|
|
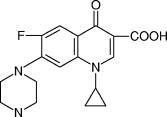
1.IntroductionTopical drug administration in the anterior segment of the eye is employed to target specific types of tissues or cells. The desirable local drug concentrations can be achieved conveniently, while the side effects and the toxicity associated with systemic applications of drugs at higher concentrations are reduced. The direct injection of drugs into the eye is the primary approach for local drug delivery in the ocular medium. Other means, such as surgical placement of drug-releasing implants, have also been utilized recently.1 Along these lines, the purpose of the present work is the application of an improved noncontact and noninvasive spectroscopic technique to identify the presence and quantify the concentration of fluoroquinolone-based antibiotics in the aqueous humor of the eye. Fluoroquinolones are antibiotics with a broad spectrum of activity. Compared to the aminoglycosides, the other most commonly used group of antibiotics, fluoroquinolones are more effective against Streptococcus or Staphylococcus epidermitis, which are responsible for about 75% of all cases of endophthalmitis. Moreover, fluoroquinolones have lower minimum inhibitory concentrations (MICs), they are not toxic for the retina and exhibit a much higher solubility than other available antibiotics. Ciprofloxacin, 1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydroquinoline-3-carboxylic acid, is a synthetic fluoroquinolone related to the nalidixic acid and it is used as an ocular antibacterial and antibiotic. It is active against Enterobacteriaceae, Pseudomonas aeruginosa, Haemophilus, and Neisseria spp., as well as against staphylococci and some other Gram-positive bacteria.2 The minimum inhibitory concentrations of ciprofloxacin in the ocular medium range between , depending on the targeted infections.3, 4 More recently, ciprofloxacin was approved for use in patients who have been exposed to the inhaled form of anthrax.5 Laser Raman spectroscopy is an inelastic light scattering technique that allows the investigation of the vibrational properties of molecules in a variety of aqueous environments. Several biomedically important molecules can be identified by Raman spectroscopy on the basis of their unique vibrational signature.6, 7 In recent years, an increased interest in Raman spectroscopy in ophthalmology8 stems from the expectation of its introduction into clinical practice in the near future. So far, a backscattering Raman collection geometry was applied to noninvasively assess the concentration of various intravitreal drugs, mainly to rabbit eyes.9, 10 Recently, we have proposed that a 90-deg scattering geometry is advantageous for the noninvasive detection of antibiotics (ceftazidime and amphotericin B) and physiological substances (glucose) in the aqueous humor of porcine eyes.11 However, this latter study was limited by uncertainties arising from the difficulties to accomplish the optimum and reproducible scattering cross section; the extracted porcine eyes could not be trussed well on the experimental pedestal and were gradually deformed. In the present work, we propose a new laser light delivery probe, adapted to an integrated CCD-based Raman spectroscopic system with improved collection optics. We demonstrate its use to monitor the presence and level of clinical concentrations of ciprofloxacin (Ciproxin®), a fluoroquinolone-based antibiotic, in an artificial anterior chamber (AAC), confined with porcine cornea. Furthermore, we quantify the concentration of ciprofloxacin in the aqueous humor, by a partial least-squares (PLS) chemometric regression algorithm.12, 13 2.Materials and MethodsThe antibiotic Ciproxin® 400 has been used following the instructions indicated in its commercial package; throughout this paper, it is referred to as ciprofloxacin, from its active substance. The chemical structure of the medicine is shown in Fig. 1 . The solutions of ciprofloxacin utilized in this work were physiological saline solutions (sodium chloride 0.9%). The Barron Artificial Anterior Chamber (AAC, Katena Products, Inc.) consists of three parts: a base with tissue pedestal, a tissue retainer, and a locking ring. The cornea is placed on the pedestal, cemented with the tissue retainer, and locked with the locking ring. The base has two ports with silicone tubing, in-line pinch clamps, and female luer-lok connectors. One port is used to inject drug solution, in our case, physiological saline solutions of ciprofloxacin, while from the other port, the air can escape so that the cornea gets the physiologic curvature. Porcine eyes, maximum post-mortem, were kindly supplied by regional abattoirs. Corneas were cut from the epithelial side and fitted to the AAC. Porcine and human corneas are very similar in size, with comparable average thickness;14 the central thickness of the human cornea is about , while its peripheral thickness is about . Moreover, a note is made of the fact that the typical size of the human eye is considered stable, with the exception of pathological conditions (i.e., microphthalmia, high hypermetropia, and high myopia), where the eye might deviate from its physiological size. Nevertheless, such size variations will not influence the scattering procedure proposed in this work. The intrachamber pressure of the AAC cell was monitored by a manometer within the physiological range of 18 to ,15 ensuring the normal curvature of the cornea during the measurements. Reference Raman spectra of the ocular drug have been acquired from aqueous solutions of ciprofloxacin placed into cylindrical (16-mm i.d., 19-mm o.d.) Pyrex optical cells. Since the key aim of any in vivo application of Raman spectroscopy is to collect, detect, and analyze as much scattered light as possible delivering the lowest laser power at the shorter acquisition time, we have optimized the area of the eye monitored and we have maximized the solid angle of collection. In order to increase the area of the laser-focusing field monitored by the collection optics, a new laser light delivery probe has been developed11 for the Raman excitation into the aqueous humor of an ocular as well as a model anterior chamber. This probe, shown in Fig. 2 , is designed to deliver the incident excitation laser beam inside the model-eye perpendicular to its optical axis, traversing the anterior chamber from one side to the other with a minimum of interaction with the surrounding lens or retina. It must be noticed that the focusing of the laser beam into the aqueous humor reduces significantly the possibility of injuring the cornea. Fig. 2Laser light delivery probe for favorable collection of the Raman photons at 90-deg scattering geometry scanning the artificial anterior chamber (ground plane vision). The laser beam inside the anterior chamber is delivered perpendicular to the optical axis for safety reasons and Raman signal optimization, as the inset photo shows. Inset: photo of the laser-illuminated artificial anterior chamber from the collecting optics side. 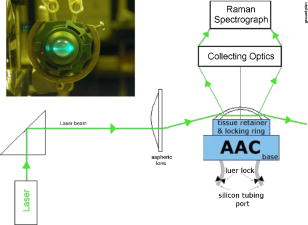 Ca. of the 514.5-nm line of a water-cooled argon ion laser (Spectra Physics, Model 2017, all lines) is employed for excitation. Utilizing the green line of an laser, we avoid resonance conditions and prevent any additional thermal effect, being outside the green light retinal hazard. The exposure time was set to . These experimental conditions have been chosen to be under the threshold lesion of the argon laser beam for the lens and the cornea, as well.16 A narrow-bandpass interference filter was used for the elimination of the laser plasma lines. The laser delivery system is composed of a right-angle prism and an aspheric lens (with an and a focal length of ). The laser beam is directed through the right-angle prism to the aspheric lens and then to the appropriately positioned AAC. The prism and the aspheric lens can be moved parallel to each other and to the optical axis. This design allows for tuning the optimum scattering geometry, for any point on the cornea, by varying the positions of the right-angle prism and the aspheric lens and scanning the whole anterior cavity. In other words, the aspheric lens has a double function; it serves as focusing lens and, in coupling with the right-angle prism, allows for beam steering. The focusing field of the laser into the anterior chamber was almost linear, with a diam of ca. . The “linearity” in this case consists of two coaxial peak-sharing cones, the focusing and the defocusing cones. However, the calculated focusing field, taking into account the geometry used for the laser delivery to the AAC, revealed a slightly perturbed coaxiality of the two cones. In the inset of Fig. 2, a photo of the AAC illuminated by the laser beam and viewed from the side of the collecting optics, is shown. The effectiveness of this laser light delivery probe was further improved with its adaptation to a CCD-based Raman spectroscopic system (T-64000, JY-Horiba). The Raman system was used in the single spectrograph configuration for the collection, analysis, and detection of the scattered light. This instrumentation has an axial entrance slit and allows for the right-angle collection of the scattering volume inside the anterior ocular cavity. The maximum length of the slit is , while the mean laser focusing field in the eye is ca. . This value of the focusing field is referred to an optimum position of the aspheric lens and the right-angle prism in order for the laser beam to cross the middle of the anterior chamber. In this work, this technique was used in this fixed optimum position and not in the scanning mode. In order to optimize the slit area per scattering volume ratio, we have employed an afocal system consisting of two lenses having focal lengths of 260 and on the slit and AAC sides, respectively, and yielding a magnification of ca. 3.25 (260/80) so that the image of the laser focusing field covers the full length of the entrance slit. This is to be compared to a magnification of 1.6 (260/160) afforded by the recently described probe.11 A holographic super-notch plus filter (HSPF-514-1.0, Kaiser Optical Systems, Inc.) was used to mask the elastic Raleigh scattering. The Raman photons were dispersed by a 600-grooves/mm 500-nm blazed holographic diffraction grating and detected by a standard cooled (at ) front-illuminated CCD (Spectraview-2D, ) detector. The nominal peak quantum efficiency of the CCD detector was 50% at and at the center of the spectral window used in this study, at . Further enhancement of the signal-to-noise ratio of the inherently weak Raman signal of the ocular medium was achieved by “binning” the CCD camera, albeit at the expense of spectral resolution. Moreover, Raman measurements need to be repeated with the position of the monochromator slightly/appropriately shifted in order to differentiate signal enhancement from any coincidental noise amplification. Last, the improvements in data acquisition and signal-to-noise ratio have been amplified by developing a partial least-squares (PLS) regression algorithm for quantitative analysis employing routines of the OPUS QUANT-2 Software by Bruker Optics. PLS is a factorial analysis technique where both spectral and analytical data are simultaneously taken into account. The number of factors (vectors or ranks) is related to the amount of relevant information in the spectra and is therefore very significant for the quality of the prediction. Validation procedures are employed to define the optimum number of ranks, thereby avoiding overfitting based on irrelevant information (e.g., random noise). With appropriate selection of calibrants, more than one component can be predicted independently from each other. 3.Results and DiscussionThe Raman spectrum of an aqueous solution of ciprofloxacin injected in an optical cell (OC) over the spectral window of 800 to is shown in Fig. 3 , in comparison to the corresponding spectrum of blank physiological saline solution. The difference spectrum reveals the rich vibrational signature of ciprofloxacin, with strongest peaks at 1393 and assigned to the stretching vibrations of the quinolone rings and aromatic , respectively.17 Note that in dilute aqueous solutions, the latter band overlaps with the bending mode of water at ca. , and its diagnostic value becomes limited. Fig. 3Raman spectra of physiological saline solution in the OC before (a) and after (b) the injection of of the antibiotic ciprofloxacin. The difference spectrum is also shown (c). 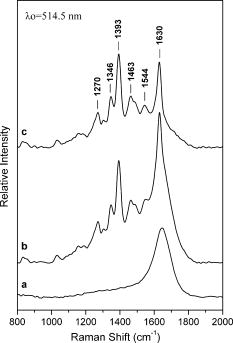 Figure 4 shows the Raman spectrum from the AAC filled with a 1-mg/mL physiological saline solution of ciprofloxacin. The Raman spectra from the AAC filled with pure physiological saline solution, of the porcine cornea, and of the polymeric material of the AAC core are also shown for reference purposes. Despite the lower resolution and the broad background due to the cornea and the bending mode of water, the ciprofloxacin band is clearly visible. Interestingly, the very strong and sharp band of the AAC core material at ca. (presumably indicative of polyoxymethylene, POM) is weak in the spectra of the aqueous solution. This is because the 90-deg scattering geometry employed allows for defining accurately the scattering volume within the aqueous medium and away from either the AAC core or the cornea tissue. Fig. 4Top: the Raman spectra of the aqueous solutions of the ciprofloxacin from the AAC. Below: the reference Raman spectra of physiological saline solution from the AAC, the porcine cornea, and the polymeric material of the AAC core. The spectrum of the cornea was acquired with the unitary binning factor, while the other three spectra was acquired with binning factor 4. 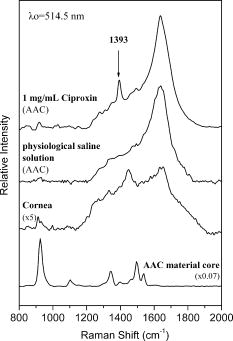 The first PLS model was based on a set of 40 Raman spectra of ciprofloxacin aqueous solutions (0 to ), acquired in standard cylindrical optical cells. This model has been cross-validated internally by the leave-one-out method. Various mathematical pretreatments were applied to the data in order to maximize the correlation coefficient and minimize the root-mean-square error of the cross validation (RMSECV). The best results were obtained in the 2nd derivative formalism (Savitsky-Golay, 13 points smoothing) over the frequency range from 763 to . Three ranks can be extracted, and the predicted versus known values of the concentration of the antibiotic allow for and [Fig. 5a ]. For any practical purpose, this error is in the same range as the threshold detection of ciprofloxacin by the present method. While this performance is adequate for most ocular applications of ciprofloxacin,3, 4 keep in mind that the model is based on spectra acquired in Pyrex optical cells, and therefore its performance is reflecting all improvements in data acquisition, except for the use of the AAC. The effect of the latter becomes evident if, instead of the aforementioned internal validation, a set of 27 spectra of ciprofloxacin (0 to ) acquired by using the AAC are employed to validate the model externally. The model now converges at two (instead of three) ranks, suggesting that some of the spectral information relevant to the concentration of the ciprofloxacin becomes buried in random noise or other spurious features induced by the AAC. Concomitantly, both the and the RMS error of the correlation remain operational but deteriorate to 98.4% and ca. , respectively [Fig. 5b]. Fig. 5Predicted versus known concentrations of ciprofloxacin in the model anterior chamber: (a) calibration set; (b) validation set.  In order to forecast the performance of a future in vivo application, it is instructive to compare the internal and external validation spectra of ciprofloxacin solutions in the optical cell and the AAC. Figure 6a depicts the set of optical cell data over the whole composition range and in the 2nd derivative formalism, i.e., as employed in the model of Fig. 5a. The use of the 2nd derivative results in the damping of broad spectral features and the relative enhancement of narrow peaks.18 As a result, the spectra are enriched in information directly related to the concentration of ciprofloxacin, and the performance of the internal validation algorithm is very high. Obviously, this advantage of the 2nd derivative pretreatment is not fully exploited in the external validation data set obtained by the AAC. The corresponding spectra [Fig. 6b] demonstrate that although the broad vibrational signature of the proteinaceous tissue is effectively filtered out by the 2nd derivative, a set of new sharp bands that is irrelevant to the presence and concentration of ciprofloxacin becomes amplified. The most prominent of these spurious bands is observed at ca. and is accompanied by weaker bands at 1345 and ca. , i.e., in overlap with the ciprofloxacin diagnostic features. In comparison to the spectra of Fig. 4, it is evident that these spurious bands are due to the polymeric backing of the AAC cell, and their nonsystematic presence in several spectra reflects uncertainties in focusing the laser beam. The external PLS validation is still capable of extracting ciprofloxacin-relevant information, but some of it is lost due to the nonsystematic overlap with the spectrum of the AAC cell material. Fig. 62nd derivative Raman spectra of ciprofloxacin aqueous solutions (0 to ) obtained under the same acquisition settings with the optical cell (a) and the AAC (b). The thick lines correspond to the 1-mg/mL solutions. 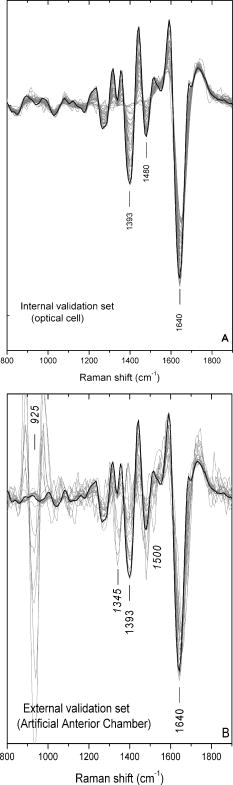 Based on the preceeding, we can anticipate that the Raman chemometric determination of ciprofloxacin in the intact eye will perform better that the AAC feasibility, and closer to that of the optical cell, allowing for a threshold of detection of the order of 25 to . Further improvements are expected if a back-thinned CCD detector is used instead. This type of detector bears a broad quantum efficiency curve, typically superior to 90%, and its use will result in an improvement of the signal-to-noise ratio by a factor of three without increasing the laser power or the spectroscopic acquisition time. 4.ConclusionsIn this work, a new 514.5-nm laser light delivery probe is developed for the in situ quantification of the concentration of medicinal substances injected in the anterior chamber of the eye. The probe is adapted to a commercial CCD-based Raman spectrometer and employs a 90-deg scattering geometry to minimize the risk of damaging the ocular tissue. Compared to earlier designs, the present setup implements a number of modifications in the collection optics to match the scattering volume with the entrance slit of the spectrometer and to improve the signal-to-noise ratio. Porcine eyes fitted to an AAC are employed to simulate the eye as an optical cell, and PLS chemometric models are developed to correlate the Raman spectra with the concentration of the drug in the aqueous humor of the eye. The probe has been evaluated by determining the concentration of ciprofloxacin, an antibiotic of the fluoroquinolone family. The concentration of this drug can be predicted with an RMS error of ca. that compares favorably with the suggested, target-dependent, minimum inhibitory concentrations of this drug (0.016 to ). Furthermore, we have demonstrated that the current performance of the method is limited by the spurious vibrational signatures of the polymeric components of the AAC cell and therefore is expected to improve if intact eyes are studied. Future studies are oriented toward the combined use of Raman spectroscopy and chemometric modeling to the early diagnosis of intraocular diseases in vivo, as well as toward the monitoring of pharmacokinetics of the administered drugs. It is anticipated that beyond the management of ocular fungal infections, this technique might be applicable to the identification of illegal substances in the aqueous humor of the eye, especially since several of these compounds are strong Raman scatterers. AcknowledgmentsFinancial support for the PENED-01/ED-559 and 04AKMON54 projects is gratefully acknowledged. The 04AKMON54 project is cofunded:
The PENED-01/ED-559 project is cofunded: in the framework of the Operational Program “Competitiveness,” Measure 8.3—Community Support Framework 2000 to 2006.ReferencesT. Yasukawa,
H. Kimura,
N. Kunou,
H. Miyamoto,
Y. Honda,
Y. Ogura, and
Y. Ikada,
“Biodegradable scleral implant for intravitreal controlled release of Ganciclovir,”
Graefe's Arch. Clin. Exp. Ophthalmol., 238 186
–190
(2000). 0721-832X Google Scholar
The Extra Pharmacopeia, 145
–147 30th ed.The Pharmaceutical Press, London (1993). Google Scholar
I. Odenholt,
E. Lowdin, and
O. Cars,
“Postadibiotic, postandibiotic sub-MIC, and subinhibitory effects of PGE-9509924, ciprofloxacin, and levofloxacin,”
Antimicrob. Agents Chemother., 47 3352
–3356
(2003). 0066-4804 Google Scholar
M. Jones,
N. Boenink,
J. Verhoef,
K. Kohrer, and
F. Schmitz,
“Multiple mutation conferring ciprofloxacin resistance in Staphylococcus aureus demonstrate long-term stability in an antibiotic-free enviroment,”
J. Antimicrob. Chemother., 45 353
–356
(2000). 0305-7453 Google Scholar
A. Meyerhoff,
R. Albrecht,
J. M. Meyer,
P. Dionne,
P. Higgins, and
D. Murphy,
“US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax,”
Clin. Infect. Dis., 39
(3), 303
–308
(2004). 1058-4838 Google Scholar
P. Caspers,
G. Lucassen,
E. Carter,
H. Bruining, and
G. Puppels,
“In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles,”
J. Invest. Dermatol., 116 434
–442
(2001). https://doi.org/10.1046/j.1523-1747.2001.01258.x 0022-202X Google Scholar
T. Koo,
A. Berger,
I. Itzkan,
G. Horowitz, and
M. Feld,
“Reagentless blood analysis by near-infrared Raman spectroscopy,”
Diabetes Technol. Ther., 1 153
–157
(1999). Google Scholar
R. Erckens,
F. Jongsma,
J. Wickested,
F. Hendrikse,
W. March, and
M. Motamedi,
“Raman spectroscopy in ophthalmology: from experimental tool to application in vivo,”
Lasers Surg. Med., 16 236
–252
(2001). 0196-8092 Google Scholar
K. Hosseini,
F. Jongsma,
F. Hendrikse, and
M. Motamedi,
“Non-invasive monitoring of commonly used intraocular drugs against endophthalmitis by Raman spectroscopy,”
Lasers Surg. Med., 32 265
–270
(2003). 0196-8092 Google Scholar
W. La Via,
J. Lambert,
M. Pelletier,
J. M. Morookian,
S. Sirk,
D. Mickiene,
T. Walsh, and
M. Borchert,
“Measurement of amphotericin B concentration by resonant Raman spectroscopy—a novel technique that may be useful for non-invasive monitoring,”
Med. Mycol., 44 169
–174
(2006). 1369-3786 Google Scholar
Th. Sideroudi,
N. Pharmakakis,
G. Papatheodorou, and
G. Voyiatzis,
“Non-invasive detection of antibiotics and physiological substances in the aqueous humor by Raman spectroscopy,”
Lasers Surg. Med., 38 695
–703
(2006). https://doi.org/10.1002/lsm.20360 0196-8092 Google Scholar
H. Martens and
T. Naes, Multivariate Calibration, John Wiley and Sons, New York (1989). Google Scholar
M. Goetz,
G. Cote,
R. Erckens,
W. March, and
M. Motamedi,
“Application of a multivariate technique to Raman spectra for quantification of body chemicals,”
IEEE Trans. Biomed. Eng., 42 728
–731
(1995). https://doi.org/10.1109/10.391172 0018-9294 Google Scholar
Y. Zeng,
J. Yang,
K. Huang,
Z. Lee, and
X. Lee,
“A comparison of biomechanical properties between human and porcine cornea,”
J. Biomech., 34 533
–537
(2001). https://doi.org/10.1016/S0021-9290(00)00219-0 0021-9290 Google Scholar
J. Ruiz-Ederra,
M. García,
M. Hernández,
H. Urcola,
E. Hernández-Barbáchano,
J. Araiz, and
E. Vecino,
“The pig eye as a novel model of glaucoma,”
Exp. Eye Res., 81 561
–569
(2005). 0014-4835 Google Scholar
F. Dannheim and
B. Rassow,
“Laser lesions of the anterior segment of the rabbit eye. Studies of cornea, iris, lens, and sclera using an argon, ruby, and YAG-laser,”
Albrecht von Graefes Arch. Klin. Exp. Ophthalmol., 205 175
–205
(1978). 0065-6100 Google Scholar
U. Neugebauer,
A. Szeghalmi,
M. Schmitt,
W. Kiefer,
J. Popp, and
U. Holzgrabe,
“Vibrational spectroscopic characterization of fluoroquinolones,”
Spectrochim. Acta, Part A, 61 1505
–1517
(2005). 0584-8539 Google Scholar
W. F. Maddams and
W. L. Mead,
“The measurement of derivative i.r. spectra –I. Background studies,”
Spectrochim. Acta, Part A, 38A 437
–444
(1982). 0584-8539 Google Scholar
|