|
|
1.IntroductionThe cochlea is the organ of hearing (Fig. 1 ). It transduces the mechanical energy of sound pressure waves into electrical energy, which is then carried to the brain via the auditory nerve. Sensation occurs when the sound pressure waves induce nanoscale vibrations of the structures within the inner ear. The dominant vibratory mechanical component of the cochlea is the basilar membrane. Its mass and stiffness vary systematically in a reciprocal fashion along the length of the cochlea, and hence acts as a cascade of passive filters. 1, 2, 3, 4 This spatial variation in mechanical properties of the basilar membrane creates a tonotopic organization to the cochlea, with the base sensitive to high-frequency tones and the apex sensitive to low-frequency tones. Thus, there is a spatial representation of frequency. Fig. 1Diagram of the mouse cochlea with the orientation of the optical fiber. A midmodiolar cross section through the cochlea cuts perpendicular to the organ of Corti. The pipette used to perfuse trypan blue to stain the basilar membrane at the base of the cochlea (BM-base) was inserted through the round window membrane (RW): basilar membrane at the apex of the cochlea (BM-apex), osseous spiral lamina (OSL-base), stria vascularis (SV-base), and auditory nerve (AN). 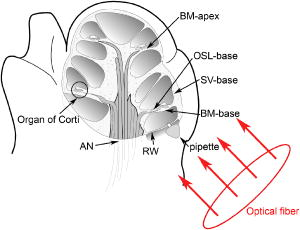 The basilar membrane consists of a filamentous layer consisting primarily of collagen fibers that run radially from medial to lateral5, 6 (i.e., perpendicular to the length of the cochlear duct). Collagen is regarded as a main source of basilar membrane stiffness and therefore plays a critical role in establishing its tuned resonant frequency map. 7, 8, 9, 10 Collagen molecules are packed together in a hexagonal manner to form fibrils, which are covalently bound together with groups of other fibrils to form collagen fibers.11, 12 Ground substance fills in the spaces between fibers. The sensory epithelium of the inner ear, located on top of the basilar membrane, is called the organ of Corti.13, 14 It contains the sensory hair cells that perform the actual sound transduction process (for review, see Ref. 14). The mechanical properties of the hair cells, as well as the other structures of the organ of Corti (the tectorial membrane, supporting cells, pillar cells, etc.) play important roles in refining cochlear tuning. However, these contributions are not as critical as that of the basilar membrane itself in determining the overall tonotopic frequency map of the cochlea. 15, 16, 17, 18, 19, 20 Lasers can induce conformational changes in collagen in many different tissues including the cornea,21 the periodontal ligament,22 the medial collateral ligament,23 the femoropatellar joint capsule,24, 25 and skin.26. Initially, there is a decrease in tissue stiffness due to photocoagulation of collagen.23, 24 Previously, we demonstrated that immediate photocoagulative effects similarly occur within the basilar membrane after laser irradiation.27 Studies in other tissues have shown that there are also delayed effects of laser irradiation on collagen.28, 29, 30 In the subsequent days to weeks after irradiation, there is an increase in tissue stiffness as existing collagen remodels and new collagen is synthesized. Ongoing changes can occur even up to after laser treatment.28 Increases in tissue stiffness of up to 100% have been shown in tendon connective tissue after laser irradiation.23 We hypothesized that cochlear laser irradiation would induce delayed changes in basilar membrane collagen similar to that found in other tissues. Using polarized light and transmission electron microscopy, we examined the effects of laser irradiation on basilar membrane collagen. Herein, we demonstrate that, 2 weeks after the irradiation procedure, collagen remodeling and new collagen deposition can be detected within the basilar membrane. Additionally, we assessed the impact of the laser irradiation procedure on auditory thresholds by measuring the auditory brainstem response (ABR). 2.Material and Methods2.1.Surgical TechniqueThe Baylor College of Medicine Institutional Animal Care and Use Committee approved the study protocol. C57BL/6 mice, weighing 20 to , age 4 to 8 weeks were anesthetized with an intraperitoneal (IP) injection of ketamine hydrochloride and xylazine hydrochloride (5 to ). Body temperature was maintained at using an electric heating pad (FHC, Bowdoinham, Maine). A retroauricular incision extending ventrally was made and the soft tissues divided to expose the tympanic bulla. The bulla was then opened and the round window membrane exposed. ABR thresholds were then measured (see the following). The membranous structures within the cochlea are nearly transparent and are bathed in an aqueous environment. To enhance laser energy absorption and achieve optical selectivity for the basilar membrane, an exogenous chromophore was applied. We chose trypan blue (Gibco Laboratories, New York), a water-soluble stain that has maximal optical absorption at , with an absorption coefficient . At the laser wavelength of available to us (see details following), the absorption coefficient of trypan blue is31 less than . Trypan blue was diluted to 0.1% in artificial perilymph (containing, in mM: , , , , 10 HEPES, 10 glucose). The pH was titrated to 7.3 and the osmolarity was . A flexible micropipette (Microfil, World Precision Instruments, Sarasota, Florida) was inserted through the round window and directed up the duct of scala tympani about . Trypan blue was gently perfused in over . As the fluid drained out the round window, it was aspirated with a cotton wisp. Thus, only the basal region of the cochlea was stained. ABR thresholds were remeasured and the cochlea was then irradiated. After laser irradiation, the incision was closed with sutures and the mouse was monitored until fully awake. The animal was then returned to its cage and maintained for another 14 to 16 days. At that point, the mouse was reanesthetized and the incision reopened. Any fluid within the middle ear space was aspirated and ABR thresholds were measured for a third time. The mouse was then sacrificed, perfused systemically with fixative, and its temporal bones harvested. 2.2.Laser IrradiationA 694-nm-long pulsed ruby laser (Epilaser, Palomar Medical Technologies, Inc., Burlington, Massachusetts) was used to irradiate the cochlea. The light was coupled from the articulated arm of the laser into a fiber optic probe to facilitate light delivery to the basilar membrane through the exposed cochlea. Light output from the optical fiber was measured with an Ophir (30A, Wilmington, Massachusetts) power meter. It was estimated that the spot size was in diameter at the cochlea. The radiant exposure applied at the focal spot was selected to be either (one pulse of ) or (six pulses of ) with a repetition rate of . We chose the dosage because it was the lowest dose used in our previous ex vivo study that caused statistically significant decreases in basilar membrane birefringence.27 We chose the dose as the lowest in this set of experiments because it is a dose commonly used in clinical practice to induce collagen changes for skin rejuvenation.32, 33 2.3.Measurement of ABR ThresholdsSine wave stimuli were generated using a digital signal processing system. The stimuli were attenuated to the appropriate intensity, and delivered acoustically to the ear by an electrostatic speaker (RP-2, PA-5, ED-1, and EC-1 Tucker-Davis Technologies). The speaker was calibrated across the frequency range of 1 to prior to each experiment using a probe-tip microphone (microphone type 8192, NEXUS conditioning amplifier, Bruel and Kjar, Demark). This was performed after connecting the microphone and speaker to an earbar inserted into the animal’s ear canal. The ABR was recorded using needle electrodes positioned at the vertex of the skull and along the ventral surface of the tympanic bulla. A ground electrode was placed in the hind leg. The signals were amplified 10,000 times using a biological amplifier (HS4/DB4, Tucker-Davis Technologies, Alachua, Florida), digitized at (RP-2, Tucker-Davis Technologies), and digitally filtered to pass frequency components in the 300 to 3000-Hz range. The stimulus for eliciting the ABR was a 5-ms sine wave tone with envelope rise and fall times of . The repetition time was and 250 trials were averaged. The peak-to-peak ABR signal was measured at stimulus intensities ranging from 10 to sound pressure level (SPL) in 10-dB steps for each frequency. These data were interpolated using a cubic spline algorithm (MATLAB, Release 13, The Mathworks, Natick, Massachusetts) off-line to identify where the signal crossed the average noise floor plus four standard deviations to determine frequency-specific thresholds. We measured ABR thresholds at frequencies between 4 to . If no ABR was detected even at our equipment limits of SPL, we arbitrarily defined the threshold to be when calculating average thresholds. Statistical analysis was performed using Excel (Microsoft, Redmond, Washington). ABR thresholds in the same mice before and after trypan blue perfusion were compared using the Student’s two-tailed paired test. The mean ABR thresholds in each group of irradiated mice were compared to each other and to ABR thresholds of all the mice before trypan blue perfusion using the Student’s two-tailed nonpaired test. Statistical significance was defined as . All presented values are mean SEM (standard error of the mean). 2.4.Polarized Light MicroscopyAfter harvesting the cochleas, they were immersed in 4% paraformaldehyde for 2 days, decalcified in 0.2-M EDTA for 2 weeks, embedded in paraffin, and cut perpendicular to the organ of Corti in sections. To enhance the native birefringence of collagen, the sections were stained with picrosirius red (Sigma Aldrich, St. Louis, Missouri). Picrosirius red is a strongly elongated, birefringent molecule that binds parallel to collagen molecules. 21, 34, 35, 36, 37 It was originally used in the textile industry to analyze fiber orientation,38 and it has been used to assess collagen organization35, 39 since the 1960s. We additionally stained the sections with hemotoxylin (Thermo Shandon, Pennsylvania) to visualize regions with minimal collagen. Once all of the cochlear cross sections were cut and stained, they were visualized using polarized light microscopy to highlight parallel arrays of collagen. Before starting this procedure, one control specimen was placed on the microscope stage and the two polarizers were rotated to maximize the birefringence of the tissue. Once this was done, the polarizers were locked into place and all of the specimens were studied in the same anatomic orientation. Thus, polarization settings were held constant for all sections. Images were captured digitally using a 6.3-Mpixel camera (Canon, EOS 10D, Tokyo 146-8501, Japan) connected to an upright microscope (Zeiss, Axioskop 2, Germany). The applied light, aperture size, and time exposure were also held constant for all images. ImageJ [National Institute of Health (NIH), http://rsb.info.nih.gov/ij/] was used to quantify the intensity of the birefringence within different collagen-containing areas of the cochlea. The color images of each turn of each cochlea were converted to 8-bit gray scale. After conversion, areas of high birefringence were white and areas of low birefringence were gray or black. Within each region to be analyzed, the area with the strongest birefringence was chosen and the signal intensity determined by averaging at least 60 pixels. The two-tailed nonpaired Student’s test was used to compare the birefringence between different regions and laser dosages. 2.5.Transmission Electron MicroscopyThe harvested cochleas were fixed in 2.5% glutaraldehyde in 0.1-M cacodylate buffer containing 2-mM at a pH 7.4, and decalcified in 0.2-M EDTA for 2 weeks. They were then washed several times with 0.1-M cacodylate buffer. The samples were postfixed in 1% osmium tetroxide in 0.1-M cacodylate buffer and then washed with cacodylate buffer. Gradual dehydration was performed from 25 to 50% ethanol and the cochleas were then stained with 2% uranyl acetate in 50% ethanol for an hour. The cochleas were further dehydrated in 100% ethanol, followed by the transitional dehydrating agent propylene oxide. The cochleas were gradually infiltrated with propylene oxide resin (Durucupan ACM Resin). This process was repeated using progressively higher resin concentrations until pure resin was infiltrated. The cochleas were oriented in resin-filled blocks, cured for 2 days at 55°, and then cut into 80-nm thin sections with an ultramicrotome. The sections were stained with 2% uranyl acetate in 50% ethanol for and with Reynold’s lead citrate for . The specimens were imaged using a transmission electron microscope (H-7500, Hitachi). 2.6.AnimalsWe studied a total of 18 mice. The first 11 mice were used to develop the technique of in vivo laser irradiation. From these 11 animals, we present only the ABR data collected prior to laser irradiation (i.e., after the surgical exposure of the cochlea and after perfusion of trypan blue into the cochlea). After the laser irradiation procedure was perfected, four mice were irradiated at and 3 mice were irradiated at . One cochlea from each of these groups was prepared for electron microscopy analysis, and the others (three and two cochleas, respectively) underwent ABR threshold testing and polarized light microscopy analysis. Four contralateral cochleas were used as controls. They were not operated on, and so were not stained with trypan blue or irradiated. One was studied with electron microscopy and three were studied with polarized light microscopy. 3.Results3.1.Polarized Light MicroscopyPolarized light microscopy revealed that the birefringence of the basilar membrane of the basal turn 2 weeks after irradiation was higher than in controls (Fig. 2 ). In a cochlea irradiated at , the organ of Corti architecture appeared normal 2 weeks following laser irradiation. Both the inner and outer hair cells were maintained throughout the cochlea at this laser dosage. At , the basilar membrane demonstrated even more birefringence than that at the lower laser dosage. However, intracochlear fibrosis and inflammatory tissue filled the scalae. The cells that normally make up the organ of Corti (hair cells and supporting cells) were not evident. Fig. 2Organ of Corti. Paraffin-embedded cochlear cross sections stained with picrosirius red and hemotoxylin were visualized using polarization microscopy to highlight collagen arrays. (A) A representative control section (cochlea not perfused or irradiated). The normal basilar membrane birefringence can be noted (*). Inner and outer hair cells are also visible (arrows). (B) A representative section of a cochlea 14 days after the laser irradiation with . Note the continued presence of hair cells (arrows) and the normal architecture of the organ of Corti. (C) A representative section of a cochlea 14 days after irradiation with . The basilar membrane birefringence appears brighter (more yellow) than that of the control cochlea. Hair cells are no longer present and scar tissue fills the intracochlear scalae (arrowheads). 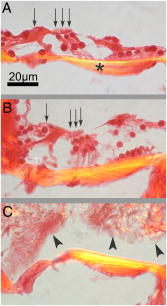 We quantified the tissue birefringence from four different regions in each cochlea (Fig. 3 ). The basilar membrane from the basal turn near the round window (BM-base) was the area stained with trypan blue and was directly in the path of the laser beam. The basilar membrane of the apical turn (BM-apex) was likely not stained by the trypan blue. The stria vascularis (SV-base) is the tissue along the lateral wall of the cochlea that produces the electrochemical gradients within the scala media necessary for normal cochlear function. The osseous spiral lamina (OSL-base) is the bone to which the basilar membrane makes its medial attachment. We only analyzed the stria vascularis and osseous spiral lamina within the basal turn of the cochlea. Fig. 3Quantification of collagen birefringence. Measurements were taken from the basilar membrane in the basal turn (BM-base), the basilar membrane in the apical turn (BM-apex), the stria vascularis in the basal turn (SV-base), and the osseous spiral lamina in the basal turn (OSL-base). The intensity of birefringence is the average pixel intensity on a scale of 0 to 255. At both 15- and laser dosages, there were statistically significant increases in the collagen birefringence in the BM-base compared to controls (*). Additionally, the collagen birefringence in the BM-base after was larger than that after (**). No changes occurred in the other regions of the cochlea. 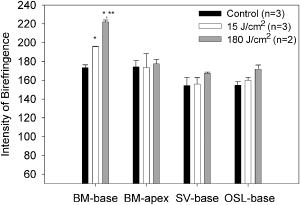 We found statistically significant increases in the average birefringence of the BM-base in cochleas irradiated with either laser dosage compared to controls ( , , and for controls, , and , respectively; ). Additionally, the effect was dose dependent in that the mean birefringence of cochleas irradiated at was larger than that of the cochlea irradiated at . There were no statistically significant changes in tissue birefringence in the BM-apex, SV-base, or OSL-base for cochleae irradiated with either laser dosage compared to controls. 3.2.Transmission Electron MicroscopyTransmission electron microscopy of the basilar membrane of a control cochlea revealed the expected normal architecture (Fig. 4 ). There were large collagen fibers oriented in a radial fashion with loose connective tissue and ground substance between them. The space between the fibers was filled with white vacuoles. This indicates that the material within these spaces leeched out of the basilar membrane during fixation, and thus did not consist of collagen. Fig. 4Transmission electron microscopy of the basilar membrane. A cross section of the basilar membrane from the basal turn is shown. (a) A representative control section demonstrates the normal organization of the basilar membrane. There are collagen fibers (*) separated by loose connective tissue, which appear predominantly as white vacuoles. (b) Fourteen days after irradiation with . The collagen fibers are slightly denser than in the control section. There are also an increased amount of thin collagen fibrils within the loose connective tissue between the fibers (arrow). (c) Fourteen days after irradiation with . At this level of irradiation, the collagen fibers remain denser than in the control section. In many areas, the fibers seem wider with less crisp borders. Thus, there is less space between adjacent collagen fibers and almost no vacuolated space. This is due to the substantial amount of thin collagen fibrils within the loose connective tissue between fibers (arrow). 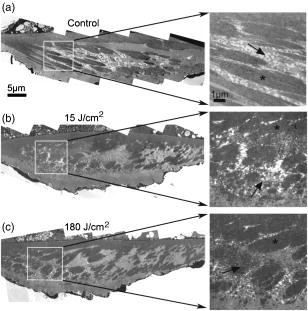 In the irradiated cochleas, the collagen fibers were denser than in controls, suggesting that remodeling of the existing collagen had occurred. Also, there were substantial numbers of new collagen fibrils within the loose connective tissue regions between the fibers. These fibrils were irregularly organized in between adjacent fibers. However, the new fibrils also appeared to condense around the borders of the preexisting collagen fibers. This may perhaps reflect the early incorporation of these new collagen fibrils into the fibers. Thus, there was a reduced amount of the vacuolated space between the fibers after , and almost no vacuolated space after . Together, these findings are consistent with new collagen deposition within the basilar membrane.40, 41 3.3.ABR Threshold ShiftsABR thresholds were measured after opening the tympanic bulla, following perfusion of the cochlea with trypan blue, and just prior to sacrifice 14 to 16 days later (Fig. 5 ). Normal thresholds were found after opening the bulla, as expected. There were no changes in ABR thresholds after trypan blue perfusion, except for 4 to increases in the ABR threshold at 22 and that were statistically significant. This indicates that the perfusion procedure and the chromophore application had only minimal effects on cochlear function. This finding is consistent with data from the guinea pig cochlea, in which only a slight high-frequency threshold shift develops when artificial perilymph is perfused through scala tympani in a careful fashion.43, 44 Presumably, this hearing loss reflects inadvertent cochlear trauma or cooling of the cochlea because of its exposure to the outside environment. Fig. 5ABR thresholds. After initially opening the bulla, the mice had normal thresholds across the frequency spectrum. After perfusing trypan blue through the round window, there were only statistically significant increases in the ABR threshold at 22 and . Two weeks after irradiating with , there were 17 to 27-dB increases in ABR thresholds that were statistically significant compared to baseline at every frequency except at . After irradiating with , there were 32 to 59-dB increases in ABR thresholds across the frequency spectrum compared to baseline. SPL was defined using a standard reference that is approximately the intensity of a 1000-Hz sinusoid that is just barely audible to a human with normal hearing42 . Postirradiation thresholds that were statistically elevated from baseline are marked (*). Thresholds statistically higher after 180 compared to are marked (**). 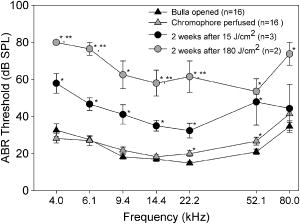 Two weeks after laser irradiation with , there were increases in ABR thresholds ranging from 17 to that were statistically significant at every frequency except at . After laser irradiation with , there were larger increases in ABR thresholds ranging from 32 to across the frequency spectrum. Statistically significant increases in ABR thresholds were found in mice irradiated with compared to those irradiated with at 4, 6, 14, and that ranged from 22 to . 4.DiscussionIn this paper we show that 2 weeks after laser irradiation, there are histologic changes consistent with collagen remodeling and new collagen deposition both by polarized light and electron microscopy. We previously demonstrated that there is a decrease in basilar membrane birefringence acutely.27 This process has been well-studied in other tissues and been found to reflect a photocoagulative effect that changes the properties of individual collagen molecules so that interpeptide hydrogen bonds break and result in collapse of their normal linear structure. 21, 28, 29, 30, 45, 46 This breakage of normal linear structure causes an acute decrease in stiffness, which likely also occurs within the basilar membrane. These studies have also shown that with time, there is new collagen deposition and reorganization that leads to an increase in tissue birefringence and stiffness. Our findings suggest that collagen within the basilar membrane undergoes similar changes. There are two potential explanations for the delayed increase in basilar membrane birefringence measured by polarization microscopy after laser irradiation. First, the new collagen fibrils that were deposited would be expected to bind additional picrosirius red molecules. This binding will increase the tissue birefringence only if the molecules are organized into arrays. Presumably, the irregularly organized fibrils between collagen fibers did not substantively detract from organization of the fibrils that were condensing around the preexisting collagen fibers. If more than 14 days of wound healing was permitted, we would expect the tissue birefringence to continue to increase as the newly deposited collagen organizes further. Second, it is possible that there was recruitment of additional binding sites for picrosirius red molecules to pre-existing molecules because of collagen remodeling.21 In any case, changes in collagen birefringence were noted only within the basal turn of the basilar membrane. Since only the basal turn was perfused with the exogenous chromophore trypan blue, the basilar membrane within the apical turn likely remained unstained and hence did not absorb the laser energy. In our previous study with an excised cochlear preparation, the entire length of the cochlea was perfused with trypan blue, and irradiation effects were noted within the basilar membrane of every turn.27 We believe the reason that significant changes in tissue birefringence did not occur within the stria vascularis and the osseous spiral lamina is that the chromophore did not substantially stain those tissues. However, in our previous study, we did note staining of these tissues and measured changes in their birefringence after laser irradiation. The reason for this difference is probably because the experimental preparations are not the same. In vivo, there is continuous perilymph flow out of the open round window since mice have a patent cochlear aqueduct. This permits the entry of cerebrospinal fluid into the inner ear from the subarachoid space.47 Therefore, the chromophore is rapidly washed out from the cochlea during perfusion and likely does not have time to penetrate into these thicker tissue structures. At the laser energy of , intense intrascalar fibrosis occurred and ABR thresholds were raised across the frequency spectrum. The normal auditory frequency range of the mouse is 1 to , and there is an exponential pattern to the basilar membrane frequency-place map of .48, 49 Based on the area targeted by the laser ( of basilar membrane at the basal end of the cochlea), one might expect that only frequencies would be affected by the laser. However, the elevation in low-frequency thresholds is not surprising given the longitudinal coupling within the cochlea that is needed to sustain traveling waves.1, 14 Modeling studies have confirmed that intrascalar scarring at the base of the cochlea can cause reduced cochlear responses at the apex.50 Additionally, we did not assess for spiral ganglion cell damage in this study, and it is possible that the entire auditory nerve could have been affected as it courses through the lower portion of the modiolus near the round window. Finally, changes in the endocochlear potential could have occurred, producing broad-spectrum hearing loss. Clearly, reducing the laser dosage appears to be critical in minimizing side effects. No evidence of intrascalar fibrosis was found at , and there were normal-appearing hair cells and organ of Corti architecture, which is quite encouraging. However, there was still a moderate elevation in ABR thresholds across the frequency spectrum. There are many potential explanations for this. As discussed, damage to spiral ganglion cells or a reduced endocochlear potential could be a factor. Additionally, changes in the tectorial membrane or hair cell mechanics could have occurred. Although difficult to prove, some of the changes in ABR thresholds that we measured may actually reflect a change in the biomechanics of the basilar membrane. Interestingly, this lower laser dosage is within a more clinically relevant range. The typical range of laser dosage used for skin rejuvination is 2 to , a technique based on the same concept of acutely photocoagulating collagen to stimulate delayed collagen remodeling and new collagen deposition. 28, 29, 30, 32, 33 The mouse basilar membrane is much thinner and more delicate than human skin, and it is probable that lower irradiation dosages may still stimulate changes in basilar membrane collagen with a lower risk of toxicity. Ultimately, our goal is to use cochlear laser irradiation to change the biomechanical properties of the basilar membrane by changing collagen organization and density. It remains to be studied if these types of laser-irradiation-induced changes within the basilar membrane do increase its stiffness, as described in other tissues. 23, 28, 29, 30 Obviously, it will be important to measure the stiffness and resonant frequency of the basilar membrane before and after irradiation to determine whether the tonotopic frequency map of the cochlea can be modulated. We predict that there should be a decrease in the resonant frequency of an irradiated region acutely because the photocoagulative effect should decrease basilar membrane stiffness. However, with time, there should be a gradual increase in the resonant frequency of that region as collagen remodeling and deposition occurs, increasing basilar membrane stiffness. If laser irradiation can safely “retune” the resonant frequency map of the cochlea, it may provide the basis for a novel therapeutic approach for the treatment of hearing loss. The most common type of sensorineural hearing loss is age-related hair cell degeneration in the high-frequency region of the cochlea. 51, 52, 53, 54 It is conceivable that using this technique, a low-frequency region of the cochlea with functional sensory hair cells might be made to resonate at a higher frequency in an effort to partially compensate for this high-frequency hearing loss. AcknowledgmentsWe wish to acknowledge Parmeswaran Diagaradjane for technical assistance with some experiments, Isaac Ayala for the artwork, NIH Grant No. DC006671 (to JSO), and a grant from the Alliance for NanoHealth/U.S. Department of Defense (USDOD) (to JSO and BA). ReferencesG. von Bekesy, Experiments in Hearing, McGraw-Hill, New York (1960). Google Scholar
R. C. Naidu and
D. C. Mountain,
“Measurements of the stiffness map challenge a basic tenet of cochlear theories,”
Hear. Res., 124
(1–2), 124
–131
(1998). https://doi.org/10.1016/S0378-5955(98)00133-6 0378-5955 Google Scholar
E. S. Olson,
“Direct measurement of intra-cochlear pressure waves,”
Nature (London), 402
(6761), 526
–529
(1999). https://doi.org/10.1038/990092 0028-0836 Google Scholar
E. S. Olson,
“Intracochlear pressure measurements related to cochlear tuning,”
J. Acoust. Soc. Am., 110
(1), 349
–367
(2001). https://doi.org/10.1121/1.1369098 0001-4966 Google Scholar
S. Iurato,
“Functional implications of the nature and submicroscopic structure of the tectorial and basilar membranes,”
J. Acoust. Soc. Am., 34 1386
–1395
(1962). https://doi.org/10.1121/1.1918355 0001-4966 Google Scholar
D. J. Lim and
H. N. Kim,
“The canaliculae perforantes of Schuknecht,”
Adv. Oto-Rhino-Laryngol., 31 85
–117
(1983). 0065-3071 Google Scholar
L. Voldrich,
“Mechanical properties of basilar membrane,”
Acta Oto-Laryngol., 86
(5–6), 331
–335
(1978). 0001-6489 Google Scholar
C. E. Miller,
“Structural implications of basilar membrane compliance measurements,”
J. Acoust. Soc. Am., 77
(4), 1465
–1474
(1985). https://doi.org/10.1121/1.392041 0001-4966 Google Scholar
W. Plassmann,
W. Peetz, and
M. Schmidt,
“The cochlea in gerbilline rodents,”
Brain Behav. Evol., 30
(1–2), 82
–101
(1987). 0006-8977 Google Scholar
G. Ehret,
“Stiffness gradient along the basilar membrane as a basis for spatial frequency analysis within the cochlea,”
J. Acoust. Soc. Am., 64
(6), 1723
–1726
(1978). https://doi.org/10.1121/1.382153 0001-4966 Google Scholar
L. Stryer,
(1975) Google Scholar
F. H. Silver, Biological Materials: Structure, Mechanical Properties, and Modeling of Soft Tissues, New York University Press, New York (1987). Google Scholar
J. S. Oghalai,
“The cochlear amplifier: augmentation of the traveling wave within the inner ear,”
Curr. Opin. Otolaryngol. Head Neck Surg., 12
(5), 431
–438
(2004). Google Scholar
C. D. Geisler, From Sound to Synapse: Physiology of the Mammalian Ear, Oxford University Press, New York (1998). Google Scholar
P. K. Legan,
A. Rau,
J. N. Keen, and
G. P. Richardson,
“The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system,”
J. Biol. Chem., 272
(13), 8791
–8801
(1997). https://doi.org/10.1074/jbc.272.13.8791 0021-9258 Google Scholar
M. Cohen-Salmon,
A. El-Amraoui,
M. Leibovici, and
C. Petit,
“Otogelin: a glycoprotein specific to the acellular membranes of the inner ear,”
Proc. Natl. Acad. Sci. U.S.A., 94
(26), 14450
–14455
(1997). https://doi.org/10.1073/pnas.94.26.14450 0027-8424 Google Scholar
D. J. Lim,
“Fine morphology of the tectorial membrane. Its relationship to the organ of Corti,”
Arch. Otolaryngol., 96
(3), 199
–215
(1972). 0003-9977 Google Scholar
D. J. Lim,
“Development of the tectorial membrane,”
Hear. Res., 28
(1), 9
–21
(1987). https://doi.org/10.1016/0378-5955(87)90149-3 0378-5955 Google Scholar
R. J. Goodyear and
G. P. Richardson,
“Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity,”
J. Neurobiol., 53
(2), 212
–227
(2002). https://doi.org/10.1002/neu.10097 0022-3034 Google Scholar
K. Legan,
R. J. Goodyear,
V. Lukashkina, and
A. N. Lukashkin,
“Loss of striated-sheet matrix, aberrant collagen fibril organization, and changes in the tectorial membrane’s frequency and level-dependent properties in the beta-tectorin null mutant mouse,”
207
(2003) Google Scholar
M. N. Asiyo-Vogel,
R. Brinkmann,
H. Notbohm,
R. Eggers,
H. Lubatschowski,
H. Laqua, and
A. Vogel,
“Histologic analysis of thermal effects of laser thermokeratoplasty and corneal ablation using Sirius-red polarization microscopy,”
J. Cataract Refractive Surg., 23
(4), 515
–526
(1997). 0886-3350 Google Scholar
G. Kesler,
R. Koren,
A. Kesler,
D. Kristt, and
R. Gal,
“Differences in histochemical characteristics of gingival collagen after ER:YAG laser periodontal plastic surgery,”
J. Clin. Laser Med. Surg., 18
(4), 203
–207
(2000). 1044-5471 Google Scholar
D. T. Fung,
G. Y. Ng,
M. C. Leung, and
D. K. Tay,
“Therapeutic low energy laser improves the mechanical strength of repairing medial collateral ligament,”
Lasers Surg. Med., 31
(2), 91
–96
(2002). https://doi.org/10.1002/lsm.10083 0196-8092 Google Scholar
K. Hayashi,
P. Hecht,
G. Thabit, 3rd,
D. M. Peters, R. Vanderby Jr., A. J. Cooley,
G. S. Fanton,
J. F. Orwin, and
M. D. Markel,
“The biologic response to laser thermal modification in an in vivo sheep model,”
Clin. Orthop. Relat. Res., 373 265
–276
(2000). https://doi.org/10.1097/00003086-200004000-00033 0009-921X Google Scholar
K. Hayashi,
J. A. Nieckarz,
G. Thabit, 3rd,
J. J. Bogdanske,
A. J. Cooley, and
M. D. Markel,
“Effect of nonablative laser energy on the joint capsule: an in vivo rabbit study using a holmium:YAG laser,”
Lasers Surg. Med., 20
(2), 164
–171
(1997). https://doi.org/10.1002/(SICI)1096-9101(1997)20:2<164::AID-LSM7>3.0.CO;2-O 0196-8092 Google Scholar
J. Tang,
F. Zeng,
H. Savage,
P. P. Ho, and
R. R. Alfano,
“Fluorescence spectroscopic imaging to detect changes in collagen and elastin following laser tissue welding,”
J. Clin. Laser Med. Surg., 18
(1), 3
–8
(2000). 1044-5471 Google Scholar
G. I. Wenzel,
B. Pikkula,
C. H. Choi,
B. Anvari, and
J. S. Oghalai,
“Laser irradiation of the guinea pig basilar membrane,”
Lasers Surg. Med., 35
(3), 174
–180
(2004). https://doi.org/10.1002/lsm.20091 0196-8092 Google Scholar
M. A. Trelles,
L. Garcia,
J. Rigau,
I. Allones, and
M. Velez,
“Pulsed and scanned carbon dioxide laser resurfacing 2 years after treatment: comparison by means of scanning electron microscopy,”
Plast. Reconstr. Surg., 111
(6), 2069
–2078
(2003). https://doi.org/10.1097/01.PRS.0000057143.53648.8B 0032-1052 Google Scholar
M. A. Trelles,
L. Garcia,
J. Rigau,
I. Allones, and
M. Velez, Plast. Reconstr. Surg., 111
(6), 2079
–2081
(2003). https://doi.org/10.1097/01.PRS.0000057143.53648.8B 0032-1052 Google Scholar
B. R. Moody,
J. E. McCarthy, and
G. J. Hruza,
“Collagen remodeling after 585-nm pulsed dye laser irradiation: an ultrasonographic analysis,”
Dermatol. Surg., 29
(10), 997
–999
(2003). 1076-0512 Google Scholar
B. R. Moody,
J. E. McCarthy, and
G. J. Hruza, Dermatol. Surg., 29
(10), 999
–1000
(2003). 1076-0512 Google Scholar
E. L. Tanzi,
C. M. Williams, and
T. S. Alster,
“Treatment of facial rhytides with a nonablative 1,450-nm diode laser: a controlled clinical and histologic study,”
Dermatol. Surg., 29
(2), 124
–128
(2003). 1076-0512 Google Scholar
G. Marque and
L. V. Wang,
“White light oblique incidence reflectometer for measuring absorption and reduced scattering spectra of tissue-like turbid media,”
Opt. Express, 1
(13), 454
–460
(1997). 1094-4087 Google Scholar
M. W. Lee,
“Combination 532-nm and 1064-nm lasers for noninvasive skin rejuvenation and toning,”
Arch. Dermatol., 139
(10), 1265
–1276
(2003). https://doi.org/10.1001/archderm.139.10.1265 0003-987X Google Scholar
M. H. Tan,
J. S. Dover,
T. S. Hsu,
K. A. Arndt, and
B. Stewart,
“Clinical evaluation of enhanced nonablative skin rejuvenation using a combination of a 532 and a laser,”
Lasers Surg. Med., 34
(5), 439
–445
(2004). https://doi.org/10.1002/lsm.20066 0196-8092 Google Scholar
L. C. Junqueira,
W. Cossermelli, and
R. Brentani,
“Differential staining of collagens type I, II and III by Sirius red and polarization microscopy,”
Arch. Histol. Jpn., 41
(3), 267
–274
(1978). 0004-0681 Google Scholar
L. C. Junqueira,
G. Bignolas, and
R. R. Brentani,
“Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections,”
Histochem. J., 11
(4), 447
–455
(1979). 0018-2214 Google Scholar
B. C. Vidal,
M. L. Mello, and
E. R. Pimentel,
“Polarization microscopy and microspectrophotometry of Sirius red, picrosirius and chlorantine fast red aggregates and of their complexes with collagen,”
Histochem. J., 14
(6), 857
–878
(1982). https://doi.org/10.1007/BF01005229 0018-2214 Google Scholar
G. E. Pierard,
“Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers,”
Matrix, 9
(1), 68
–71
(1989). 0934-8832 Google Scholar
J. M. Preston and
P. C. Tsien,
“The cellulose-dye complex. Part V-A: comparison of orientation factors derived from dichroism and other parameters,”
J. Soc. Dyers Colour., 66 361
–365
(1950). 0037-9859 Google Scholar
F. Sweat,
H. Puchtler, and
S. I. Rosenthal,
“Sirius red F3BA as a stain for connective tissue,”
Arch. Pathol., 78 69
–72
(1964). 0363-0153 Google Scholar
D. Goldberg,
M. Tan,
M. D. Sarradet, and
M. Gordon,
“Nonablative dermal remodeling with a 585-nm, 350-microsec, flashlamp pulsed dye laser: clinical and ultrastructural analysis,”
Dermatol. Surg., 29
(2), 161
–163
(2003). 1076-0512 Google Scholar
D. Goldberg,
M. Tan,
M. D. Sarradet, and
M. Gordon, Dermatol. Surg., 29
(2), 163
–164
(2003). 1076-0512 Google Scholar
C. W. van Wyk,
H. A. Seedat, and
V. M. Phillips,
“Collagen in submucous fibrosis: an electron-microscopic study,”
J. Oral Pathol. Med., 19
(4), 182
–187
(1990). 0904-2512 Google Scholar
C. A. Smith and
J. A. Vernon, Handbook of Auditory and Vestibular Research Methods, C. C. Thomas, Springfield, IL (1976). Google Scholar
J. S. Oghalai,
“Chlorpromazine inhibits cochlear function in guinea pigs,”
Hear. Res., 198
(1–2), 59
–68
(2004). https://doi.org/10.1016/j.heares.2004.03.013 0378-5955 Google Scholar
A. L. Nuttall,
D. M. Marques, and
M. Lawrence,
“Effects of perilymphatic perfusion with neomycin on the cochlear microphonic potential in the guinea pig,”
Acta Oto-Laryngol., 83
(5–6), 393
–400
(1977). 0001-6489 Google Scholar
D. T. Fung,
G. Y. Ng,
M. C. Leung, and
D. K. Tay,
“Investigation of the collagen fibril distribution in the medial collateral ligament in a rat knee model,”
Connect. Tissue Res., 44
(1), 2
–11
(2003). 0300-8207 Google Scholar
D. T. Fung,
G. Y. Ng,
M. C. Leung, and
D. K. Tay,
“Effects of a therapeutic laser on the ultrastructural morphology of repairing medial collateral ligament in a rat model,”
Lasers Surg. Med., 32
(4), 286
–293
(2003). https://doi.org/10.1002/lsm.10161 0196-8092 Google Scholar
J. F. Willott, Handbook of Mouse Auditory Research: From Behavior to Molecular Biology, CRC Press, Boca Raton, FL (2001). Google Scholar
R. R. Fay,
“Comparative psychoacoustics,”
Hear. Res., 34
(3), 295
–305
(1988). https://doi.org/10.1016/0378-5955(88)90009-3 0378-5955 Google Scholar
M. Muller,
K. von Hunerbein,
S. Hoidis, and
J. W. Smolders,
“A physiological place-frequency map of the cochlea in the CBA/J mouse,”
Hear. Res., 202
(1–2), 63
–73
(2005). https://doi.org/10.1016/j.heares.2004.08.011 0378-5955 Google Scholar
C. H. Choi and
J. S. Oghalai,
“Predicting the effect of post-implant cochlear fibrosis on residual hearing,”
Hear. Res., 205
(1–2), 193
–200
(2005). https://doi.org/10.1016/j.heares.2005.03.018 0378-5955 Google Scholar
R. Jonsson and
U. Rosenhall,
“Hearing in advanced age. A study of presbyacusis in 85-, 88- and 90-year-old people,”
Audiology, 37
(4), 207
–218
(1998). 0020-6091 Google Scholar
J. S. Milne,
“Hearing loss in the elderly: a 17-year longitudinal study,”
Clin. Otolaryngol. Allied Sci., 14
(5), 457
(1989). Google Scholar
E. K. Moscicki,
E. F. Elkins,
H. M. Baum, and
P. M. McNamara,
“Hearing loss in the elderly: an epidemiologic study of the Framingham Heart Study Cohort,”
Ear Hear., 6
(4), 184
–190
(1985). https://doi.org/10.1097/00003446-198507000-00003 0196-0202 Google Scholar
K. E. Pedersen,
U. Rosenhall, and
M. B. Moller,
“Changes in pure-tone thresholds in individuals aged 70–81: results from a longitudinal study,”
Audiology, 28
(4), 194
–204
(1989). 0020-6091 Google Scholar
|

