|
|
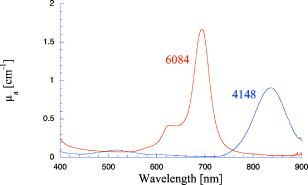
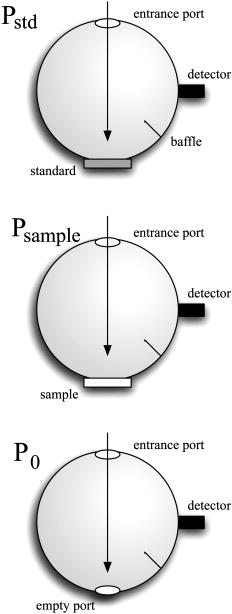
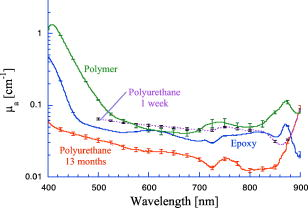
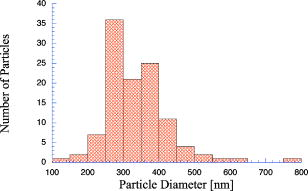
1.IntroductionPhantoms that simulate the optical characteristics of tissues are commonly used to mimic light distributions in living tissue. Tissue phantoms are often designed and utilized for three purposes: to simulate light distributions with a geometry of physical tissue,1 for the calibration of optical devices,2, 3 and for recording a reference measurement with an optical measurement device.4 For all three uses, the absorption and scattering properties of the created tissue phantom are the primary design factor. Optical tissue phantoms are necessary to calibrate steady-state optical measurements on real tissues to establish quantitative information.3 Optical device calibration requires using several phantoms with the optical properties that span the typical range of the tissue to be simulated. Furthermore, phantoms that are optically stable are also suitable for use as a reference measurement taken in conjunction with measurements on living tissues. Several types of phantoms to mimic the optics of human tissue have been described in the literature, including homogenized milk,5 nondairy creamer,6 wax,7 and a blood and yeast suspension.8 Suspensions of oils/fats in an aqueous solution, such as Intralipid9 and a water soluble dye (India ink10), appear to be the most commonly used phantom materials. Difficulties arise in creating inhomogeneities using liquid phantoms such as layered structures. A desire for solid phantoms led to the development of suspensions of Intralipid and India ink in agar,11 agarose,12 and polyacrylamide13 that could be cut and shaped to provide inhomogeneities in the phantoms. The inclusion of inhomogeneities limited the selection of dyes used to waterproof inks to prevent diffusion of dyes between regions.11 However, these phantoms mostly suffer from relatively short useable lifetimes, which are usually limited to no more than two months. More robust phantoms made of rubbers or plastics have been described that give long-term optical stability and greater shaping flexibility. Phantom compositions of silicone,1, 14 polyester,15, 16 polyurethane,17 and epoxy resin18 have been described. There may be no great advantage of one material over another. For example, silicone more closely matches the mechanical properties of tissue and may be cast into arbitrary shapes, whereas epoxy, polyurethane, and polyesters are easier to machine after casting. Typically, the selection of a material is determined by the choice of absorbers and their stability in that medium. The choice of scattering agents in solids is usually limited to aluminum oxide ,1, 14 titanium dioxide,15 and silicon dioxide,16, 18 polyester,1 polystyrene, or latex microspheres. The Mie theory can be used to predict the scattering properties of microspheres with knowledge of the relative refractive index, size distribution, and number density. Unfortunately, microspheres tend to be much more expensive than aluminum oxide or titanium dioxide particles. One primary difference between aluminum oxide and titanium dioxide particles may be the maximum attainable value for (the cosine for the mean angle of scattering), which describes the scattering anisotropy. Firbank and Deply found that is limited to , whereas can reach in polyester resin.15 Arridge 19 has shown that any arbitrary scattering angle distribution can be theoretically matched using an appropriate distribution of particle sizes. Our goal was to make durable stable optical phantoms with predefined optical properties at two wavelengths, 690 and , that are commonly used for oximetry.20, 21 To our knowledge, previous phantom designs have been developed for a single wavelength or for use with a single broadband absorbing dye that determines the absorption spectrum. We wanted to use component materials that would be photostable. Moreover, the dyes needed to have absorption spectra with minimal overlap. We encountered two problems during initial material selection experiments. First, dyes that absorb strongly at also exhibited comparable absorption at , and second, dyes that bleached during the curing process of the binding medium. The dyes we selected for our phantoms determined the bulk material used to suspend the dyes and scatterers. A method is needed to determine the scattering characteristics of the phantoms. Inverse adding doubling (IAD) was used to find the scattering and absorption of a slab of turbid material using total reflection and total transmission measurements. This method is applicable to homogeneous turbid slabs with any optical thickness, albedo, or phase function. The slab may have a different index of refraction from its surroundings and may or may not be bounded by glass. The optical properties are obtained by iterating an adding-doubling solution of the radiative transport equation until the calculated values of the reflection and transmission match the measured ones. Alternatively, spatially22, 23, 24 and/or temporally25, 26, 27 resolved measurements of diffuse light distributions can also be used to quantitatively determine absorption and reduced scattering coefficients. We present a method for fabricating solid optical tissue phantoms that are castable and photostable. These optical phantoms are designed to be suitable reference standards at two distinct wavelengths and whose optical properties have been carefully measured. The final materials were verified by making several optical phantoms with differing quantities of dye and scattering particles. Phantoms made without added scatterers were made to verify the stability of the dyes’ absorption properties before and after the curing process and over a duration of 14 months. 2.Materials and Methods2.1.Component Material SelectionThe optical phantoms consist of three components: polyurethane, absorbing chromophores, and the scattering agent. Selection of the three primary components were driven by our choice of absorbing dyes. We used a phthalocyanine dye (Epolight 6084, Epolin Incorporated, Newark, New Jersey) as the absorber at . The absorption at was achieved using a palladium dye (Epolight 4148). The dye manufacturer lists these dyes for use in optical filters and laser goggle applications and suggested polyurethane as a casting material. We verified that both dyes lost their absorption during the curing process in epoxy resin (Marine-grade epoxy resin, Side A 314 resin and Side B 109 hardener, Tap Plastics, Incorporated, Dublin, California). Both dyes exhibit narrow absorption bands [full width at half maximum (FWHM) 32 and for Epolight 6084 and 4148, respectively] that allow nearly independent absorption at the wavelengths by Epolight 6084 and by Epolight 4148 when cured in polyurethane (Fig. 1 ). Titanium dioxide (Ti-Pure R-900, Dupont Chemicals, Wilmington, Delaware) was assumed to behave as a pure scatterer with negligible absorption between 650 and . Finally, we used a two part polyurethane system (WC-781 A/B, BJB Enterprises Incorporated, Tustin, California) that had a pot life to suspend the titanium dioxide and absorbing dyes. This polyurethane is a clear rigid casting resin that is designed to be tintable and pigmentable. This polyurethane is formed by mixing part A with part B in a ratio of 100/85 by weight (100/88 by volume). The pot life for the polyurethane was a convenient duration, because it allowed sufficient time to mix and degas but still cured quickly enough that no apparent settling of the scatterers took place. The viscosity of the mixed polyurethane is . The demolding time is . Optical measurements could be made at . However, complete curing of the polyurethane requires 5 to 7 days at room temperature or at 71 to . 2.2.Initial Studies2.2.1.Absorber characterizationThe absorption coefficients as a function of dye concentration were measured in cured polyurethane (WC-781 A/B, BJB Enterprises Incorporated, Tustin, California). Stock solutions of the two powdered dyes were prepared in xylene with concentrations of of Epolight 6084 and of Epolight 4148. Stock solutions of the dyes were pipetted and mixed in five quantities of 15, 25, 35, 40, and of Epolight 6084 stock into of polyurethane part A, and five quantities ranging from 50 to in increments of Epolight 4148 stock into of polyurethane part B. The absorbance of each concentration of Epolight 6084 in part A was measured using a dual-beam Cary 100 Bio Spectrophotometer (Varian Scientific Instruments Incorporated, Walnut Creek, California) with a cuvette of polyurethane part A in the path of the reference beam. Likewise, the absorbance of Epolight 4148 for each concentration was measured with a cuvette of polyurethane part B in the reference beam path. Then the two parts of the polyurethane were randomly combined with the concentration of dye in part A randomly assigned to each concentration of dye in part B. The samples are thoroughly mixed together and poured into 1-cm cuvettes. Degassing of the mixtures was performed by placing the samples into a desiccator vacuum chamber (Nalgene 5311 desiccator, Nalge Nunc International, Rochester, New York) that was connected to a vacuum pump (Speedivac 2, Edwards High Vacuum International, England) capable of reducing the pressure to less than . After full curing, the absorbance of the cuvettes was measured on the Cary spectrometer. These measurements used polyurethane without added absorbers cured in an identical cuvette in the reference beam path. A difference of absorbance measurements with the Cary Spectrophotometer was used to determine the absorption coefficient of polyurethane. Two samples of polyurethane were cast without any added absorbers, one as a thin slab and another as a tall cylinder. After of curing, the samples were removed from their respective molds. The surfaces of each sample were hand polished starting with grit aluminum oxide sandpaper (AngstromLap, Fiberoptic Center, Incorporated, New Bedford, Massachusetts) and finishing with grit paper to create an optically smooth surface. The final thickness of the slab and cylinder of polyurethane were respectively 0.41 and . The difference between the two samples relative to air (i.e., nothing in the reference beam path) produced a lower noise signal than when the thin slab was placed in the reference arm of the spectrometer and the cylinder in the sample arm. 2.2.2.Titanium dioxide scatteringA stock solution of titanium dioxide consisted of of titanium dioxide suspended in 60-mL ethanol. Before dilution into the polyurethane, the titanium dioxide stock solution was shaken by hand then sonicated for at least five minutes to ensure complete suspension of particles. To test the scattering coefficients of different concentrations of titanium dioxide in polyurethane, we added different amounts of stock solution into polyurethane in two separate batches. The first batch had two samples with concentrations of 2.5 and ; we added of Epolight 6084 dye and of Epolight 4148 dye for final absorption coefficients, and to both samples. For the second batch consisting of two samples with concentrations of 0.5 and , we added of Epolight 6084 dye and of Epolight 4148 dye for final absorption coefficients, and to each sample. From each sample in both batches, a small portion of the final mixture was poured into a weighing boat, and degassed for about 8 min. This provided a thin disk used for the inverse adding-doubling method. The IAD method (described in a later section) was used to characterize the scattering coefficient for each of the previous samples. Scanning electron microscopy was used to evaluate the size distribution of the particles. Three samples were prepared by diluting the stock solution of with additional ethanol. A drop of the diluted stock was pipetted onto a clean microscope cover slip and allowed to evaporate, leaving a film of particles. The horizontal width of 112 randomly selected particles was measured from five separate scanning electron microscopy (SEM) images using ImageJ software. The scale bar was used to establish the width of a pixel within each image. 2.3.Phantom DesignOur two wavelength phantoms had four variables to specify( , and ) but only three free parameters, since the reduced scattering coefficients at the two wavelengths are related by the use of a single scattering agent. In other words, we could select the optical properties at the first wavelength, but the reduced scattering at the second wavelength has a constant proportional relationship to the reduced scattering at the first wavelength with respect to scatterer concentration. To compensate, we condensed the desired optical properties into three parameters , and the reduced albedo at the second wavelength . The reduced albedo was defined by The desired absorption and reduced scattering properties at the second wavelength would be modified by the following method, which kept the reduced albedo constant for . A flow chart of this process was diagrammed in Fig. 2 . The wavelength dependence of the reduced scattering coefficient for our particles was determined using IAD and fit by the equation . The effective reduced scattering coefficient at was calculated given the desired reduced scattering coefficient at . The modified absorption coefficient was calculated using the desired reduced albedo and the dependent reduced scattering coefficient at .Fig. 2Process for maintaining the four desired optical properties with only three independent variables. The reduced scattering of the first wavelength establishes the reduced scattering at the second wavelength. A new absorption coefficient is calculated at the second wavelength that holds the desired reduced albedo at the second wavelength, constant relative to the new optical properties. 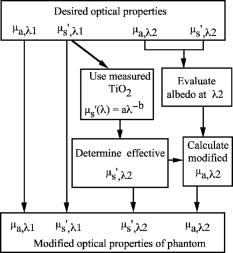 2.4.Fabrication Method of Tissue PhantomsThe optical properties of the tissue phantoms were chosen to represent a range of different optical properties at the 690- and 830-nm wavelengths. The absorption coefficient at these two wavelengths would be varied to correspond to physiologic relevant values for the sum total of contributions by fractions of water, oxygenated and deoxyhemoglobin, and a wavelength constant background absorption. For all the phantoms, the scattering coefficient would be held constant. However, the wavelength dependence of the scattering from differs from tissue in that the ratio of scattering coefficients at 690 to for does not equal the target ratio for tissue. To account for this effect, the target absorption coefficients at were adjusted as described in the previous section. Finally, a single set of optical properties was used to make 20 identical phantoms. The sequence for phantom preparation was as follows using the stock solutions of dye and described earlier. We made cylindrically shaped phantoms in diameter and about in height. This corresponded to polyurethane by mixing of part A and of part B. In addition, an extra of part A and of part B were prepared for making thin slabs for optical property characterization. The proportions of each part of polyurethane were determined by weight, and the addition of either dye or was neglected since their contribution was less than 1 part in 200 for all phantoms. Absorbers were added to the polyurethane in part A while the scatterers were mixed to part B. Since the scattering of all phantoms were to be identical, of stock was mixed into of part B polyurethane, which was a sufficient quantity for all phantoms. Part A of the polyurethane was weighed out for a single phantom into a polyethylene container. Using stock of Epolight 4148, the near-infrared dye was first pipetted into part A and stirred in with a glass rod until visibly homogeneous. This dye had a pale violet tint when mixed into the polyurethane but was not noticeable after the addition of the Epolight 6084 stock, which produces a strong turquoise blue tint. When both dyes were homogeneously stirred into part A, about was placed in a cuvette for an absorbance measurement on the Cary spectrometer using a cuvette of part A polyurethane without dye in the reference arm to verify proper absorption attenuation. At this point, of the part A with dye was set aside in a plastic weighing boat dish. The remaining portion of polyurethane part A with dye was weighed again to calculate the appropriate quantity of the part B with to be added by weight. While on the scale, part B was poured into part A. The final amount of part B was added drop by drop using a wooden tongue depressor. The large flat area of the tongue depressor allowed drops to be controlled so that the weight of any drops could be controlled with precision of about . The polyurethane was thoroughly mixed using a tongue depressor to scrape the container sides/bottom and stirred for about 2 min. Most of the mixture was then poured into 16-oz HDPE molds (16-oz specimen containers, Fisher Scientific International, Incorporated, Fair Lawn, New Jersey). The remaining material was poured into two plastic weighing boats to cast thin disks of differing thickness and about in diameter. The set-aside of polyurethane part A and dye was treated identical as the previous, except it had of transparent part B added to it. Also, these samples were mixed then poured into 1-cm pathlength cuvettes for casting. It was necessary to remove entrapped bubbles in the polyurethane introduced by the mixing together of components. Up to five phantoms or weighing boat slabs could be degassed at a time. This restricted us to mixing no more than two sets of optical properties together at a single time, since for each set, a phantom and two slabs were made. The second set was mixed while the first set was degassing. Degassing was done by placing the samples in a desiccator/vacuum chamber (Nalgene 5311 desiccator, Nalge Nunc International, Rochester, New York). The chamber was connected to a vacuum pump (Speedivac 2, Edwards High Vacuum International, England) capable of reducing the pressure to less than . Samples were degassed for about , at which time bubbles stopped forming. After degassing, the phantoms were set onto a level surface and covered. The phantoms were solid after and could be removed from the mold. However, at , the polyurethane is relatively soft, such that fingerprints can be embedded on the surface with moderate pressure. In general, we waited before removing the samples from the casting mold. The phantoms in the specimen container molds were removed by flexing the walls of the container and then pushing on the bottom while holding the phantom upside down. The samples cast in weighing boats were removed by peeling the weighing boat, which tore away from the polyurethane. The phantoms were finished by leveling the top surface of the polyurethane block. A lathe was used to mill the meniscus lip from the top of each phantom until the shiny surface was completely removed. Then each phantom was sanded by hand in two steps: rough finishing with 200-grit wet sandpaper and fine finishing with 600-grit wet sandpaper. In each sanding step, the paper was wet with water and placed on a glass surface while the polyurethane block was sanded in a circular motion. Elimination of visible surface scratches were used as a completion condition. 2.5.Testing of Phantom Optical PropertiesAbsorption of each phantom was measured using the Cary spectrometer after casting. The clear samples cured in cuvettes for each phantom were measured, referenced to cured polyurethane without dyes in a cuvette. Four absorbance measurements of each sample were recorded. Additional measurements of the same cuvette samples were recorded 13 and 14 months later to establish long-term stability. Inverse adding doubling was used to find the scattering and absorption of a slab of turbid material using total reflection and total transmission measurements. Two measurements on each of the two thin slabs for each phantom were made. Total diffuse reflection measurements were made using a 203.2-mm-diam integrating sphere (IS-080-SF, Labsphere, Incorporated, North Sutton, New Hampshire) with a 12.7-mm-diam detector port and a 31.75-mm-diam sample port with a baffle between ports. Light was guided to the phantom disk through a optical fiber (0.22 numerical aperture) placed about above the sample centered with the port hole. A 4-mm-diam steel tube coated with multiple coats of flat white paint was used to sleave the fiber through a 6.35-mm top port down to the phantom disk. An Oriel mercury lamp was used for the source and a (0.38 numerical aperture) delivered light from the integrating sphere to a scanning grating detector system of a Fluorolog-3 spectrofluorometer (Instruments S.A., Incorporated, Edison, New Jersey). Signals above were often poor as a consequence of lower power from the lamp at longer wavelengths and lesser efficiency of the photomultiplier tube above . The detector fiber was connected to the integrating sphere using an SMA connector surrounded by white shielding in the 12.7-mm port. Measurements were referenced to a 99% Spectralon reflectance standard (Labsphere, Incorporated, North Sutton, New Hampshire) and a dark measurement where the sample port was open and the fiber illuminating black felt on the optical table was below the port. Four measurements were made on each disk; two measurements on each side. The phantom disks were placed flush with the sample port. Figure 3 shows the sphere measurements for total reflection, and Fig. 4 shows the reference sphere calibration measurements. Fig. 4Diagrams of various measurements needed to determine the fraction of diffuse illumination and the sphere reflection efficiency. 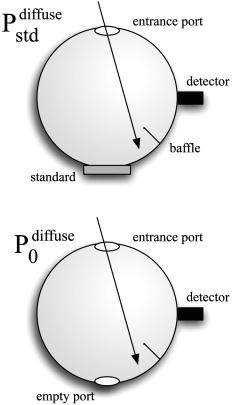 Total diffuse transmission measurements were made with another identical integrating sphere. In this setup, only two ports were open, the 25.4-mm-diam sample port and 12.7-mm-diam detector port with a baffle between ports. For this arrangement, the phantom disk was placed on the top port, and light was collected with the same detector fiber we previously described. Light was delivered to the phantom disk with the fiber. The end of the fiber was positioned just above the phantom disk centered with the port hole. Again, four measurements were recorded on each disk; two measurements on each side. Measurements were referenced to 100% with the fiber illuminating the open port hole, and a dark measurement with an open port but with the fiber removed from the sample porthole. Figure 5 shows the sphere arrangement for total diffuse transmission measurements. Fig. 5Diagrams of various integrating sphere transmission measurements that are needed. The empty port on the bottom of the spheres should be identical to the size of the entrance port in the sphere reflection measurements. 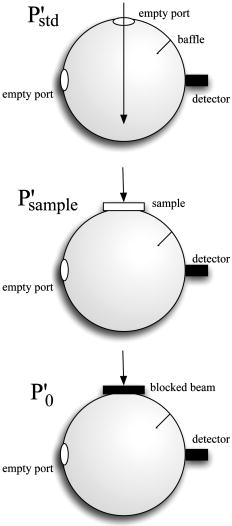 Two other values were needed to calculate the scattering properties. The thickness of each phantom disk was measured using a depth gage (ID-C112EB, Mitutoyo Corporation, Japan). The refractive index of the cured polyurethane was measured at using an Abbé refractometer at 1.468. We assumed negligible change in the refractive index over the spectral range of 500 to . The total diffuse reflectance and transmittance measurements in terms of percentage at all wavelengths were input into the IAD program. The total diffuse reflection was calculated using The total diffuse transmission was calculated usingThe batch variability was measured on 12 out of 20 phantoms from a single batch to establish the variability in our fabrication method. A thin slab was cut and sanded flat from 12 of the phantoms. The thickness of each slab was measured with a depth gage. Total diffuse reflection and transmission measurements were recorded for each of the 12 slabs, along with reference measurements as previously described. Inverse adding doubling was used to determine the absorption and reduced scattering coefficient for each slab over the wavelength range of 650 to . 3.Results and Discussion3.1.Absorption CharacterizationBoth dyes exhibit stable absorption properties in polyurethane after a period of 14 months from casting. The visible/near-infrared (NIR) spectrum for the absorption coefficient of Epolight 6084 and 4148 is shown in Fig. 1. The absorption coefficient at relates only to the concentration of Epolight 6084 by the relation where and is the absorption coefficient for polyurethane at after 13 months when the Epolight 4148 is present at a concentration less than . Likewise, when Epolight 6084 is less than , the absorption coefficient at depends solely on the concentration of Epolight 4148 by the linear relationwhere and is the absorption coefficient for polyurethane at after 13 months. The linear relations for the absorption coefficient are applicable for Epolight 6084 up to a concentration of at least , and for Epolight 4148 up to . A comparison of the absorption from each dye in solution in one part of the polyurethane and then after casting is shown in Fig. 6 for Epolight 6084 and Fig. 7 for Epolight 4148. The absorption spectrum of both dyes was unaffected by curing.Fig. 6The absorption coefficient at as a function of dye concentration of Epolight 6084 in part A of the polyurethane (circles) and after curing in polyurethane (squares). The fitting line is , where and is the absorption coefficient of polyurethane at one week after casting. 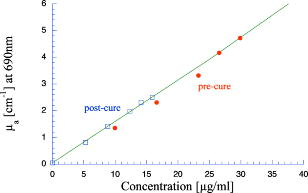 Fig. 7The absorption coefficient at as a function of dye concentration of Epolight 4148 in part B of the polyurethane (circles) and after curing in polyurethane (squares). The fitting line is , where and is the absorption coefficient of polyurethane at one week after casting. 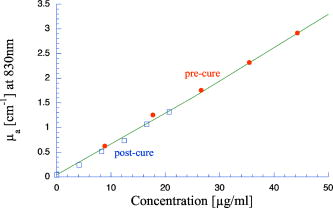 The tissue phantoms are limited by a minimum intrinsic absorption coefficient that is due to the absorption of the binding medium. The absorption coefficient of polyurethane at a time point one week after casting is greater than after 13 months from casting, as shown in Fig. 8 . The change of absorption at these time points is over the wavelength range of 500 to . The polyurethane absorption is stable between 13- and 14-month time points, but it is not known how gradual or when exactly the drop in absorption occurred between the one-week and 13-month time points. The polyurethane exhibited less absorption than two other resins (an epoxy resin and a polymer resin), which showed significant absorption occurring below , thus giving each a yellow tint as shown in Fig. 8. 3.2.Scatterer CharacterizationThe scattering coefficients were determined from diffuse reflection and transmission measurements. A typical spectra is shown in Fig. 9 . This illustrates the wavelength separation in the absorption of the two dyes in the reflected and transmitted light. The reduced scattering coefficient as a function of wavelength (Fig. 10 ) was obtained using inverse adding doubling, using the total diffuse reflection and transmission measurements shown in Fig. 9. For comparison, the absorptioncoefficient obtained from a spectrometer measurement of a sample without titanium dioxide is also shown in Fig. 10 with the absorption coefficient calculated by IAD. The reduced scattering coefficient relates to the scattering coefficient , and the cosine of the mean angle of scattering from the incident direction of light by the equation . The reduced scattering coefficient is an equivalent optical parameter that is one over the mean free path between scattering events when the scattering phase function is isotropic. Since two measurements are recorded, only absorption and the reduced scattering coefficient can be determined. The anisotropy and the scattering coefficient were not determinable separately without another measurement of the ballistic photons. Our slabs (ranging from 2 to ) were sufficiently thick that all transmitted light was completely diffuse. Fig. 9Typical reflectance and transmission measurements used to derive optical properties using the inverse adding-doubling method. 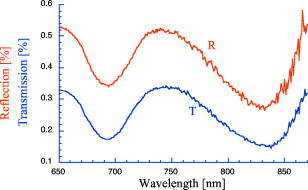 Fig. 10The reduced scattering and absorption coefficient spectra for the reflection and transmission data in Fig. 9 are shown in comparison to the spectrometer measurement of absorption for the same phantom made without added scatter. The error bars are the standard deviation of three spectrometer measurements through the clear phantom. Above , the sensitivity of the detector drops precipitously, causing error in the resultant optical properties. The reduced scattering is fit by the relation . 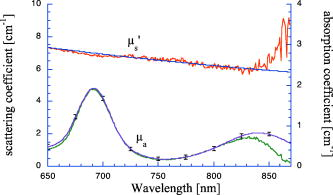 The derived scattering properties for the titanium dioxide follow the relations where , and is the concentration of the titanium dioxide. The different slopes correspond to the altered scattering efficiencies of the titanium dioxide particles at 690 and . The size distribution of the titanium dioxide particles is shown in Fig. 11 having a mean of . If we approximate the particles as spheres, then the Mie theory can be used to predict the scattering anisotropy for the average particle size. Three-fourths of all particles were between 250 and in diameter. An average of the value calculated in increments across the range 250 and in particle diameter is at the wavelength and at the wavelength.3.3.Phantom Optical PropertiesA set of ten optical phantoms were made with differing levels of absorption but with the same scattering properties. The variation among the ten phantoms in the scattering coefficient at 690 and is shown in Fig. 12 . The measured absorption coefficients at 690 and of the ten samples are shown relative to the predicted absorption from Eqs. 1, 2 in Fig. 13 . The difference between predicted and the measured absorption coefficients for the ten phantoms was plus or minus 3% at and 6% at . Fig. 12The variation in reduced scattering coefficient for ten different phantoms at (circles) and (squares) measured by the IAD method. All samples were designed to have the same reduced scattering properties. The error bars are the standard deviation of four sets of reflection and transmission measurements for each sample. 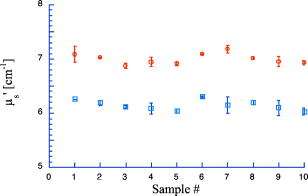 Fig. 13The predicted absorption coefficient using Eqs. 1, 2 are shown relative to the spectrometer measured absorption coefficient for the ten different phantoms. 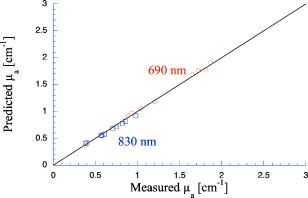 The reproducibility of a phantom with a single set of absorption and scattering properties is shown in Fig. 14 for 12 phantoms made from a single batch. Both absorption and scattering varied by plus or minus 3% between 600 and . Between 500 and , the percentage absorption variation increases but the absolute error remains constant. At wavelengths longer than , the error increased (as previously stated) due to lamp power and decreased detector efficiency. Since both the Epolight 6084 and 4148 were mixed together in one part of the polyurethane and titanium dioxide to the other part before combining the two parts, the actual percentage variation in absorption at should be comparable to that at . Since all 20 phantoms come from a single large batch where all the dye was added to part A of the polyurethane and the was added to part B for all 20 samples, the variation in the samples can only come from two sources: weighing errors for the combination to the two parts of polyurethane, or inhomogeneities in our large solutions of components. The largest weighing error was less than 2% for all phantoms, including the ten with different absorption properties. Fig. 14The absorption and reduced scattering coefficient spectra for 12 of the 20 identical phantoms. The error bars show the standard deviation for each optical property among the 12 phantoms and are approximately between 600 and . 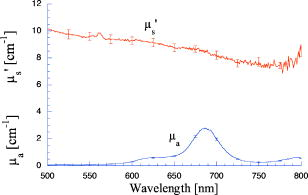 To minimize error due to inhomogeneities, the part of the polyurethane with was regularly stirred to prevent settling of the scatterers before mixing with the part with added absorbers. However, after combining the two parts of the polyurethane together, the phantom cannot be stirred once the degassing step begins. Samples were held under vacuum at a minimum of eight minutes, but can be degassed for 10 to . Though the pot life of the polyurethane was a half-hour, by 30 to the polyurethane was still liquid but warming and noticeably beginning to gel. It is doubtful that full degassing of phantoms can be performed with a pot life less than half an hour, since gelling of the polyurethane will inhibit gas from escaping. It took about to make all 20 phantoms with two people working. The timeliest part of the process was degassing, because we were limited to only four or five phantoms in a vacuum chamber due to space constraint. 4.ConclusionsWe present a method to fabricate tissue phantoms that simulate the optical properties of living tissue at 690- and wavelengths. The optical properties of the phantoms are consistent in a period of 14 months from the date of casting. The two molecular dyes provide independent absorption between our chosen wavelengths for absorption coefficients up to 5 and at 690 and , respectively. These dyes exhibit stable absorption through the curing process of polyurethane. In addition, the high molecular extinction coefficient of each dye allows us to keep the addition of dye stock to less than 0.1% of the volume of the polyurethane. The polyurethane itself is the only component that changed optical properties after casting. The absorption due to the polyurethane decreases approximately by a factor of 2 over the visible spectrum. This change is assumed to occur gradually, though we do not have evidence to support this belief. Though polyurethane slowly changes absorption properties, the effect is to make the material more colorless, which helps to make any added dye the more dominant absorber. Polyurethane as a phantom material is easy to work with and handle. The material becomes solid by after mixing but is not fully cured. We find that the polyurethane could not be machined for at least . Even sanding of the material before is futile because ripples form on the surface. This polyurethane may be cast into any shape, but the material of the mold determines how easy it is to extract the polyurethane after curing. Polyethylene containers readily release after curing. Attempts to cast the polyurethane between glass slides proves more problematic. Spray-on mold release (Ease Release 400, Mann Formulated products, Easton, Pennsylvania) allows the polyurethane to release from glass but the thin film of mold release causes surface roughness to the polyurethane, making it like frosted glass. Polycarbonate molds behave more like glass in terms of demolding. The mold release allows polyvinylchloride (pvc) pipe to act as a mold too, but only the polyethylene works well without the mold release. Degassing of the polyurethane is a necessary though time-consuming step in the phantom preparation. Significant bubble formation occurs during each mixing step. For our phantoms, at least three mixing steps occur for each phantom, since we have two dyes and two components of polyurethane. Bubbles that are not removed provide many inhomogeneities, and may act as large scatterers. Removal of the bubbles allows for consistency in the finished product that otherwise would not be obtainable. We determine four linear relationships [Eqs. 1 through 4] that describe the absorption coefficient as a function of dye concentration and the reduced scattering coefficient as a function of the titanium dioxide concentration. These relationships predict the measured absorbing and scattering properties with less than 4% error. The optical properties of the phantoms are shown to be stable over a period of 14 months, making the phantoms suitable for use as reference standards. ReferencesR. Bays,
G. Wagnieres,
D. Robert,
J. Theumann,
A. Vitkin,
J. Savary,
P. Monnier, and
H. van den Bergh,
“Three-dimensional optical phantom and its application in photodynamic therapy,”
Lasers Surg. Med., 21 227
–234
(1997). 0196-8092 Google Scholar
P. R. Bargo,
S. A. Prahl, and
S. L. Jacques,
“Optical properties effects upon the collection efficiency of optical fibers in different probe configurations,”
IEEE J. Sel. Top. Quantum Electron., 9 314
–321
(2003). https://doi.org/10.1109/JSTQE.2003.811287 1077-260X Google Scholar
G. Zonias,
L. T. Perelman,
V. Backman,
R. Manahoranm,
M. Fitzmaurice,
J. Van Dam, and
M. S. Feld,
“Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo,”
Appl. Opt., 38 6628
–6637
(1999). 0003-6935 Google Scholar
P. R. Bargo,
S. A. Prahl, and
S. L. Jacques,
“Collection efficiency of a single optical fiber in turbid media,”
Appl. Opt., 42 3187
–3197
(2003). 0003-6935 Google Scholar
B. Wilson,
Y. Park,
Y. Hefetz,
M. Patterson,
S. Madsen, and
S. Jacques,
“The potential of time-resolved reflectance measurements for the noninvasive determination of tissue optical properties,”
Proc. SPIE, 1064 97
–106
(1989). 0277-786X Google Scholar
J. Linford,
S. Shalev, and
J. Bews,
“Development of a tissue equivalent phantom for diaphanography,”
Med. Phys., 13 869
–875
(1986). https://doi.org/10.1118/1.595948 0094-2405 Google Scholar
B. C. Wilson,
T. J. Farrell, and
M. S. Patterson,
“An optical fiber based diffuse reflectance spectrometer for noninvasive investigation of photodynamic sensitizers in vivo,”
Proc. SPIE, ISO6 219
–232
(1990). 0277-786X Google Scholar
B. Chance,
J. Haselgrove,
N. G. Wang,
M. Maris, and
E. Sevick,
“Photon dynamics in tissue imaging,”
Proc. SPIE, 1525 68
–82
(1991). 0277-786X Google Scholar
S. T. Flock,
S. L. Jacques,
B. C. Wilson,
W. M. Star, and
M. J. C. van Gemert,
“Optical properties of Intralipid: a phantom medium for light propagation studies,”
Lasers Surg. Med., 12 510
–519
(1992). 0196-8092 Google Scholar
S. J. Madsen,
M. S. Patterson, and
B. C. Wilson,
“The use of India ink as an optical absorber in tissue-simulating phantoms,”
Phys. Med. Biol., 37 985
–993
(1992). https://doi.org/10.1088/0031-9155/37/4/012 0031-9155 Google Scholar
R. Cubeddu,
A. Pifferi,
P. Taroni,
A. Torricelli, and
G. Valentini,
“A solid tissue phantom for photon migration studies,”
Phys. Med. Biol., 42 1971
–1979
(1997). https://doi.org/10.1088/0031-9155/42/10/011 0031-9155 Google Scholar
G. Wagnieres,
S. Cheng,
M. Zellweger,
N. Utke,
D. Braichotte,
J. Ballinni, and
H. van den Bergh,
“An optical phantom with tissue-like properties in the visible for use in PDT and fluorescence spectroscopy,”
Phys. Med. Biol., 42 1415
–1426
(1997). https://doi.org/10.1088/0031-9155/42/7/014 0031-9155 Google Scholar
M. N. Lizuka,
M. D. Sherar, and
I. A. Vitkin,
“Optical phantom materials for near infrared laser photocoagulation studies,”
Lasers Surg. Med., 28 237
–243
(2001). https://doi.org/10.1002/lsm.1044 0196-8092 Google Scholar
M. Lauldi,
A. Colombo,
B. Farina,
S. Tomatis, and
R. Marchesini,
“A phantom with tissue-like optical properties in the visible and near-infrared for use in photomedicine,”
Lasers Surg. Med., 28 237
–243
(2001). https://doi.org/10.1002/lsm.1044 0196-8092 Google Scholar
M. Firbank and
D. T. Delpy,
“A design for a stable and reproducible phantom for use in near infra-red imaging and spectroscopy,”
Phys. Med. Biol., 38 847
–853
(1993). https://doi.org/10.1088/0031-9155/38/6/015 0031-9155 Google Scholar
U. Sukowski,
F. Shubert,
D. Grosenick, and
H. Rinneberg,
“Preparation of solid tissue phantoms with defined scattering and absorption properties for optical tomography,”
Phys. Med. Biol., 41 1823
–1844
(1996). https://doi.org/10.1088/0031-9155/41/9/017 0031-9155 Google Scholar
M. L. Vernon,
J. Frechette,
Y. Painchaud,
S. Caron, and
P. Beaudry,
“Fabrication and characterization of a solid polyurethane phantom for optical imaging through scattering media,”
Appl. Opt., 38 4247
–4251
(1999). 0003-6935 Google Scholar
M. Firbank,
O. Motaki, and
D. T. Delpy,
“An improved design for a stable and reproducible phantom for use in near infra-red spectroscopy and imaging,”
Phys. Med. Biol., 40 955
–961
(1995). https://doi.org/10.1088/0031-9155/40/5/016 0031-9155 Google Scholar
S. R. Arridge,
P. van der Zee,
D. T. Delpy, and
M. Cope,
“Particle sizing in the Mie scattering region: singular value analysis,”
Inverse Probl. Eng., 5 671
–689
(1989). 1068-2767 Google Scholar
A. Duncan,
J. H. Meek,
M. Clemence,
C. E. Elwell,
P. Fallon,
L. Tyszczuk,
M. Cope, and
D. T. Delpy,
“Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy,”
Pediatr. Res., 39 889
–894
(1996). 0031-3998 Google Scholar
N. Okui and
E. Okada,
“Wavelength dependence of crosstalk in dual-wavelength measurement of oxy- and deoxy-hemoglobin,”
J. Biomed. Opt., 10 011015
(2005). https://doi.org/10.1117/1.1846076 1083-3668 Google Scholar
T. J. Farrell,
M. S. Patterson, and
B. C. Wilson,
“A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo,”
Med. Phys., 19 879
–888
(1992). https://doi.org/10.1118/1.596777 0094-2405 Google Scholar
R. Bays,
G. Wagnieres,
D. Robert,
D. Braichotte,
J. F. Savary,
P. Monnier, and
H. van den Bergh,
“Clinical determination of tissue optical properties by endoscopic spatially resolved reflectometry,”
Appl. Opt., 35 1756
–1766
(1996). 0003-6935 Google Scholar
A. Kienle,
L. Lilge,
M. S. Patterson,
R. Hibst,
R. Steiner, and
B. C. Wilson,
“Spatially resolved absolute diffuse reflectance measurements for noninvasive determination of the optical scattering and absorption coefficients of biological tissue,”
Appl. Opt., 35 2304
–2314
(1996). 0003-6935 Google Scholar
J. B. Fishkin,
O. Coquoz,
E. R. Anderson,
M. Brenner, and
B. J. Tromberg,
“Frequency-domain photon migration measurements of normal and malignant tissue optical properties in a human subject,”
Appl. Opt., 36 10
–20
(1997). 0003-6935 Google Scholar
B. J. Tromberg,
O. Coquoz,
J. B. Fishkin,
T. Pham,
E. R. Anderson,
J. Butler,
M. Cahn,
J. D. Gross,
V. Venugopalan, and
D. Pham,
“Noninvasive measurements of breast tissue optical properties using frequency-domain photon migration,”
Philos. Trans. R. Soc. London, Ser. B, 352 661
–668
(1997). https://doi.org/10.1098/rstb.1997.0047 0962-8436 Google Scholar
G. Mitic,
J. Kölzer,
J. Otto,
E. Plies,
G. Sölkner, and
W. Zinth,
“Time-gated transillumination of biological tissues and tissue-like phantoms,”
Appl. Opt., 33 6699
–6710
(1994). 0003-6935 Google Scholar
|