limits from 0 to .For a single velocity of red blood cells, the power spectrum and even the flow spectrum show a specific distribution in the region to and scales linearly with the cell velocity (neglecting multiple Doppler events). This means that cells with high velocities contribute to both lower and higher Doppler frequencies simultaneously, whereas the lower velocities contribute to only the lower Doppler frequency parts. Based on these findings, partial integrals of the flow spectrum can be used to distinguish between high and low velocities of red blood cells, even if the resulting partial flows do not satisfy Eq. 2 exactly. This approach was used by Dörschel and Müller4 extended by differentiation of the power spectrum. From a physiological point of view, it is known that in a closed vessel system the total cross section increases from the arteries to the superficial dermal plexus and then decreases in the veins. In a closed system, the flow is constant and the velocity is proportional to . This means that the expected velocity is lower in the smaller vessels than in the larger ones.5 Consequently, it is assumed that low frequencies increasingly represent the blood flow of the superficial dermal plexus, whereas the higher frequencies correspond to the larger capillaries in deeper skin layers. In this paper, an analysis of the Doppler flow measurements is used based on frequency-selective calculations of the blood flow without differentiation of the power spectra. This method is used to study the kinetics of the visible vasodilation caused by the topical application of benzyl nicotinate. The flowmeter was modified to avoid any direct contact between the laser diode and the skin surface treated with a gel containing benzyl nicotinate. The data of the blood flow measurements were compared with two other biological parameters, skin color and temperature, which were measured to evaluate their suitability for use instead of the flowmetry. Conflicting results have been reported in the literature concerning the relationship between skin color and blood flow.1, 6, 7 However, a relationship between the skin temperature and the microcirculation has been described in some detail.1, 8, 9 2.Materials and Methods2.1.VolunteersThe study was performed on eight healthy male volunteers (mean age: ). All volunteers had a skin of phototype10 II or III. Approval was obtained for these experiments from the Ethics Committee of the Charité Hospital. All volunteers signed informed consent forms before the trials began. 2.2.Laser Doppler FlowmetryThe microcirculation of blood was measured using the laser Doppler flowmeter developed at the LMTB GmbH (Berlin, Germany). This device consists of a laser diode built into a small handpiece. The laser radiation at a wavelength of was focused perpendicularly onto the skin. The backscattered light was collected from an area of approx. . The remitted light provided information about the blood flow due to the Doppler effect and was determined for using two photodiodes. Both diodes detect the backscattered light signal. The signal of one diode was dc lowpass filtered and used only for normalization. The signal of the other diode was highpass filtered, and the ac signal was used for the laser Doppler flow measurement. This Doppler-shifted signal was sampled by a 16-bit analog-to-digital (A/D) converter with a sample frequency of . Normalization was performed using the quotient of the ac and dc signals for each point. A power density spectrum was automatically calculated from the measured Doppler signals using discrete implementation of the fast Fourier transformation (2048 data). The power density spectrum was corrected using a previously detected electronic noise spectrum measured without laser light to reduce noise, which originated in the electronics. Then a related flow spectrum for the total flow was calculated following Eq. 2. On the basis of this flow spectrum, several partial flows can be calculated by integrating over a certain frequency region (integral borders , low, and , high) of the flow spectrum: Two different partial blood flows were calculated as mean values using integration limits and : 0.1 to and 5 to , respectively.In this study, a metal ring with an inner diameter of , an outer diameter of , and a height of was used as a spacer between the handpiece and the skin surface. In this manner, direct contact was avoided between the treated skin area and the laser diode. The site on the skin, which was covered by the modified handpiece, was marked and used in subsequent measurements. 2.3.Measurement of Skin Temperature and RednessThe skin temperature was measured using an IR thermometer without direct contact with the skin (Raynger MX, Schlender Messtechnik, Berlin, Germany). This thermometer determines the temperature based on the emitted radiation taking into consideration the emissivity of the skin. In preliminary experiments, a decrease in temperature of approx. was observed immediately after topical application of the gel on the excised porcine skin model. The temperature reached the same value after as the untreated area and remained constant. This means that the measurement of skin temperature is only affected by the blood flow or more after topical treatment with the gel. The redness of the skin was determined using the colorimeter Spectropen (Fa. Dr. Lange, Berlin, Germany). Based on the Commission Internationale de I’Eclairage (CIE) color space, parameter was used as a value for the red part of the skin color. The effect of the topically applied gel on the measurement of color has already been studied in preliminary experiments using the excised porcine skin model. No influence was observed on any of the parameters , , or . 2.4.Topical Application of the GelIn these experiments the gel (Wärme-Gel-Ratiopharm, Ratiopharm GmbH, Ulm, Germany) used contained 1% benzyl nicotinate and 4.5% (2-hydroxyethyl)-salicylate. It also contained polyacrylic acid, trometamol, macrogol-glycerol-ricinoleate, rosemary oil, dwarf pine oil, isopropyl alcohol, disodium salt of edetic acid, and purified water. The gel was applied homogeneously using a syringe without the needle, as reported previously,11 onto skin areas of on the flexor forearm of each volunteer. 2.5.Protocol of MeasurementThe volunteer was made to lie down in a horizontal position before the first measurement and remained in this position during the entire experiment. In this way, the forearm was kept at the same level as the heart. Volunteers were not allowed to be in any way physically active during the measurement. Two skin areas of , apart, were marked on the right flexor of the forearm. There were two regions: the skin area, later treated with the gel and the untreated area (control area). Blood flow measurements, skin temperature, and redness were recorded five times before topical application of the gel. The gel was applied on the marked skin area near the hand . Then the blood flow, skin temperature and degree of redness were measured on both skin areas ( and ): The studies were performed under standard conditions at room temperature and at a relative humidity of . 2.6.Evaluation of DataThe mean values and standard deviations of blood flows, skin temperature, and color, measured five times prior to topical application of the gel, were calculated using the software program Microsoft Excel 97. The data obtained on the control area were compared with those of the area treated later with the gel . After topical application of the gel, the parameters measured were correlated with the time, both for the treated area and the control area , as shown in Fig. 1 . In the case of both blood flow measurements (at 0.1 to 5 and 5 to ), the baseline was calculated as the mean value of the parameter measured on the control area . The values obtained from the treated area were corrected for this baseline. The maximal value was determined and half of this value was calculated. The corresponding points in time of were determined both for the increase and the decline of the signal. The period of the signal was calculated as the difference between and . Skin temperature and skin color are to a large extent dependent on physiological effects. The difference was calculated between the values measured on the treated area and those on the control area . These differences were used to determine the values of , , and for both the skin temperature and skin color analogously, as described for the blood flow. 2.7.StatisticsNonparametric Wilcoxon tests were performed to compare the data using the software program SPSS 11.0. Before topical application, blood flow, skin temperature, and redness values, measured on the skin area prior to treatment with the gel, were compared with those determined on the control area . The values for measured on the treated area after topical application were compared with those previously determined on the same site. The method of linear regression was used to evaluate the relationship between the parameters , , and , which were determined for the various biological responses to the gel. 3.ResultsBefore topical application of the gel, no significant difference in blood flow, skin temperature, and redness was recorded on the control area and the application area ( , data not shown). The ratio of the blood flows measured at 5 to 30 and 0.1 to was (mean value standard deviation). Measurements made in the application area increased significantly after treatment with the gel containing benzyl nicotinate . The findings for the results obtained for a volunteer are shown in Fig. 2 . Both of the blood flow measurements show a rapid increase to a higher level [Fig. 2a]. The values varied around this level until they decreased with increasing time. In contrast, the skin temperature decreased slightly immediately after topical application of the gel [Fig. 2b]. A similar decrease in temperature was observed in the preliminary experiments immediately after topical application of the gel on the excised porcine skin model (data not shown). Both the cutaneous temperature and the redness increased until a plateau was reached. The values showed small variations around this level until they eventually decreased with time. Fig. 2Kinetics of the measurements performed on volunteer 5 after correction for (a) both blood flows and (b) skin temperature and redness. 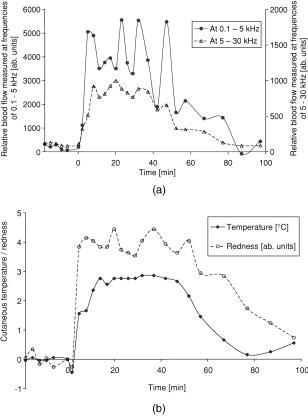 Table 3Values of the period Δt=(trise−tdelay) determined for both blood flows (measured at 0.1 to 5 and 5 to 30kHz ), skin temperature and color.
*tdelay>
95min
. The maximal values , the time to reach the half of this value , and the period of this increase were determined for each biological parameter, i.e., the blood flow, cutaneous temperature, and redness. The values obtained are summarized in the Tables 1, 2, 3 . A linear proportionality between the appearance of the maximal values was observed only for the blood flow data (coefficient of correlation , data not shown). This relationship was also reflected by a nearly constant ratio of the values measured at 5 to 30 and 0.1 to (mean value standard deviation . Table 1Values of ymax determined for both blood flows (measured at 0.1 to 5 and 5 to 30kHz ), skin temperature, and color.
Table 2Values of trise determined for both blood flows (measured at 0.1 to 5 and 5 to 30kHz ), skin temperature, and color.
In addition, a significant difference in the time of an increase was observed only for the blood flow measurements . Half of was reached 0.4 to earlier for the blood flow measured at 0.1 to than for the microcirculation determined at 5 to . A linear correlation between the values of the period was only observed for both blood flows (coefficient of correlation , data not shown). 4.DiscussionSkin microcirculation is affected by several parameters, e.g., pressure to the probe,12 skin temperature,8, 9 position,13 physical activity,9 sex,14, 15 and race.16, 17, 18. In this study, the effect of the topically applied gel containing benzyl nicotinate on the blood flow, skin temperature, and redness was determined under standardized conditions, avoiding any influence by the aforementioned physiological parameters. The data measured on the treated area were corrected using the values obtained simultaneously from the control area. The validity of using the control area as a baseline was evaluated by measurements on both skin areas before topical application of the gel. Significant differences were not found in any of the biological parameters (data not shown). In general, the topically applied benzyl nicotinate caused an increase in each biological parameter with increasing time. Such a vasodilation effect has been reported for several topically applied alkyl nicotinates measured by laser Doppler velocimetry 7, 19, 20, 21 and laser Doppler perfusion imaging.22 Benzyl nicotinate was found to bind to human serum albumin, whereas some other esters were hydrolyzed.23 Taking account of the fact that the small vessels in the superficial dermal plexus correspond to low velocities, and the larger deeper vessels to higher velocities, the two partial integrals at 0.1 to 5 and 5 to should correlate to the vessel size and deepness of the vessels with a remaining uncertainty. In which case, the partial integrals at frequency 0.1 to correspond mainly to the superficial capillaries, keeping in mind that there are still parts of the higher velocities included in the signal. However, the blood flow measured at 0.1 to showed an increase earlier than that determined at 5 to . This lag can be explained by a good correlation of the blood flow at 0.1 to to the microcapillaries near the surface and the blood flow at 5 to to the deeper capillaries. The distance must be overcome between both types of capillaries, presumably by the transport of the drug into the blood from the upper to the deeper capillaries. This assumption is supported by the nearly constant ratio and a linear proportionality between the maximal values of both blood flows. The relationship between both blood flows was additionally confirmed by comparing the parameters and . The increase in the ratio of the blood flows measured at 5 to 30 and 0.1 to from (before application) to ( reached after application) indicates a greater effect of the benzyl nicotinate on the superficial microcapillaries compared to the deeper capillaries. Presumably, the drug was mainly absorbed by the microcapillaries near the surface and only partially transported to the deeper plexus. The deviation at high levels, measured for both blood flow data sets [see Fig. 2a], might be caused by the adjustment of the blood flow. Therefore, the time behavior was evaluated using the time points to reach half of the maximal values. In contrast, the time to reach maximal blood flow and the area under the curve were determined in the case of measurements using other flowmeters with a lower sensitivity.15, 24, 25 A slight decrease, especially in the skin temperature and possibly also the redness, was detected directly after topical application [see Fig. 2b]. This effect might be due to isopropyl alcohol, which is a component of the gel. The decreased skin temperature can be explained by the evaporation of isopropyl alcohol from the skin surface, which requires heat. This theory is supported by the results of preliminary experiments on the excised porcine skin model, where a similar decrease in temperature was observed immediately after topical application of the gel (data not shown). In addition, isopropyl alcohol is known to penetrate through the horny layer quickly.26 A slight vasoconstriction could be caused by the alcohol reaching the microcapillaries. A similar blanching effect was observed after topical application of ethyl alcohol.27 The initial decrease in skin temperature and redness might be a reason for the fact that no agreement could be found for both sets of blood flow data with any of the other biological parameters ( , , and , see Tables 1, 2, 3). The efficiency of the insulating barrier function of the horny layer with respect to the thermal internal interaction of the body with the environment might be another reason for this observation. 5.ConclusionA lag in the increase of blood flow at different frequencies after application of nicotinates could be used to confirm not only the assumed correlation between the lower Doppler frequency and the superficial microcapillaries but also the higher Doppler frequencies and the larger capillaries lying in deeper skin layers. A linear proportionality between the blood flow of the superficial microcapillaries and the larger capillaries in deeper skin layers indicates that the drug is transported via the blood. No correlation of the blood flow with either the skin temperature or the redness was observed, which shows that these biological parameters are unsuitable for measuring the kinetics of the microcirculation after topical application of drugs. ReferencesE. Berardesca,
J. L. Leveque,
P. Masson, European Group for Efficacy Measurements on Cosmetics and Other Topical Products (EEMCO Group),
“EEMCO guidance for the measurement of skin microcirculation,”
Skin Pharmacol. Appl. Skin Physiol., 15
(6), 442
–456
(2002). 1422-2868 Google Scholar
M. D. Stern,
“In vivo evaluation of microcirculation by coherent light scattering,”
Nature (London), 254 56
–58
(1975). https://doi.org/10.1038/254056a0 0028-0836 Google Scholar
G. Nilsson,
E. G. Salerud,
T. Strömberg, and
K. Wårdell,
“Laser Doppler perfusion monitoring and imaging,”
Biomedical Photonics Handbook, 15-1
–15-24
(2003) Google Scholar
K. Doerschel and
G. Müller,
“Velocity resolved laser Doppler blood flow measurement in skin,”
Lasermed., 12 163
–171 1996). Google Scholar
E. Witzleb,
“Funktionen des Gefäßsystems,”
Physiologie des Menschen, 505
–572
(1990) Google Scholar
J. E. Wahlberg and
G. Nilsson,
“Skin irritancy from propylene glycol,”
Acta Derm Venereol, 64 286
–290
(1984). 0001-5555 Google Scholar
P. M. Dowd,
M. Whitefield, and
M. W. Greaves,
“Hexyl-nicotinate-induced vasodilation in normal human skin,”
Dermatologica, 174
(5), 239
–243
(1987). 0011-9075 Google Scholar
O. Stuttgen,
A. Ott, and
U. Flesch,
“Measurement of skin temperature,”
Cutaneous Investigation in Health and Disease, 275
–322
(1989) Google Scholar
E. Tur,
“Cutaneous laser Doppler flowmetry in general medicine,”
Bioengineering of the Skin: Cutaneous Blood Flow and Erythema, 133
–153
(1995) Google Scholar
T. B. Fitzpatrick,
“The validity and practicality of sun-reactive skin types I through VI,”
Arch. Dermatol., 124 869
–871
(1988). https://doi.org/10.1001/archderm.124.6.869 0003-987X Google Scholar
H. -J. Weigmann, J. Lademann,
H. Meffert,
H. Schaefer, and
W. Sterry,
“Determination of the horny layer profile by tape stripping in combination with optical spectroscopy in the visible range as a prerequisite to quantify percutaneous absorption,”
Skin Pharmacol. Appl. Skin Physiol., 12 34
–45
(1999). 1422-2868 Google Scholar
A. N. Obeid,
N. J. Barnett,
G. Dougherty, and
G. Ward,
“A critical review of laser Doppler flowmetry,”
J. Med. Eng. Technol., 14 178
–181
(1990). 0309-1902 Google Scholar
L. Bernardi,
M. Rossi,
P. Fratino,
G. Finardi,
E. Mevio, and
C. Orlandi,
“Relationship between changes in human skin blood flow and autonomic tone,”
Microvasc. Res., 37 16
–27
(1989). https://doi.org/10.1016/0026-2862(89)90069-1 0026-2862 Google Scholar
J. Harvell,
I. Hussona-Saeed, and
H. I. Maibach,
“Changes in transepidermal water loss and cutaneous blood flow during the menstrual cycle,”
Contact Dermatitis, 27
(5), 294
–301
(1992). 0105-1873 Google Scholar
E. Oestmann,
A. P. Lavrijsen,
J. Hermans, and
M. Ponec,
“Skin barrier function in healthy volunteers as assessed by transepidermal water loss and vascular response to hexyl nicotinate: intra- and inter-individual variability,”
Br. J. Dermatol., 128
(2), 130
–136
(1993). 0007-0963 Google Scholar
C. J. Gean,
E. Tur,
H. I. Maibach, and
R. H. Guy,
“Cutaneous responses to topical methyl nicotinate in black, oriental, and Caucasian subjects,”
Arch. Dermatol. Res., 281
(2), 95
–98
(1989). 0340-3696 Google Scholar
E. Berardesca and
H. I. Maibach,
“Racial differences in pharmacodynamic response to nicotinates in vivo in human skin: black and white,”
Acta Derm Venereol, 70
(1), 63
–66
(1990). 0001-5555 Google Scholar
F. Kompaore,
J. P. Marty, and
C. Dupont,
“In vivo evaluation of the stratum corneum barrier function in Blacks, Caucasians and Asians with two non invasive methods,”
Skin Pharmacol., 6
(3), 200
–207
(1993). 1011-0283 Google Scholar
H. Tanojo,
E. Boelsma,
H. E. Junginger,
M. Ponec, and
H. E. Bodde,
“In vivo human skin permeability enhancement by oleic acid: a laser Doppler velocimetry study,”
J. Controlled Release, 58
(1), 97
–104
(1999). 0168-3659 Google Scholar
E. Boelsma,
C. Anderson,
A. M. Karlsson, and
M. Ponec,
“Microdialysis technique as a method to study the percutaneous penetration of methyl nicotinate through excised human skin, reconstructed epidermis, and human skin in vivo,”
Pharm. Res., 17
(2), 141
–147
(2000). https://doi.org/10.1023/A:1007505011474 0724-8741 Google Scholar
B. M. Ross,
B. Hughes,
S. Turenne,
M. Seeman, and
J. J. Warsh,
“Reduced vasodilatory response to methylnicotinate in schizophrenia as assessed by laser Doppler flowmetry,”
Eur. Neuropsychopharmacol., 14 191
–197
(2004). 0924-977X Google Scholar
A. Caselli,
T. Hanane,
B. Jane,
S. Carter,
L. Khaodhiar, and
A. Veves,
“Topical methyl nicotinate-induced skin vasodilation in diabetic neuropathy,”
J. Diabetes Complications, 17
(4), 205
–210
(2003). 1056-8727 Google Scholar
A. Steiner,
J. M. Mayer, and
B. Testa,
“Nicotinate esters: their binding to and hydrolysis by human serum albumin,”
J. Pharm. Pharmacol., 44
(9), 745
–749
(1992). 0373-1022 Google Scholar
P. Elsner and
H. I. Maibach,
“Cutaneous responses to topical methyl nicotinate in human forearm and vulvar skin,”
J. Dermatol. Sci., 2
(5), 341
–345
(1991). 0923-1811 Google Scholar
E. Tur,
H. I. Maibach, and
R. H. Guy,
“Percutaneous penetration of methyl nicotinate at three anatomic sites: evidence for an appendageal contribution to transport?,”
Skin Pharmacol., 4
(4), 230
–234
(1991). 1011-0283 Google Scholar
V. Shahi and
J. L. Zatz,
“Effect of formulation factors on penetration of hydrocortisone through mouse skin,”
J. Pharm. Sci., 67
(6), 789
–792
(1978). 0022-3549 Google Scholar
P. H. Yu,
C. Y. Fang, and
L. E. Dyck,
“Cutaneous vasomotor sensitivity to ethanol and acetaldehyde: Subtypes of alcohol-flushing response among Chinese,”
Alcohol Clin. Exp. Res., 14
(6), 932
–936
(1990). 0145-6008 Google Scholar
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||