|
|
1.IntroductionCurrently, we are investigating the feasibility of using meta-tetrahydroxyphenyl chlorin –mediated photody namic therapy (PDT) for nasopharyngeal carcinomas (NPC) as an alternative or additional treatment modality to current treatment protocols using combined chemotherapy, radiotherapy, and brachytherapy. The few published studies of PDT for nasopharyngeal cancer show a very variable clinical outcome. In studies where nasopharyngeal carcinomas were included in a larger series of head and neck cancers, light dosimetry was not systematically employed and treatment parameters varied per patient.1, 2, 3 Other studies used manual positioning of the laser light through an endoscope.4, 5 The only example in the literature with a systematic approach to light delivery is from Lofgren 6 using the photosensitizer Photofrin. They used a specially developed rigid light delivery system that is inserted through the nose into the nasophanryngeal cavity with a beam deflector (mirror) at the distal end to direct light upward. Results from five patients were reported with variable clinical results. All published studies used the first-generation photosensitizer Photofrin or hematoporphyrin derivative (HpD). The side effect of long-term generalized photosensitization induced by this drug, combined with the variable outcome of tumor response, resulted in the cessation of these studies. We believe that the previous attempts to perform PDT in the nasopharynx were all hampered by inadequate light delivery due to the difficult accessibility of the nasopharyngeal cavity. This seriously decreased the clinical effectiveness of these preliminary clinical experiments. The nasopharyngeal cavity is located behind the septum and above the soft palate; it can be accessed through the nose or the oral cavity. Previously, we developed a flexible silicone device, the Rotterdam nasopharynx applicator7 (RNA), to accurately position brachytherapy sources via the nose. Based upon this RNA concept, an applicator was developed to facilitate an accurate, controllable, and reproducible approach to the delivery and monitoring of light in the nasopharyngeal cavity. The applicator was successfully evaluated in optical phantoms and volunteers.8 Controlled light delivery is essential for local control of the tumor, while limiting PDT-induced tissue damage to critical structures. In vivo monitoring of the fluence rate is also essential because in hollow organs the actual fluence rate at the tissue surface is strongly increased due to reflections and backscattering from the tissue. This fluence rate amplification within a hollow cavity varies strongly with its shape and tissue optical properties and has been reported to be as high as a factor of 7.5 for the bladder.9 In situ fluence rate measurements in other PDT applications—such as the bladder, the oesophagus,10 the oral cavity,11 the thoracic cavity,12 and the trachea13—emphasized the need for dedicated light delivery systems with an integrated in situ fluence rate measurement. Such an approach may contribute significantly to a standardization of clinical response and a decrease in complications due to overtreatment. Furthermore, fluence rate measurements can also reflect tissue changes in physiology as a direct result of PDT-induced tissue damage during treatment. Different fluence rates can also have a dramatic influence on the physiological response to PDT. In this study, in vivo fluence rate measurements were conducted during a phase I/II study with the primary aim of lowering the drug dose and reducing the drug light interval in order to reduce the duration of skin photosensitization and hospitalization. Patients were divided into two drug-dose (DD)–drug-light-interval (DLI) groups. For group 1, the DD equals and the DLI is as recommended for the treatment of squamous cell carcinoma of the head and neck. 14, 15, 16, 17 For group 2, the DD is and the DLI is to investigate the PDT response and the time course of skin photosensitivity. These clinical data will be presented elsewhere. Here we report on the effectiveness of shielding by the applicator in the risk areas, such as the soft palate, tongue, and tonsils. We also report on fluence rate measurements in the tumor target area to investigate interpatient variations, the fluence rate distribution in the nasopharyngeal cavity, and PDT-induced changes in fluence rate during -mediated PDT. 2.Materials and MethodsFive patients were initially treated in a pilot study at the Netherlands Cancer Institute with good clinical results. Due to the much higher incidence of NPC in Indonesia, the multicenter phase I/II study was subsequently carried out in Indonesia at the ear, nose, and throat (ENT) departments of the Dr. Sardjito Hospital in Yogyakarta and the Dr. Cipto Mangunkusumo Hospital in Jakarta. 2.1.ApplicatorThe applicator is depicted in Fig. 1a and the applicator with respect to anatomical locations is schematically shown in Fig. 1b. The nasopharynx PDT applicator consists of two 220-mm long silicone tubes positioned through the nasal cavity, covering the nasopharynx up to the upper part of the oropharynx. Both tubes were interconnected at the distal end by a small silicone bridge, which abuts proximally to the nasal septum and approximates the shape of the nasopharyngeal cavity.7 The lateral distance between the tubes is . The applicator bridge is made out of medical grade silicone (Meproplastik, Biotool, Hengelo, The Netherlands) and remains in situ for the duration of the treatment (approximately ). The bridge is perforated with two 5-mm holes to enhance flexibility. The inner diameter of the two silicone tubings (o.d. , i.d. , Barendrecht, The Netherlands) can accommodate a standard flexible implant tube (o.d. , Nucletron, Veenendaal, The Netherlands) for improved guidance of the isotropic probes. Furthermore, both silicon tubes house additional black tubes for guidance and shielding of the cylindrical diffusers, these shielding tubes end at the point where the bridge is in contact with the septum. This enables variable diffuser lengths to be used without adjustment of the laser output power; thus diffuser lengths are tailored to individual patients preventing light from entering the nasal cavity. A removable flexible black silicon patch is applied to protect the whole soft palate and prevents light from entering the oral cavity. The black patch can also be tailored to fit the patient, depending on the oronasopharyngeal dimension. A 60-mm or 40-mm cylindrical diffuser (Biolitec, Bonn, Germany) connected to a single port 652-nm diode laser (Ceralas PDT, Biolitec, Bonn, Germany) was used as the light source. Fig. 1Basic shape of the nasopharyngeal light applicator device and (b) position of the light application device with respect to the anatomical location. For treatment, an optical cylindrical diffuser fiber (1) that emits diffusely over a 40- to 60-mm length was used. The NPC target area (2) is usually localized along the outer curve of the applicator. The thick black line represents the black silicon patch intended to shield light from critical healthy areas such as the soft palate (3). 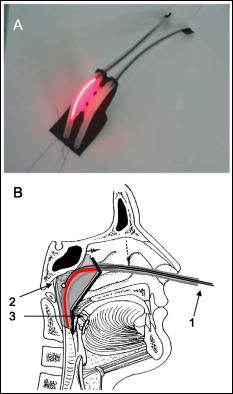 2.2.DosimetryThe dosimetry device is constructed out of a 19-in. chassis with universal ac power supply (PXI-1045 18-Slot 3U, National Instruments) with an 2.5-GHz embedded controller (PXI-8187 P4, National Instruments). Two two-channel power measurement modules (PX2000-306-SS, BFI Optilas, Alphen aan de Rijn, The Netherlands) were used to detect the light from the isotropic detectors. The four isotropic detectors were connected via connectors to the power measurement modules and calibrated in an integrating sphere with a well-defined fluence rate. This calibration unit was incorporated into the chassis. Light-emitting diodes that generate the calibration fluence rate in the integrating sphere were controlled by a high-resolution analog output board (PXI-6704, National Instruments). The fluence rate in the integrating sphere was monitored by a digital multimeter unit (PXI-4070, National Instruments) connected to a photodiode to ensure a constant and reproducible fluence rate. The fluence rate at the surface of the applicator was measured using a fiber-optic isotropic detector (model IP tip o.d. 0.85-mm, Medlight, Switzerland) that collects the sum of the primary incident light from the cylindrical diffuser and the tissue backscattered light. All modules were simultaneously controlled by a single program, which enables calibration, real-time fluence and fluence rate measurements, and data storage. Prior to clinical application, the NPC PDT applicator was first tested in the absence of tissue, that is, in optical tissue phantoms in air, and in five volunteers (data will be presented elsewhere). The fluence rate distribution over the applicator surface in air and tissue optical phantom was found to be homogeneous [standard deviation (SD)/mean is 3.8% and 18.3%, respectively]. 2.3.PatientsFourteen patients were enrolled in the study complying with the following inclusion criteria: male or nonpregnant, nonlactating female; at least 18 years of age and legally competent; a Karnofsky performance status of at least 70%; histologically confirmed recurrent nasopharyngeal carcinoma, type TI, II, III, or IV; with a discrete tumor that was endoscopically visible and accessible for unrestricted surface illumination using a nasopharyngeal applicator; and had exhausted all conventional treatment options. The procedure was performed according to a protocol approved by the local medical ethics committee and with the patient’s written informed consent. After decongestion (R/Xylometazoline HCL1%) and topical anaesthesia, the applicator was introduced in the nose and nasopharynx trans-orally over four French guide tubes secured by a small silicone flange. The applicator remained fixed in a stable position for the illumination period, during which the patient was seated in an ENT chair. Previous studies using -PDT on early stage squamous cell carcinomas of the head and neck14, 15, 16 demonstrated excellent clinical and cosmetic results using a fixed incident fluence rate of and a total fluence (“light dose”) of . Two separate illuminations were therefore performed in order to deliver a total fluence of per diffuser length (i.e., ) over the entire nasopharyngeal cavity at a fixed diffuser output power of . The latter corresponds to a fluence rate at the surface of the applicator of approximately as measured in air. Fluence rate measurements were performed using four isotropic detectors: three located inside the nasopharyngeal cavity, and one manually held detector to measure the fluence rate on the critical structures, that is, the soft palate, hard palate, tongue, left and right tonsils, and the oropharynx near the distal end of the PDT applicator. As seen in Fig. 2 , two isotropic detectors were placed in the tube adjacent to the cylindrical diffuser, that is, one in the middle and one at the end of the diffuser, and a third detector was positioned in the empty tube. Phantom experiments showed that approximately 5% of the maximum fluence was delivered to the empty tube located from the diffuser. To investigate the extent of this cross talk between illumination channels, the third detector was moved during treatment progressively from the distal to the proximal end of the empty channel. Fig. 2Schematic diagram of the position of the isotropic detectors as placed in the NPC PDT applicator. The silicon bridge connecting both tubes is seen from above. Positions 1 and 2 indicate the location of the two isotropic detectors that remained fixed in position next to the diffuser during PDT. Position 3 indicates the location of the third isotropic detector that is repositioned from the beginning, to the middle, and finally to the end of the applicator. The first illumination was always on the left side, after which the diffuser plus detectors were switched from tube to tube. 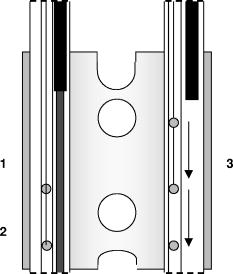 3.ResultsAll patients received two illuminations: one in the left tube and one in the right tube. For each illumination the cylindrical diffuser output power was fixed at , and a calculated total fluence of was delivered for each tube. Table 1 shows the average fluence of the first group of patients , delivered to the risk area locations: soft palate, hard palate, tongue, left and right tonsils, and oropharynx near the distal end of NPC PDT applicator. The fluence was calculated by multiplying the measured fluence rate by the exposure time without taking into account possible changes in fluence rate during PDT. No PDT-induced tissue damage was seen in any of the risk areas up to the three-month follow-up. Table 1Average delivered fluence to the risk areas.
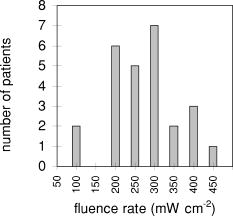
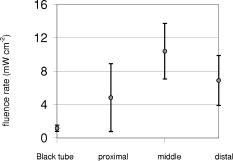
Probes 1 and 2, located in the same channel as the diffuser, measured the fluence rate during the two-sided illumination in 14 patients. Two software data storage failures occurred during the first illumination, thus resulting in a total of 52 measurements. The histogram in Fig. 3 shows the distribution of the initial fluence rate (average of the first ) measured during the start of each of the two illuminations for all patients at the middle of the diffuser. The average fluence rate recorded by probe 1 was ranging from a minimum of up to a maximum of . This results in an average fluence at the tissue surface of . A similar trend was observed for probe 2, however the fluence rate at the distal location of the diffuser was significantly lower (23%) ( test ) than the average initial fluence rate at the middle of the diffuser. The average fluence rate recorded by probe 2 was SD 63: the maximum and minimum were 375 and , respectively. The average fluence rate distribution over the applicator surface was less homogeneous as compared to the measurements in air and in phantoms and was found to be 21% for the SD/mean (range 1.2 to 56.0%). Figure 4a shows the fluence rate as a function of exposure time of a 61-year-old male patient with an undifferentiated persistent NPC (T2a N1 M0). The average fluence rates at the middle and end of the diffuser were and , respectively, and varied little during treatment time. Figure 4b shows the fluence rate as a function of exposure time of a 45-year-old female patient with an undifferentiated persistent NPC (T4 N2 M0). In this patient, a strong decrease in fluence rate was observed over the exposure time. The initial fluence rates dropped by 33 and 35%, respectively, for probes 1 and 2. A similar pattern was observed during the second illumination. The small, transient dips in fluence rate coincide with the patient’s swallowing and regurgitative spasm. Figure 5 shows the change in fluence rate during PDT of all subjects and both illuminations as a function of the initial fluence rate, that is, the average over the first . No correlation between these parameters was observed ( , Spearman’s coefficient , , 95% confidence interval for to 0.022); furthermore, no significant differences between the two DD-DLI groups were observed. Fig. 4An example of the fluence rate as measured by detectors 1 and 2, as a function of exposure time, during of the first illumination (left side), for two different patients, A and B. The grey and black lines represent the fluence rate as measured by probe 1 and 2, respectively. 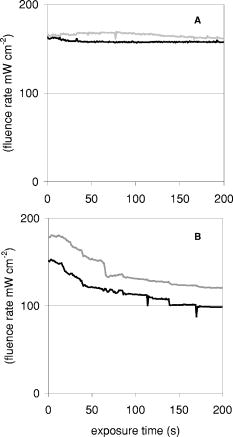 Fig. 5PDT-induced change in fluence rate during treatment of all patients. A decrease in fluence rate during illumination corresponds to a negative percentage. Closed circles represent the seven patients from group 1: DD equals , DLI is . The open circles represent group 2: DD equals , DLI is . 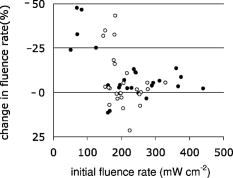 Figure 6 shows the results of the average of all fluence rate cross-talk measurements of all patients. On average, a fluence rate (for the middle location) of is delivered contralateral to the illumination tube to the empty tube. This corresponds to an additional fluence of , that is, 4% of the average . 4.DiscussionIn this study, fluence rate measurements were performed in risk and target areas during -mediated NPC PDT, using a novel light applicator with incorporated isotropic detectors at strategic locations in the nasopharyngeal cavity. In the majority of clinical studies using -PDT in head and neck cancer patients, dosimetry was based upon microlense total output power and the treatment surface area, aiming to deliver an incident fluence rate of and a total fluence of , without taking backscattering into account. 14, 15, 16, 17 Only one study10 measured the tissue surface fluence rate by means of isotropic detectors placed at the tumor center and periphery during illumination. This study demonstrated that the fluences delivered to the oral cavity were 133 to 545% of the incident fluence. Good clinical responses without long-term side effects of the PDT were observed in all these head and neck studies. It is therefore evident that the fluence rates used result in sufficient PDT efficacy and can be safely employed in the oral cavity. However, the wide variation in the total delivered fluence for a given prescribed incident fluence, as shown by Tan, 10 suggests that many patients treated with this protocol received much higher light doses than would be required for tumor control. This could be a severe drawback for PDT in areas where critical normal tissues are involved, and tissue regeneration may not be so complete as in the oral cavity. To ensure maximal ablation of NPC with minimal normal tissue damage, it was therefore decided to include dosimetric parameters in PDT protocols for NPC. Our measurements demonstrated a wide range of total tissue fluence rated for a given incident fluence rate, as was previously seen in the oral cavity.11 We note that while the dosimetric parameters used in the present study were similar to those used previously in the oral cavity, the average measured fluence (rates) in the nasopharynx were relatively high , . Despite the variation in tissue surface fluences between NPC patients, significant necrotic slough over the entire illuminated cavity was seen in all patients, without major complications. This may be explained by the fact that the underlying structures in the nasopharyngeal cavity is highly scattering bone, which protects the deeper structures—such as blood vessels, nerves (optic nerve), or brain tissue—from PDT-induced tissue damage.18 We note that while the dosimetric parameters used in the present study are similar to those used previously in the oral cavity, the average measured fluence rate and fluence are relatively high , . Other organs that lack an underlying bone structure, such as the esophagus and the bronchus, are more prone to PDT complications such as fistulas. 19, 20, 21, 22 Several studies have shown that the efficacy of -PDT can be optimized by lowering the fluence rate in order to reduce the rate and extent of oxygen depletion during PDT.23, 24, 25 While it is difficult to directly translate these preclinical findings into the clinic, the use of a lower fluence rate may be a way of further enhancing the response of tissue to PDT. We note that high fluence rates have generally been used in clinical -PDT of the nasophrangeal and oral cavities. Results could potentially be improved by lowering the fluence rate. A larger randomized trial may benefit from standardization of the low fluence rate during the illumination period. The interpatient variations in fluence rate originate from differences in tissue optical properties and anatomic geometry. Absorbing properties of tissues can vary according to differences in blood flow and tissue oxygenation. Large variations between patients were also seen in the dimensions of the oronasopharynx. In some cases, the applicator bridge had to be reduced in size in order to insert the applicator properly. In phantom measurements in air, the fluence rate remained constant over the length of the diffuser, but in vivo the fluence rate at the distal end of the diffuser was 23% lower than the average fluence rate at the middle of the diffuser. Despite these low fluence rates, substantial necrosis was still observed to beyond the diffuser tip. We believe that this decrease can be explained by integrating cavity theory. In the nasopharyngeal cavity, approximately 75% of the surface consists of scattering tissue and only 25% comprises the highly absorbing black patch protecting the soft pallet; whereas toward the orophanrynx, the ratio between the scattering tissue and the patch is more likely to be 1, resulting in a lower fluence rate. To improve the light distribution profile, we are currently developing patches that are highly scattering on the inside and black on the side of the nasopharynx. A number of factors influence the variation in fluence rate that we observe during illumination. These include increased tissue hemoglobin content, changes in tissue oxygenation due to oxygen consumption during PDT, changes in perfusion, or, to a lesser extent, decreased scattering.26 These parameters were not measured directly in the present study, but they may play a role in the PDT-induced clinical response. For example, the absorption contrast between oxyhemoglobin and deoxyhemoglobin reaches its maximum at around . A 50% drop in oxygenation during illumination would increase the absorption coefficient by a factor of 3.4, clearly altering the cavities’ optical properties because blood is the major absorber at this wavelength. No PDT-induced tissue damage was observed in any of the risk areas, demonstrating sufficient shielding. However delayed regeneration of normal oropharyngeal mucosal was observed in five out of six patients in group 1. In this group, all patients where treated with diffuser lengths of 5 to . It was therefore decided to use 4-cm diffuser lengths for the second group of patients to ensure full closure of the soft palate and the oropharynx. The drawback of using shorter diffuser lengths is the risk of insufficient coverage of tumor margins. Prior knowledge, based on computed tomagraphy and magnetic resonance imaging, of how far the tumor extends into the oropharynx would further improve treatment planning with respect to the optimal cylindrical diffuser length. Another treatment planning aspect that should be taken into consideration is that, due to tissue backscattering, the fluence rate profile broadens along the length of the diffusers. Based on phantom measurements,8 it is estimated that an additional 5 to of necrosis can be expected at the distal end of the diffuser. Measuring the fluence rate at the risk areas required the use of a tongue spatula to expose the region of interest. In combination with the inserted NPC applicator, this induced stress and regurgitative spasm to the patients. Because the initial measurements demonstrated only low fluence rates in shielded areas, these measurements were discontinued for the second group of patients. 5.ConclusionIn conclusion, our findings demonstrate that the applicator is easily inserted and enables for the delivery of a reasonably homogeneous light distribution (i.e., SD/mean equals 34%) in the target area with adequate shielding of the risk area. Interpatient variations in fluence rate stress the need for in vivo dosimetry to allow for correction of differences in optical properties and anatomic geometry between patients and thus deliver a comparable amount of light available for absorption. Eleven out of 13 patients seen three months after PDT were biopsy-proven tumor free. In a future planned randomized phase III study, dosimetry will be fine-tuned further and will be based upon in vivo real-time fluence measurements and delivering a standardized fluence to the tissue surface. Furthermore, the use of lower fluence and fluence rate may further improve the complete response rate. ReferencesE. F. Stranadko,
M. I. Garbuzov,
V. G. Zenger,
A. N. Nasedkin,
N. A. Markichev,
M. V. Riabov, and
I. V. Leskov,
“Photodynamic therapy of recurrent and residual oropharyngeal and laryngeal tumors,”
Vestn. Otorinolaringol., 3 36
–39
(2001). 0042-4668 Google Scholar
V. G. Schweitzer,
“Photodynamic therapy for treatment of head and neck cancer,”
Otolaryngol.-Head Neck Surg., 102 225
–232
(1990). 0194-5998 Google Scholar
Z. Q. Sun,
“Photodynamic therapy of nasopharyngeal carcinoma by argon or dye laser—an analysis of 137 cases,”
Zhonghua Zhong Liu Za Zi, 14 290
–292
(1992). Google Scholar
B. Kulapaditharom and
V. Boonkitticharoen,
“Photodynamic therapy for residual or recurrent cancer of the nasopharynx,”
J. Med. Assoc. Thai, 82 1111
–1117
(1999). 0125-2208 Google Scholar
M. C. Tong,
C. A. van Hasselt, and
J. K. Woo,
“Preliminary results of photodynamic therapy for recurrent nasopharyngeal carcinoma,”
Eur. Arch. Otorhinolaryngol., 253 189
–192
(1996). 0937-4477 Google Scholar
L. A. Lofgren,
S. Hallgren,
E. Nilsson,
A. Westerborn,
C. Nilsson, and
J. Reizenstein,
“Photodynamic therapy for recurrent nasopharyngeal cancer,”
Arch. Otolaryngol. Head Neck Surg., 121 997
–1002
(1995). 0886-4470 Google Scholar
P. C. Levendag,
R. Peters,
C. A. Meeuwis,
L. L. Visch,
D. Sipkema,
C. de Pan,
P. I. Schmitz,
“A new applicator design for endocavitary brachytherapy of cancer in the nasopharynx,”
Radiother. Oncol., 45
(1), 95
–98
(1997). 0167-8140 Google Scholar
H. Nyst,
R. L. P. Van Veen,
I. B. Tan,
R. Peters,
S. Spaniol,
D. J. Robinson,
P. C. Levendag, and
H. J. C. M. Sterenborg,
“Performance of a dedicated light delivery and dosimetry device for photodynamic therapy of nasopharyngeal carcinoma: phantom and volunteer experiments,”
Google Scholar
H. P. A. Marijnissen,
H. Jansen, and
W. M. Star,
“Treatment system for whole bladder wall photodynamic therapy with in vivo monitoring and control of light dose rate and dose,”
J. Urol. (Baltimore), 142 1351
–1355
(1989). 0022-5347 Google Scholar
R. L. Van Veen,
M. C. Aalders,
K. L. Pasma,
P. D. Siersema,
J. Haringsma,
W. Van De Vrie,
E. E. Gabeler,
D. J. Robinson, and
H. J. Sterenborg,
“In situ light dosimetry during photodynamic therapy of Barrett’s esophagus with 5-aminolevulinic acid,”
Lasers Surg. Med., 31 299
–304
(2002). https://doi.org/10.1002/lsm.10129 0196-8092 Google Scholar
I. B. Tan,
H. Oppelaar,
M. C. Ruevekamp,
R. B. Veenhuizen,
A. Timmers, and
F. A. Stewart,
“The importance of in situ light dosimetry for photodynamic therapy of oral cavity tumors,”
Head Neck, 21 434
–441
(1999). 1043-3074 Google Scholar
P. Baas,
L. Murrer,
F. A. Zoetmulder,
F. A. Stewart,
H. B. Ris,
N. van Zandwijk,
J. L. Peterse, and
E. J. Rutgers,
“Photodynamic therapy as adjuvant therapy in surgically treated pleural malignancies,”
Br. J. Cancer, 76 819
–826
(1997). 0007-0920 Google Scholar
L. H. P. Murrer,
H. P. A. Marijnissen,
P. Baas, and
W. M. Star,
“Applicator for light delivery and in situ light dosimetry during endobronchial photodynamic therapy: first measurements in humans,”
Lasers Med. Sci., 12 253
–259
(1997). 0268-8921 Google Scholar
M. P. Copper,
I. B. Tan,
H. Oppelaar,
M. C. Ruevekamp, and
F. A. Stewart,
“Meta-tetra(hydroxyphenyl)chlorin photodynamic therapy in early-stage squamous cell carcinoma of the head and neck,”
Arch. Otolaryngol. Head Neck Surg., 129
(7), 709
–711
(2003). 0886-4470 Google Scholar
C. Hopper,
A. Kubler,
H. Lewis,
I. B. Tan, and
G. Putnam,
“THPC-mediated photodynamic therapy for early oral squamous cell carcinoma,”
Int. J. Cancer, 111
(1), 138
–146
(2004). 0020-7136 Google Scholar
A. K. D’Cruz,
M. H. Robinson, and
M. A. Biel,
“THPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients,”
Head Neck, 26
(3), 232
–240
(2004). 1043-3074 Google Scholar
K. F. Fan,
C. Hopper,
P. M. Speight,
G. A. Buonaccorsi, and
S. G. Bown,
“Photodynamic therapy using THPC for malignant disease in the oral cavity,”
Int. J. Cancer, 73
(1), 25
–32
(1997). 0020-7136 Google Scholar
A. C. Kubler,
W. Stenzel,
M. Ruhling,
B. Meul, and
J. H. Fischer,
“Experimental evaluation of possible side effects of intra-operative photodynamic therapy on rabbit blood vessels and nerves,”
Lasers Surg. Med., 33
(4), 247
–255
(2003). 0196-8092 Google Scholar
J. Etienne,
N. Dorme,
G. Bourg-Heckly,
P. Raimbert,
J. F. Flejou,
“Photodynamic therapy with green light and -tetrahydroxyphenyl chlorin for intramucosal adenocarcinoma and high-grade dysplasia in Barrett’s esophagus,”
Gastrointest. Endosc., 59
(7), 880
–889
(2004). 0016-5107 Google Scholar
S. Andrejevic Blant,
P. Grosjean,
J. P. Ballini,
G. Wagnieres,
H. van den Bergh,
C. Fontolliet, and
P. Monnier,
“Localization of tetra(-hydroxyphenyl)chlorin (Foscan) in human healthy tissues and squamous cell carcinomas of the upper aero-digestive tract, the esophagus and the bronchi: a fluorescence microscopy study,”
J. Photochem. Photobiol., B, 61
(1–2), 1
–9
(2001). 1011-1344 Google Scholar
J. F. Savary,
P. Monnier,
C. Fontolliet,
J. Mizeret,
G. Wagnieres,
D. Braichotte, and
H. van den Bergh,
“Photodynamic therapy for early squamous cell carcinomas of the esophagus, bronchi, and mouth with -tetra (hydroxyphenyl) chlorin,”
Arch. Otolaryngol. Head Neck Surg., 123
(2), 162
–168
(1997). 0886-4470 Google Scholar
L. H. Murrer,
K. M. Hebeda,
J. P. Marijnissen, and
W. M. Star,
“Short- and long-term normal tissue damage with photodynamic therapy in pig trachea: a fluence-response pilot study comparing Photofrin and THPC,”
Br. J. Cancer, 80
(5–6), 744
–755
(1999). 0007-0920 Google Scholar
S. Coutier,
L. N. Bezdetnaya,
T. H. Foster,
R. M. Parache, and
F. Guillemin,
“Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (THPC)-sensitized HT29 xenografts in nude mice,”
Radiat. Res., 158
(3), 339
–345
(2003). 0033-7587 Google Scholar
H. Tsutsui,
A. J. MacRobert,
A. Curnow,
A. Rogowska,
G. Buonaccorsi,
H. Kato, and
S. G. Bown,
“Optimisation of illumination for photodynamic therapy with THPC on normal colon and a transplantable tumour in rats,”
Lasers Med. Sci., 17
(2), 101
–109
(2002). 0268-8921 Google Scholar
S. Coutier,
S. Mitra,
L. N. Bezdetnaya,
R. M. Parache,
I. Georgakoudi,
T. H. Foster, and
F. Guillemin,
“Effects of fluence rate on cell survival and photobleaching in meta-tetra-(hydroxyphenyl)chlorin-photosensitized Colo 26 multicell tumor spheroids,”
Photochem. Photobiol., 73
(3), 297
–303
(2001). 0031-8655 Google Scholar
A. Amelink,
A. van der Ploeg van den Heuvel,
W. J. de Wolf,
D. J. Robinson, and
H. J. Sterenborg,
“Monitoring PDT by means of superficial reflectance spectroscopy,”
J. Photochem. Photobiol., B, 79
(3), 243
–251
(2005). 1011-1344 Google Scholar
|