|
|
1.IntroductionCataract is any opacity in the crystalline lens of the human eye, which leads to reduced light transparency and may cause irreversible loss of vision. Although the problem of cataract-dependent blindness in the industrialized world has been largely solved through surgical treatments, which remove the cloudy and opaque natural lens and implant an intraocular lens (IOL) made from polymer, cataract is still by far the most common cause of blindness worldwide today. The World Health Organization (WHO) estimates the number of cataract operations worldwide to be 12 million cases in the year 2000, and predicts a rise to 32 million cataract operations by 2020.1 The postoperative complication with the highest incidence is posterior capsule opacification (PCO), the so-called secondary cataract. PCO is caused by proliferation and migration of retained lens epithelial cells (LEC) into the optical axis, and leads to a progressive deterioration and disturbances in visual activity (Fig. 1 ). Fig. 1Cataract and secondary cataract-related opacification of the human eye lens. (a) Cataract and (b) posterior capsule opacification (PCO, secondary cataract). 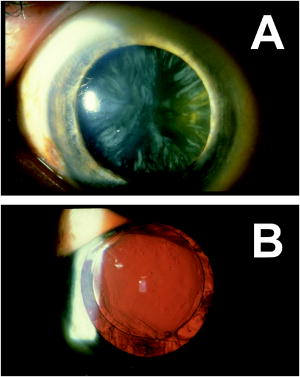 Despite recent progress in cataract surgical procedures and the development of IOLs, which reduce PCO incidence, the formation of secondary cataracts still remains as a most common problem in modern cataract treatment. The incidence of PCO varies, depending on the IOL materials and the age of patients, between 10 to 50% within 3 to 5 years after IOL implantation.2, 3, 4 In particular, in pediatric implantations, the rate of PCO is considerably higher; more or less, the occurrence of PCO is even unavoidable.5, 6, 7, 8, 9 Currently, the only effective treatment of PCO is Nd:YAG laser posterior capsulotomy, where high energy light beams are used to cause photodisruption of the opacified tissue membrane, which results in a significantly improved visual acuity and contrast sensitivity.2 This procedure, however, sometimes lead to serious complications, including damages of the IOL optic,10, 11, 12, 13 increased intraocular pressure,14, 15, 16, 17 or even retinal detachment.18, 19, 20, 21, 22 To overcome these problems, in recent years extensive efforts have been made in IOL design to reduce the mitosis or migration of LEC. Improved IOL designs, 23, 24, 25, 26, 27, 28, 29 materials,30, 31 surface modification,32, 33, 34, 35, 36 or even combinations with a sustained drug delivery system have been explored.37, 38, 39, 40, 41, 42 The rapid progress in drug delivery systems attracted considerable interest during the recent decade. In numerous experimental studies, antimetabolites, immunotoxins, and antiinflammatory agents such as 5-fluorouracil, daunomycin, thapsigargin, indomethacin, and others have been reported to significantly inhibit LEC growth in cell cultures. 41, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 In view of these results, several attempts to prevent PCO using the IOLs themselves as sustained drug delivery devices have been reported.37, 38, 39, 42 However, a major disadvantage of such preprogrammed passive devices is the lack of any dynamic response. Further, the release of any substances affecting the wound healing after cataract surgery is not desirable. A drug-loaded IOL that offers noninvasively triggered drug release would overcome all the current limitations. In this study, we present a novel photocontrolled drug delivery device for PCO treatment, an IOL with a drug chemically attached to the polymer backbone. A challenging demand is that daylight needs to pass through the implanted IOL for years without triggering the drug release. Two-photon absorption (TPA)-induced photocleavage of the linker site is employed to release the immobilized drug, a photochemical event that is extremely unlikely to occur even in bright sunlight, but is easy to obtain by short laser pulses. 2.Concept for Photocontrolled Drug Release Polymers for Intraocular LensesAn IOL material has to meet a wide variety of properties to make it suitable for secondary cataract treatment (Table 1 ). First of all, it should be a fully transparent polymer having an index of refraction high enough to not make the IOLs thicker than they are today for the same refractivity. The material properties should not be significantly different from those materials currently in use, mainly acrylic and silicone polymers. Photochemical triggering of the drug release needs to be noninvasive, an important aspect if ambulant treatments are considered in the future. The implanted IOL should not release any drug until triggered from the outside, which may be years after implantation. Further, a single IOL should carry a sufficient amount of drug to enable more than a single secondary cataract treatment. The challenge is to release the drug in a chemically nonmodified form. The solubility of common drugs in polymers is poor, and in turn, their diffusion constants are quite low. The concept employed should not be restricted to a single chemical compound, but should be suitable for a variety of drugs. Last but not least, the linker system employed needs to have a high TPA coefficient. The cornea effectively blocks UV radiation to reach the implanted photocontrolled drug delivery IOL (PCDD-IOL) but light in the green, at the double wavelength required for the photochemical reaction, passes easily (Fig. 2 ). Due to the geometry of the laser beams employed for TPA-triggered drug release inside a small volume of the PCDD-IOL, the required high intensities are reached. Fig. 2Scheme of two-photon absorption (TPA)-triggered drug release from a polymeric intraocular lens that contains a photocontrolled drug depot (PCDD-IOL). (a) Visible light from natural sources does not reach the intensities inside the PCDD-IOL so that any TPA-dependent process must be considered, exposure to short laser pulses focused in or on the PCDD-IOL pass the intensity thresholds for TPA-dependent photoreactions easily. The UV absorbing cornea blocks UV photons effectively to reach the PCDD-IOL. (b) Visible wavelengths pass the cornea with only few losses and are focused in the PCDD-IOL to obtain the intensities required forTPA-induced release of the drug from the polymer. 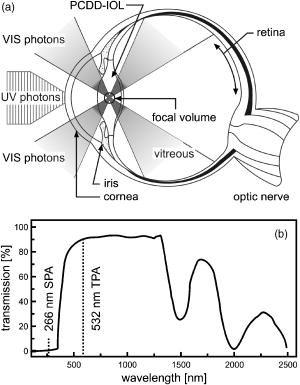 Table 1Figure of merit for intraocular lenses with phototriggered drug release.
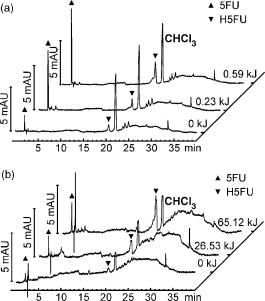
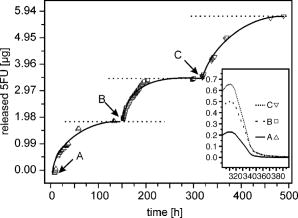
2.1.Functional Building Blocks and Polymer SynthesisFor the photocleavable linker system, which attaches the therapeutic drug 5-fluorouracil (5FU) to the polymer backbone, a coumarin molecule is chosen (Fig. 3 ). The acrylic polymer serves two functions; first it is the IOL material, and second it is the drug reservoir. For this purpose, a copolymer (PC) from a nonmodified monomer and a monomer with a coumarin side group is used. The drug employed is 5FU , which is effective for secondary cataract treatment, but has a very low solubility and diffusion constant in the used PC polymer. To overcome this problem, a side group is attached that improves both parameters. The formed linkage in the formed drug-diffusion modulator complex (DD) is hydrolysable, and upon contact with chamber water the 5FU is released in its original form. In a photochemical reaction, the DD complex is attached through a cycloaddition reaction to the coumarin side group, and a polymer with photocontrolled drug delivery (PCDD) capabilities is obtained. Fig. 3Functional building blocks for photocontrolled drug delivery intraocular lenses (PCDD-IOL). The polymer backbone is a copolymer (PC) obtained from a polymer monomer and a polymer monomer with a photosensitive linker molecule attached at a molar ratio of about 9:1. The drug complex (DD) comprises the drug molecule and an optional diffusion modulator molecule , which is attached through an easily hydrolysable linkage. is required, as the solubility and diffusion constant of the nonmodified drug in the polymer is often very poor. The photocontrolled drug delivery polymer (PCDD) is finally obtained by coupling the drug complex (DD) to the copolymer (PC) by means of a photocleavable linkage. 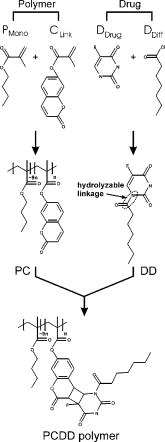 The photoreversibility of cyclobutane systems is well known. 53, 54, 55, 56, 57, 58 UV photons as a trigger cannot be employed here due to the strong UV absorption of the cornea, which limits the penetration depth of the light. We employ a TPA-induced process that enables a cleavage of the UV-active linkage behind the cornea as a photochemical barrier. The required intensities are achieved by focusing a pulsed laser system to a spot inside the PCDD material. Since the probability for a TPA-dependent reaction is relatively low, the process requires considerably high photon densities. This is because the device is not affected by ordinary light.59, 60, 61, 62 Compared with conventional sustained-release intraocular drug delivery devices based on biodegradable polymers, this novel photomodulated delivery device offers temporal and spatial control over the release of the drug. The required or desired drug levels can be easily controlled by dosing the irradiation energy. The material properties should be equivalent or superior to polymethylmethacrylate (PMMA), which is currently one of the standard materials for IOL manufacturing. As a first example, we have chosen n-butylmethacrylate (NBMA) as a polymer backbone, which provides the unique mechanical and optical properties of the acrylic polymers. The fabrication of the device consists of three different steps, as sketched in Fig. 2. The copolymer matrix bearing photoreactive moieties was obtained by copolymerization of NBMA and monomeric methylmethacrylate modified with coumarin . The coumarin side group acts as a molecular linker due to its capability for reversible photocyclizations/photoreversions in dependence on the wavelengths of light used.63, 64, 65 We have shown that coumarin photodimers used in these experiments can be cleaved by two-photon absorption.66 The model drug, 5-fluorouracil (5FU), was modified with heptanoic acid resulting in the drug precursor (DD), 1-heptanoyl-5-fluorouracil (H5FU). While possibilities of direct photochemical crossdimerization of coumarin derivatives and 5FU were reported in several studies,67, 68, 69 the utilization of alkylated 5FU provides some advantages such as improved solubility, miscibility, and diffusion properties in organic solvents and hydrophobic polymers, respectively. The diffusion modulator is attached to the drug by a hydrolysis-labile ester bond that is cleaved on exposure to water at the target site. Finally, the polymer-drug conjugate (PCDD) was obtained using photochemical cycloaddion reaction. Analysis by UV/VIS spectroscopy shows that the typical absorption band of coumarin at corresponding to the double bond besides the carbonyl group,70 is missing in the absorption spectrum of the obtained polymer-drug conjugate. This indicates a complete cyclodimerization of all coumarin moieties to -photoproducts. In the spectrum, no absorbance in the visible region is observed, which guarantees the excellent transparency of the polymer for its use in IOLs. Because the temperature at which 5% of the polymer decomposed is much higher than the glass-transition temperature , it is possible to fabricate IOLs via compression molding. The high value of guarantees that the IOL is stable after implantation. With the data from elemental analysis, we can calculate a drug loading in the polymer of about 6% (wt/wt). 2.2.Phototriggered Drug ReleaseBoth single-photon absorption (SPA) and TPA-induced processes were investigated. Polymer films for testing were fabricated from a blend of the PCDD polymer-drug conjugate and PMMA (for SPA 3:1 wt/wt and for TPA 1:1 wt/wt, respectively) by the solvent casting technique. During the irradiation of the obtained films with UV light, the absorption band with a maximum at , which corresponds to the coumarin moieties, increased [Fig. 4a ], which indicates the cleavage of the cyclobutane ring and the related drug release. TPA-induced photocleavage of the PCDD material by intense pulses is observed at much higher energies. The spectral changes during the course of irradiation with an average pulse intensity of /pulse are shown in Fig. 4b. The observed spectral changes of SPA- and TPA-induced processes are similar. Fig. 4Single-photon and two-photon absorption-dependent drug release from PCDD-IOL material. Difference absorption spectra of a PBMAOCH5FU film during photocontrolled drug release. (a) Single-photon absorption: UV light is used to trigger release. (b) Two-photon absorption: a frequency-doubled Nd:YAG-laser ( , /pulse, ) is employed. (c) The initial photocleavage rate is plotted as a function of the incident intensity at . A slope of about 2 is derived, which corresponds to a two-photon reaction. 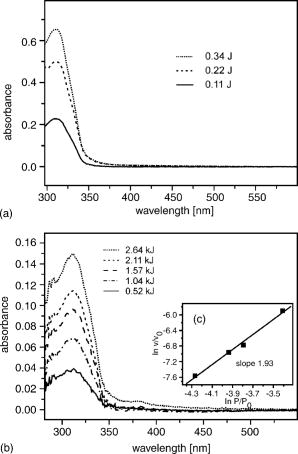 The dependence of the initial photocleavage rate on the incident intensity was determined to confirm the TPA nature of the process induced by the pulses. The initial rates of photocleavage in the model IOL were derived from the changes in the absorption at , and the incident intensities range from /pulse at a repetition rate of were employed. The experimentally obtained slope of 1.93 in the log-log plot, shown in Fig. 4c, corresponds nicely to the theoretically expected value of 2,59, 61, 62 and indicates that the cleavage of the PCDD conjugate is solely induced by TPA. We analyzed the identity and quantity of the delivered H5FU by HPLC analysis (Fig. 5 ). From solutions of PCDD in chloroform, samples were analyzed before and after irradiation with the energies given. In both cases, the obtained HPLC profiles are very similar and show only two products, which are H5FU and its hydrolysis product 5FU, released during photocleavage without any side products. The total amount of delivered H5FU and 5FU, respectively, correlates exactly with the absorption changes at measured by UV/VIS spectroscopy. 2.3.Multidose Drug DeliverySecondary cataract is observed many months even years after IOL implantation. Even after treatment, secondary cataract may reappear. Due to this, a multidose capability of the PCDD IOL is desired. To demonstrate an external triggered repeated drug release, the model IOL was irradiated in a stepwise manner, and the release pattern of 5FU was monitored using UV/VIS spectroscopy. The result depicted in Fig. 6 shows the cumulative amount of the discharged 5FU after three consecutive steps of irradiation with a constant energy dose of . During each irradiation step, approximately of 5FU were released from approximately PCDD material. The amount of released drug was strictly proportional to the applied energy dose. This was also confirmed by HPLC analysis. In view of the low volume inside the capsular back, the LD50 of 5-fluorouracil, which was reported for rabbit lens epithelial cells (RLEC) to be ,71 can easily be obtained. 2.4.In Vitro TestIn in-vitro cell tests, IOLs were tested to show that the drug-loaded material itself is nontoxic to the cells, but as soon as it is photochemically activated, the drug release is triggered and the cell count is reduced significantly. PCDD polymer disks of radii with a thickness of carrying approximately of 5FU each were used for the tests. Such samples were incubated with pancreatic carcinoid cells (line Bon 1) and the proliferation of the cells was analyzed (Fig. 7 ). Fig. 7Cell tests. Proliferation of (a) control cells and (b) cells treated with the activated IOL, both after in culture. The treated cells show a significantly lower cell density. 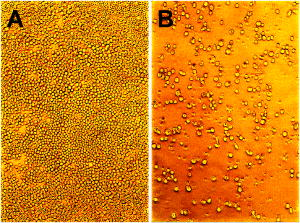 The data obtained clearly indicate that IOLs that were not photochemically activated have no significant influence on the cell proliferation. However, photoactivated IOLs cause a significant reduction of 29% of the cell mass compared to an untreated control group (Table 2 ). It should be mentioned that the in-vitro tests are for screening purposes, as in-vivo lens epithelial cells may show a different sensitivity toward 5FU. Table 2Cell test: The protein content after 7days was analyzed with the RC-DC Protein Assay. The statistic correlation performed with a Turkey multicomparison test shows a significant difference between nonactived and activated PCDD-IOLs (p less than 0.001).
2.5.Summary and ConclusionWe have developed a photocontrolled multidose drug delivery device that may be applied in the form and having the function of an IOL. This combination we named PCDD-IOL. The drug delivery is photochemically triggered by TPA, which enables ambulant treatment of readily available laser sources. The multidose capability of the PCDD-IOLs was demonstrated in vitro. Cell tests confirmed that the PCDD-IOL material itself shows not cytotoxicity until photochemical stimulation. Such materials will be tested in rabbits in the near future. The presented materials and concept are a new improved route in secondary cataract treatment. 3.MethodsChemicals were purchased from Fluka (Taufkirchen, Germany) (5-fluorouracil HPLC grade), Acros (Nidderau, Germany) (heptanoyl chloride 99%, hydroxycoumarin 99%), Lancaster (Frankfurt, Germany) (methacryloyl chloride 97%), Riedel de Haen (triethylamine purum), Fisher Scientific (Nidderau, Germany) (acetonitrile HPLC grade), Aldrich (Taufkirchen, Germany) (butyl methacrylate 99%), and Merck (Darmstadt, Germany) (silica gel 60), and used as received. THF was dried over sodium and stored under argon until used. Azobisisobutyronitrile (AIBN) (BASF, Ludwigshafen, Germany) was recrystalized from ethanol. 3.1.Syntheses3.1.1.1-Heptanoyl-5-fluorouracil (H5FU)5-fluorouracil (5FU) and potassiumhydroxide (KOH) were dissolved in methanol and stirred at room temperature (RT). After , the methanol was removed in vacuum and the residue was suspended in dry acetonitrile. heptanoylchloride was added at to the solution. After the addition, the reaction was warmed to RT and stirred for a further . The solvent was removed and the crude product was dried under vacuum. Extraction with ethylacetate returned the H5FU product (yield , 75%).72 3.1.2.7-Methacryloyloxycoumarin (MAOC)To a solution of 7-hydroxycoumarin, triethylamine and dry tetrahydrofuran (THF), methacryloylchloride was added under argon gas and heated for . After cooling to RT, the solution was stirred for another . The crude product was purified by flash chromatography on silica gel using (40:1) as an eluent. MAOC was eluted at . An amount of MAOC was obtained (yield 65%).73 3.1.3.Poly(n-butylmethacrylate-co-7-methacryloyloxycoumarin) (PBMAOC)n-butylmethacrylate, MAOC, and azobisisobutyronitrile (AIBN) were dissolved in dry THF and reacted for at . After precipitating the crude product twice in methanol, PBMAOC was collected (yield 70%).73 3.1.4.H5FU loaded polymer (PBMAOCH5FU)In a mixture of chloroform-acetone (1:2 v/v) PBMAOC, H5FU and benzophenone were dissolved and irradiated in a Rayonet-type photoreactor (12 Eversun , Osram, Munich, Germany, concentrically installed) under continuous stirring for . Precipitating the crude product twice in methanol returned of the PBMAOCH5FU polymer (yield 91%).74 3.2.Preparation of Polymer FilmsPolymers or polymer blends were dissolved in chloroform to the highest concentration possible by stirring overnight. The solutions were filtered through Teflon filters (P819.1, Roth, Karlsruhe, Germany). A few milliliters of the filtered chloroform solution were pipetted on a glass plate. By means of a coating knife, a wet film of 0.4 or thickness was obtained ( casting). After solvent evaporation (typically for ), a polymer film was obtained. Samples were prepared by either punching out or cutting out the desired pieces from the prepared polymer films. 3.3.Phototriggered Drug Release: Single-Photon AbsorptionSingle-photon absorption (SPA)-induced drug release was analyzed in solutions. Samples were prepared by dissolving of PBMAOCH5FU in . The solution was through a Teflon filter to remove any scattering particles. Excitation light of wavelengths from a fluorescence spectrometer (RF-1502, Shimadzu, Duisberg, Germany) was used to induce SPA-dependent drug release in the test solutions directly in the spectrometer, where the resulting absorption spectra were recorded. The test samples were continuously stirred (Telemodul 20P and mini, Labortechnik, Oberschleißheim, Germany), but during recording of the absorption spectra, the stirring was switched off. The intensity of the light in the fluorescence spectrometer was . Polymer films for SPA-induced drug release were made from a mixture of PBMAOCH5FU and PMMA as described before. A wet thickness of was used. Light of wavelength (MinUVIS, Desaga, Heidelburg, Germany, ) was used to induce the drug release by light-induced cycloreversion of the coumarin linker system. 3.4.Phototriggered Drug Release: Two-Photon AbsorptionSolutions for two-photon absorption (TPA)-induced drug release tests were prepared by dissolving PBMAOCH5FU in . The solutions were filtered through Teflon filters before use. An Infinity 40-100 mode-locked Nd:YAG laser (Coherent, Rödermark, Germany) emitting pulses at at a repetition rate of was used to excite the samples. The pulse energy was and the beam diameter was . Polymer films for TPA-induced drug release were made from PBMAOCH5FU and PMMA (1:1 wt/wt) blends. A wet thickness of was used. To induce the cycloreversion of the coumarin system via two photon absorption, the Infinity 40-100 system described earlier was used. Pulse energies ranging from /pulse to /pulse were used. 3.5.Quantitative Analysis of Released DrugHPLC analysis of the released drug was done on a Hewlett-Packard Model 1050 system equipped with a diode array detector. The trace was used for quantitative determinations. A reversed phase column (Nucleosil, , RP18, , Bischoff, Leonberg, Germany) equipped with a precolumn ( , Bischoff) was employed for trapping the polymer content. A water / acetonitrile gradient was used. 3.6.Multistep Drug ReleaseA polymer film comprising PBMAOCH5FU and PMMA at a ratio of 1:3 (wt/wt) was prepared from a chloroform solution as described earlier. A suitably cut piece of the polymer film was mounted on one side of the inner walls of a fluorescence cuvette (101 QS, Hellma, Müllheim/Baden). The cuvette was filled with of water. The film was irradiated with light from a MinUVIS (Desaga, ) until the desired energies were reached. After light-dependent release of 5FU from the polymer matrix, the drug diffuses from the polymer film into the aqueous solution. The UV/VIS spectra of the aqueous solution were recorded in an UVIKON 922 (Kontron, Munich, Germany) spectrophotometer. Accompanying HPLC analyses of small samples taken from the cuvettes were done as described before. 3.7.Cell TestPolymer films for cell tests were prepared from 1:3 (wt/wt) mixtures of PBMAOCH5FU and PMMA dissolved in chloroform. A coating knife was used to prepare the films as described earlier. Test disks having diam and a thickness of were punched out of the polymer foil and used for the cell tests. The drug release was triggered by light with wavelength having a total energy of . 2000 Bon 1 cells in Dulbecco's modified Eagle's medium and Ham's F-12 medium containing 10% fetal calf serum (PAA, Cölbe, Germany) and 1% penicillin/streptomycin were seeded in 24 well plates (Greiner Bio-One, Frickenhausen, Germany). The polymer disks were added after in a way that any contact of the polymer disks with the Bon cells was omitted. No polymer disks were added to the control samples. The cell cultures were incubated at in a saturated atmosphere containing 5% (HS incubation cabin, Heraeus, Hanau, Germany). The cells were observed for . Then the cells were washed three times with phosphate buffered saline (PBS). Then, 3-(N-Morpholino) propanesulfonic acid containing 0.1% Triton-X-100 was added to each well to homogenize the cells. of the suspension was analyzed with the RC-DC Protein Assay (Bio-Rad, Hercules, California) to determine the protein content following a modified Lowry method.75 The protein content correlates to the total cell count. The statistic correlation was performed with a Turkey multicomparison test. AcknowledgmentsWe thank L. Hesse, Eye Clinic Heilbronn, for supplying the cataract photos (Fig. 1). The advice of A. Greiner, Chemistry University of Marburg, on polymer synthesis is gratefully acknowledged. This work was financially supported through BMBF grant 13N8978. ReferencesA. Foster,
“Cataract and vision 2020—the right to sight initiative,”
Br. J. Ophthamol., 85 635
–637
(2001). 0007-1161 Google Scholar
D. J. Apple,
K. D. Solomon,
M. R. Tetz,
E. I. Assia,
E. Y. Holland,
U. F. Legler,
J. C. Tsai,
V. E. Casteneda,
J. P. Hoggatt, and
A. M. Kosick,
“Posterior capsule opacification,”
Surv. Ophthalmol., 37 73
–116
(1992). 0039-6257 Google Scholar
D. A. Schaumberg,
M. R. Dana,
W. G. Christen, and
R. J. Glynn,
“A systematic overview of the incidence of posterior capsule opacification,”
Ophthalmology, 105 1213
–1221
(1998). 0161-6420 Google Scholar
D. J. Apple,
N. Mamalis,
K. Loftfield,
J. M. Googe,
L. C. Novak,
D. Kavka-Van Norman,
S. E. Brandy, and
R. J. Olson,
“Complications of intraocular lenses. A historical and histopathological review,”
Surv. Ophthalmol., 29 1
–54
(1984). 0039-6257 Google Scholar
M. E. Wilson,
“Intraocular lens implantation: Has it become the standard for care for children?,”
Ophthalmology, 103 1719
–1720
(1996). 0161-6420 Google Scholar
S. K. Pandey,
M. E. Wilson,
R. H. Trivedi,
A. M. Izak,
T. A. Macky,
L. Werner, and
D. J. Apple,
“Pediatric cataract surgery and intraocular lens implantation: Current techniques, complications and management,”
Int. Ophthalmol. Clin., 41 175
–196
(2001). 0020-8167 Google Scholar
T. Kohnen,
R. Pena-Cuesta, and
D. D. Koch,
“Secondary cataract formation following pediatric intraocular lens implantation: results,”
Ger. J. Ophthalmol., 5 171
–175
(1996). 0941-2921 Google Scholar
J. Zwaan,
P. B. Mullaney,
A. Awad,
S. al-Mesfer, and
D. T. Wheeler,
“Pediatric intraocular lens implantation. Surgical results and complications in more than 300 patients,”
Ophthalmology, 105 112
–118
(1998). 0161-6420 Google Scholar
M. E. Wilson,
D. J. Apple,
E. C. Bluestein, and
X. H. Wang,
“Intraocular lenses for pediatric implantation: biomaterials, designs, and sizing,”
J. Cataract Refractive Surg., 20 584
–591
(1994). 0886-3350 Google Scholar
P. E. Bath,
K. J. Hoffer,
D. Aron-Rosa, and
Y. Dang,
“Glare disability secondary to YAG laser intraocular lens damage,”
J. Cataract Refractive Surg., 13 309
–313
(1987). 0886-3350 Google Scholar
B. Dick,
O. Schwenn, and
N. Pfeiffer,
“Schadensausmaß bei verschiedenen Intraokularlinsen durch die Neodymium: YAG-Laser Behandlung—Eine experimentelle Studie,”
Klin. Monatsbl. Augenheilkd., 211 263
–271
(1997). 0023-2165 Google Scholar
H. M. Clayman,
F. G. Karrenberg, and
J. M. Parel,
“Intraocular lens damage from the neodymium-YAG laser,”
Ann. Ophthalmol., 16 551
–556
(1984). 0003-4886 Google Scholar
T. J. Newland,
M. L. McDermott,
D. Eliott,
L. D. Hazlett,
D. J. Apple,
R. J. Lambert, and
P. R. Barrett,
“Experimental neodymium: YAG laser damage to acrylic, poly (methyl methacrylate), and silicone intraocular lenses,”
J. Cataract Refractive Surg., 25 72
–76
(1999). 0886-3350 Google Scholar
C. E. Jahn and
M. Emke,
“Long-term elevation of intraocular pressure after neodymium: YAG laser posterior capsulotomy,”
Ophthalmologica, 210 85
–89
(1996). 0030-3755 Google Scholar
M. C. Kraff,
D. R. Sanders, and
H. L. Lieberman,
“Intraocular pressure and the corneal endothelium after neodymium-YAG laser posterior capsulotomy. Relative effects of aphakia and pseudophakia,”
Arch. Ophthalmol. (Chicago), 103 511
–514
(1985). 0003-9950 Google Scholar
S. Fourman and
J. Apison,
“Late-onset elevation in intraocular pressure after Neodymium-YAG laser posterior capsulotomy,”
Arch. Ophthalmol. (Chicago), 109 511
–513
(1991). 0003-9950 Google Scholar
R. K. Parrish and
A. Somovic,
“Myopia and unexpected intraocular pressure elevations after neodymium-YAG capsulotomy,”
Arch. Ophthalmol. (Chicago), 103 1277
–1278
(1985). 0003-9950 Google Scholar
D. M. Fastenberg,
P. L. Schwartz, and
H. Z. Lin,
“Retinal detachment following neodymium-YAG laser capsulotomy,”
Am. J. Ophthalmol., 97 288
–291
(1984). 0002-9394 Google Scholar
S. R. Leff,
J. C. Welch, and
W. Tasman,
“Rhegmatogenous retinal detachment after YAG laser posterior capsulotomy,”
Ophthalmology, 94 1222
–1225
(1987). 0161-6420 Google Scholar
L. Rickman-Barger,
C. W. Florine,
R. S. Larson, and
R. L. Lindstrom,
“Retinal detachment after neodymium:YAG laser posterior capsulotomy,”
Am. J. Ophthalmol., 107 531
–536
(1989). 0002-9394 Google Scholar
R. F. Steinert,
C. A. Puliafito,
S. R. Kumar,
S. D. Dudak, and
S. Patel,
“Cystoid macular edema, retinal detachment, and glaucoma after Nd:YAG laser posterior capsulotomy,”
Am. J. Ophthalmol., 112 373
–380
(1991). 0002-9394 Google Scholar
M. U. Dardenne,
G. J. Gerten,
K. Kokkas, and
O. Kermani,
“Retrospective study of retinal detachment following neodymium: YAG laser posterior capsulotomy,”
J. Cataract Refractive Surg., 15 676
–680
(1989). 0886-3350 Google Scholar
C. P. Born and
D. K. Ryan,
“Effect of intraocular lens optic design on posterior capsular opacification,”
J. Cataract Refractive Surg., 16 188
–192
(1990). 0886-3350 Google Scholar
J. E. Downing,
“Long-term discission rate after placing posterior chamber lenses with convex surface posterior,”
J. Cataract Refractive Surg., 12 651
–654
(1986). 0886-3350 Google Scholar
N. Mamalis,
A. S. Crandall,
E. Linebarger,
W. K. Sheffield, and
M. J. Leidenix,
“Effect of intraocular lens size on posterior capsule opacification after phacoemulsification,”
J. Cataract Refractive Surg., 21 99
–102
(1995). 0886-3350 Google Scholar
O. Nishi,
K. Nishi, and
K. Sakanishi,
“Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens,”
Ophthalmic Surg. Lasers, 29 587
–594
(1998). 1082-3069 Google Scholar
Q. Peng,
N. Visessook,
D. J. Apple,
S. K. Pandey,
L. Werner,
M. Escobar-Gomez,
R. Schoderbek,
K. D. Solomon, and
A. Guindi,
“Surgical prevention of posterior capsule opacification Part 3: Intraocular lens optic barrier effect as a second line of defense,”
J. Cataract Refractive Surg., 26 198
–213
(2000). 0886-3350 Google Scholar
R. G. Martin,
D. R. Sanders,
J. Souchek,
M. G. Raaman, and
M. DeLuca,
“Effect of posterior chamber intraocular lens design and surgical placement on postoperative outcome,”
J. Cataract Refractive Surg., 18 333
–341
(1992). 0886-3350 Google Scholar
T. Nagamoto and
G. Eguchi,
“Effect of intraocular lens design on migration of lens epithelial cells onto the posterior capsule,”
J. Cataract Refractive Surg., 23 866
–872
(1997). 0886-3350 Google Scholar
P. G. Ursell,
D. J. Spalton,
M. V. Pande,
E. J. Hollick,
S. Barman,
J. Boyce, and
K. Tilling,
“Relationship between intraocular lens biomaterials and posterior capsule opacification,”
J. Cataract Refractive Surg., 24 352
–360
(1998). 0886-3350 Google Scholar
H. Hayashi,
K. Hayashi,
F. Nakao, and
F. Hayashi,
“Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and acrylic intraocular lens implantation,”
Arch. Ophthalmol. (Chicago), 116 1579
–1582
(1998). 0003-9950 Google Scholar
T. A. Wesendahl,
G. U. Auffarth,
S. Brown, and
D. J. Apple,
“Textur von IOL-oberflächen: Ein neues konzept zur nachstarprevention,”
Ophthalmologe, 90 140
(1993). 0941-293X Google Scholar
J. Ygge,
M. Wenzel,
B. Philipson, and
P. Fagerholm,
“Cellular reactions on heparin surface-modified versus regular PMMA lenses during the first postoperative month. A double masked and randomized study using specular microphotography,”
Ophthalmology, 97 1216
–1223
(1990). 0161-6420 Google Scholar
Y. Tamada and
Y. Ikada,
“Fibroblast growth on polymer surface and biosynthesis of collagen,”
J. Biomed. Mater. Res., 28 783
–789
(1994). 0021-9304 Google Scholar
S. Umezawa and
K. Shimizu,
“Biocompatibility of surface-modified intraocular lenses,”
J. Cataract Refractive Surg., 19 371
–374
(1993). 0886-3350 Google Scholar
L. Hesse,
L. Freisberg,
H. Bienert,
H. Richter,
C. Kreiner, and
C. Mittermayer,
“Reduction of cataract by plasma etching of intraocular lenses. An animal experiment study,”
Ophthalmologe, 94 821
–825
(1997). 0941-293X Google Scholar
M. R. Tetz,
M. W. Ries,
C. Lucas,
H. Stricker, and
H. E. Volcker,
“Inhibition of posterior capsule opacification by an intraocular-lens-bound sustained drug delivery system: an experimental animal study and literature review,”
J. Cataract Refractive Surg., 22 1070
–1078
(1996). 0886-3350 Google Scholar
O. Nishi,
K. Nishi,
I. Saitoh, and
K. Sakanishi,
“Inhibition of migrating lens epithelial cells by sustained release of ethylenediaminetetraacetic acid,”
J. Cataract Refractive Surg., 22 863
–868
(1996). 0886-3350 Google Scholar
O. Nishi,
K. Nishi,
T. Morita,
Y. Tada,
E. Shirasawa, and
K. Sakanishi,
“Effect of intraocular sustained release of indomethacin on postoperative inflammation and posterior capsule opacification,”
J. Cataract Refractive Surg., 22 806
–810
(1996). 0886-3350 Google Scholar
P. Chollet,
F. Malecaze,
P. Le Toan,
H. Lamche, and
J. L. Arne,
“Annexin V-coated intraocular lenses,”
J. Cataract Refractive Surg., 22 818
–824
(1996). 0886-3350 Google Scholar
G. Duncan,
I. M. Wormstone,
C. S. Liu,
J. M. Marcantonio, and
P. D. Davies,
“Thapsigargin-coated intraocular lenses inhibit human lens cells growth,”
Nat. Med. (N.Y.), 3 1026
–1028
(1997). 1078-8956 Google Scholar
O. Nishi,
K. Nishi,
Y. Yamada, and
Y. Mizumoto,
“Effect of indomethacin-coated posterior chamber intraocular lenses on postoperative inflammation and posterior capsule opacification,”
J. Cataract Refractive Surg., 21 574
–578
(1995). 0886-3350 Google Scholar
M. M. Ismail,
J. L. Alio, and
J. M. Ruiz Moreno,
“Prevention of secondary cataract by antimiotic drugs: Experimental study,”
Ophthalmic Res., 28 64
–69
(1996). 0030-3747 Google Scholar
J. M. Ruiz,
M. Medrano, and
J. L. Alio,
“Inhibition of posterior capsule opacification by 5-fluorouracil in rabbits,”
Ophthalmic Res., 22 201
–208
(1990). 0030-3747 Google Scholar
P. J. McDonnel,
W. Krause, and
B. M. Glaser,
“In vitro inhibition of lens epithelial cell proliferation and migration,”
Ophthalmic Surg., 19 25
–30
(1988). 0022-023X Google Scholar
W. J. Power,
D. Neylan, and
L. M. Collum,
“Daunomycin as an inhibitor of human lens epithelial cell proliferation in culture,”
J. Cataract Refractive Surg., 20 287
–290
(1994). 0886-3350 Google Scholar
M. Weller,
P. Wiedemann,
R. Fischbach,
C. Hartmann, and
K. Heimann,
“Evaluation of daunomycin toxicity on lens epithelium in vitro,”
Int. Ophthalmol., 12 127
–130
(1988). 0165-5701 Google Scholar
P. Sourdile and
Y. Ducournau,
“Effect of daunomycin on epithelial cells of the crystalline lens,”
Ophthalmologe, 4 107
–108
(1990). 0941-293X Google Scholar
C. Hartmann,
P. Wiedemann,
K. Gothe,
M. Weller, and
K. Heimann,
“Prevention of secondary cataract by intracapsular administration of the antimitotic daunomycin,”
Ophthalmologe, 4 102
–106
(1990). 0941-293X Google Scholar
O. Nishi,
K. Nishi,
T. Fujiwara, and
E. Shirasawa,
“Effects of diclofenac sodium and indomethacin on proliferation and collagen synthesis of lens epithelial cells in vitro,”
J. Cataract Refractive Surg., 21 461
–465
(1995). 0886-3350 Google Scholar
C. M. Hans and
A. L. Galand,
“Mitomycin against posterior capsular opacification: an experimental study in rabbits,”
Br. J. Ophthamol., 80 1087
–1091
(1996). 0007-1161 Google Scholar
U. U. Inan,
F. Ozturk,
S. Kaynak,
E. Kurt,
L. Emiroglu,
E. Ozer,
S. S. Ilker, and
C. Guler,
“Prevention of posterior capsule opacification by intraoperative single-dose pharmacologic agents,”
J. Cataract Refractive Surg., 27 1079
–1087
(2001). 0886-3350 Google Scholar
C. H. Krauch,
S. Farid, and
G. O. Schenck,
“Photo--cyclodimerisation von cumarin,”
Chem. Ber., 99 625
–633
(1966). 0009-2940 Google Scholar
N. Yonezawa,
T. Yoshida, and
M. Hasegawa,
“Symmetric and asymmetric photocleavage of cyclobutane rings in head-to-head coumarin dimers and their lactone-opened derivatives,”
J. Chem. Soc., Perkin Trans. 1, 1 1083
–1086
(1983). 0300-922X Google Scholar
N. K. Mal,
M. Fujiwara, and
Y. Tanaka,
“Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica,”
Nature (London), 421 350
–353
(2003). https://doi.org/10.1038/nature01362 0028-0836 Google Scholar
M. Mustafa,
“Dimerization reaction in sunlight,”
Chem. Rev. (Washington, D.C.), 51 1
–23
(1951). https://doi.org/10.1021/cr60158a001 0009-2665 Google Scholar
A. Lendlein,
H. Jiang,
O. Jünger, and
R. Langer,
“Light-induced shape-memory polymers,”
Nature (London), 434 879
–882
(2005). https://doi.org/10.1038/nature03496 0028-0836 Google Scholar
M. Zharnikov,
Y. Ouchi,
M. Hasegawa, and
A. Scholl,
“X-ray absorption spectromicroscopy study of UV-photoinduced surface modification and anisotropy in polyimide films,”
J. Phys. Chem. B, 108 859
–863
(2004). 1089-5647 Google Scholar
M. Göppert-Mayer,
“Elementary processes with two quantum jumps,”
Ann. Phys., 9 273
–294
(1931). 0003-3804 Google Scholar
M. J. Wirth and
H. O. Fatunmbi,
“Very high detectability in two-photon spectroscopy,”
Anal. Chem., 62 973
–976
(1990). https://doi.org/10.1021/ac00208a015 0003-2700 Google Scholar
W. Kaiser and
B. G. C. Garret,
“Two-photon excitation in ,”
Phys. Rev. Lett., 7 229
–231
(1961). https://doi.org/10.1103/PhysRevLett.7.229 0031-9007 Google Scholar
J. D. Bhawalkar,
G. S. He, and
P. N. Prasad,
“Nonlinear multiphoton processes in organic and polymeric materials,”
Rep. Prog. Phys., 59 1041
–1070
(1996). https://doi.org/10.1088/0034-4885/59/9/001 0034-4885 Google Scholar
Y. Chen and
C. F. Cho,
“Reversible photodimerization of coumarin derivatives dispersed in poly(vinly acetate),”
J. Polym. Sci., Part A: Polym. Chem., 33 2705
–2714
(1995). 0887-624X Google Scholar
Y. Chen and
J. D. Wu,
“Preparation and photoreaction of copolymers derived from N-(1-phenylenthyl) acrylamide and 7-Acrzloyloxy-4-methyl coumarin,”
J. Polym. Sci., Part A: Polym. Chem., 32 1867
–1875
(1994). 0887-624X Google Scholar
Y. Chen and
K. H. Chen,
“Synthesis and reversible photocleavage of novel polyurethanes containing coumrain dimmer components,”
J. Polym. Sci., Part A: Polym. Chem., 35 613
–624
(1997). 0887-624X Google Scholar
H. C. Kim,
S. Kreiling,
A. Greiner, and
N. Hampp,
“Two-photon-induced cycloreversion of coumarin photodimers,”
Chem. Phys. Lett., 372 899
–903
(2003). 0009-2614 Google Scholar
K. Mori,
O. Murai,
S. Hashimoto, and
Y. Nakamura,
“Highly region- and stereoselective photocycloaddition between coumarin and thymine by molecular recognition,”
Tetrahedron Lett., 37 8523
–8526
(1996). 0040-4039 Google Scholar
S. C. Shim,
C. S. Ra, and
K. H. Chae,
“Photocycloaddition of 5, 7-dimethoxycoumarin to 5-fluorouracil.,”
Bull. Korean Chem. Soc., 1 121
–123
(1980). 0253-2964 Google Scholar
P. N. Confalone and
D. L. Confalone,
“The design and synthesis of monofunctional psoralens structurally related to methoxslen and trioxalen,”
Tetrahedron, 39 1265
–1271
(1983). 0040-4020 Google Scholar
J. S. Seixax de Melo,
R. S. Becker, and
A. L. Macanita,
“Photophysical behaviour of coumarins as a function of substitution and solvent: Experimental evidence for the existence of a lowest lying state,”
J. Phys. Chem., 98 6054
–6058
(1994). https://doi.org/10.1021/j100075a002 0022-3654 Google Scholar
X. Su,
S. Li, and
J. Zheng,
“Inhibition of rabbit lens epithelial cell proliferation,”
Zhonghua Yan Ke Za Zhi, 32 339
–341
(1996). Google Scholar
P. Jolimaitre,
C. André-Barres,
M. Malet-Martino,
R. Martino, and
I. Rico-Lattes,
“A new method for the synthesis of 5-fluorouracil prodrugs,”
Synlett, 11 1829
–1831
(1999). Google Scholar
J. S. Chung,
H. S. Kim, and
K. H. Chae,
“Preparation of photo-crosslinkable polymers having coumrain side groups and their properties,”
Korea Polym. J., 3 12
–18
(1995). Google Scholar
H.-C. Kim,
S. Kreiling,
S. Härtner,
L. Hesse,
A. Greiner, and
N. Hampp,
“Two-photon absorption induced drug delivery from polymers for medical applications,”
Proc. SPIE, 5323 327
–334
(2004). 0277-786X Google Scholar
O. H. Lowry,
N. J. Rosebrough,
A. L. Farr, and
R. J. Randall,
“Protein measurement with the Folin phenol reagent,”
J. Biol. Chem., 193 264
–275
(1951). 0021-9258 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||