|
|
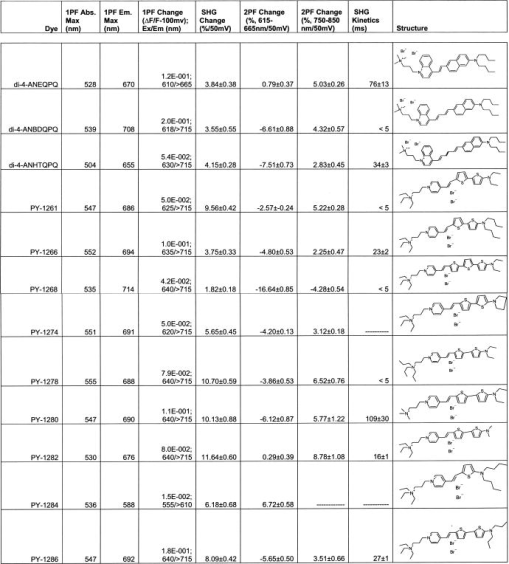
1.IntroductionTraditional methods for monitoring electrical activity in complex systems, such as the neurons in living brains, use electrodes and therefore preclude the acquisition of high-resolution spatiotemporal maps of activity. Even for single cells, the use of patch-clamp technology, although very sensitive and capable of recording single action potentials, is invasive and does not permit recordings from very thin processes such as axons or dendritic spines. This has prompted the development of voltage-sensitive dyes (VSDs) whose optical properties change in response to membrane potential. These membrane-specific molecular probes can then be imaged with high-speed cameras or laser scanning microscopes, and the time courses at multiple points within a specimen can be analyzed. Since the initial work on the squid giant axon,1, 2 a variety of VSDs have been developed to study transmembrane potential (TMP) primarily by linear optical measurements such as absorbance or fluorescence. These dyes can be excited with visible light and provide an effective method to image cell membranes and their physiology. Nonlinear optical phenomena are observed when a high-intensity laser interacts with an optical material and are characterized by a probability that is proportional to the incident light intensity raised to a power greater than one. The use of these phenomena to detect TMP changes has been demonstrated. 3, 4, 5, 6, 7, 8, 9, 10, 11 In this work, the methods of choice are second harmonic generation (SHG) and two-photon excitation fluorescence (2PF), which are both nonlinear optical processes taking place in proportion to the square of the incident light intensity. 2PF is the nonlinear form of one-photon excitation fluorescence and operates on a similar principle. In 2PF, two photons excite a fluorophore into a state corresponding to twice their individual energies; the fluorophore then relaxes to the lowest energy electronic excited state before emitting a fluorescent photon. The emission spectrum for this nonlinear process is the same as in one-photon excitation. In contrast, SHG occurs instantaneously, when two photons are converted into one of twice the energy. SHG does not involve an excited state and therefore conserves energy; the harmonic photon is emitted coherently. There are several other interrelated differences between the two methods. The first is based on the order of the term that generates each optical phenomenon. The polarization of an optical material in the presence of a high-intensity electric field is a power series with coefficients associated with the material’s higher-order electric susceptibilities. 2PF comes from the imaginary portion of the third-order term, depending linearly on the concentration of chromophore. SHG comes from the second-order term, depending quadratically on the concentration of the SHG-active chromophores, or “harmonophores.” Also, SHG is confined to loci lacking a center of symmetry, as provided, for example, by a cell membrane leaflet.12 2PF does not have this symmetry constraint. The chromophore used to generate both nonlinear phenomena can be synthesized in several forms. Previous work from this laboratory has shown that some aminonaphthylethenylpyridinium (ANEP)–based dyes [Fig. 1a, 1b, 1c ] can exhibit a relative signal change of 43% per for SHG signals.13 It was also shown that SHG and 2PF signal sensitivity are wavelength dependent and scale linearly with applied voltage change.10 However, the kinetics of some previous dyes was too slow to follow action potentials.10 The hydrophobic tail of the dye, characteristics of the chromophore, and the charge on the head may all influence the relative signal change and its speed. Indeed, FM4-64 [Fig. 1d], a dye that provides SHG responses to membrane potential fast enough to follow action potentials,7, 11 has a similar styryl chromophore with ethyl groups attached to both the amino tail and the quaternary ammonium head. However, FM4-64 has a relatively small sensitivity to membrane potential of only 7.5 to 10% per . Fig. 1Molecular structures of dyes used in previous work: (a) di-4-ANEPPS; (b) Ebis(di-4-ANEP); (c) di-4-ANEPPDHQ; (d) FM4-64. 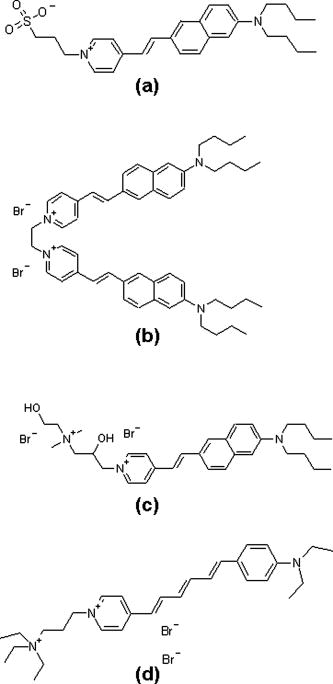 Here, we adopt a progressive alteration of dye characteristics while screening signal sensitivity and kinetics. In particular, we have developed a new class of dyes with a chromophore consisting of a pyridine and multiple thiophene groups. The charge is oriented around the pyridine in the ground state and shifts during excitation. Some of these new dyes yield significantly improved fast responses to membrane potential for SHG as well as 2PF. The dyes under consideration show specific excitation by 1064-nm femtosecond pulses that can be generated with a relatively inexpensive and stable fiber laser.14 We believe that the combination of the better dye responses and the simpler optical setup afforded by the fiber laser will allow this nonlinear optical microscopy to become a more widespread tool for the neuroimaging of electrical activity. 2.Materials and Methods2.1.ImagingFor full details of our nonlinear imaging microscope, we refer the reader to previously published descriptions.13, 15 Our current microscope combines a Fluoview (Olympus) scan-head and an Axiovert (Zeiss) inverted microscope constructed for multiple imaging methodologies including wide-field, one-photon excitation fluorescence imaging, as well as SHG and 2PF imaging. Our laser sources include a Mira (Coherent) Ti:Sapphire laser and a Femtopower (Fianium) fiber laser operating at .14 For excitation, we routinely use an IR-Achroplan (Zeiss) , 0.8 NA water-immersion objective, although for shorter wavelengths, we also use an A-Plan (Zeiss) , 0.65 NA air objective. Fluorescence is imaged (for linear imaging) or collected (for nonlinear imaging) back through the excitation objective, while SHG is collected in the forward, transmitted light direction by a condenser, either 0.8 NA or 0.55 NA (Zeiss). For optimal collection, the numerical aperture of the condenser should be at least of the numerical aperture of the excitation objective,16, 17 although in practice, a condenser of slightly lower numerical aperture will often suffice. Since the wavelength of second harmonic light is precisely half that of the excitation light, the choice of filter for SHG imaging is greatly simplified and the primary concern is the blocking of the excitation light that is also transmitted through the sample as well as fluorescence that is emitted forward. 2.2.CellsNIE-115 mouse neuroblastoma were cultured in Dulbecco modified Eagle's medium (DMEM) with 10% fetal bovine serum and 1% antibiotic-antimycotic (Invitrogen, Carlsbad, California) in a 60-mm plastic dish (353002, Becton Dickinson, Franklin Lakes, New Jersey). The culture was maintained at with 5% for a period of prior to experimentation. For experimentation, the growth medium was replaced with of Earle’s Balanced Salt Solution (EBSS, Sigma, St. Louis) with HEPES (Merck Biosciences AG, Laufelfingen, Switzerland). The internal buffer, injected into the patch pipette, was a potassium aspartate ( , Sigma, St. Louis), sodium chloride ( , Sigma, St. Louis) and HEPES solution at a pH of 7.35. The pH was adjusted with potassium hydroxide and hydrochloric acid. 2.3.DyesThe series of dyes used in this work are shown in Table 1 . The top three rows in the table show more highly conjugated variants of the aminonaphthylethenylpyridinium (ANEP) chromophore. These were all synthesized by J. P. Wuskell. The remaining entries in the table are members of a new class of aminothiophene chromophores synthesized by P. Yan. They display a variety of polar heads, hydrophobic tails, and chromophore lengths so as to facilitate the exploration of structure-sensitivity relationships. 2.4.ElectrophysiologyFor characterizing the voltage sensitivity of SHG and 2PF, we used a slow switching protocol as previously described13, 15 with a step. For investigating the time dependence of the voltage sensitivity, we instead triggered a step wave form for the voltage clamp approximately halfway through the page scan; the protocol for determining signal time dependence was previously described in detail.10 3.ResultsTypical images for SHG and 2PF of dye-stained cells are shown in Fig. 2 . The SHG images are typically more sharp at the membrane because the signal from submicroscopic structures such as filopodia, which contribute a haze to the 2PF signal, are not allowed in SHG.18 Also noteworthy in Fig. 2 is the loss of signal near the axes bisecting the 10 o’clock to 4 o’clock regions of the cell. This corresponds to the polarization dependence of the nonlinear optical signals and can be minimized through the use of circularly polarized laser light. Fig. 2Two forms of nonlinear optical images acquired in parallel of a single cell stained with PY-1261 and excited with a 1064-nm fiber laser: (a) second harmonic generation; (b) two-photon excitation fluorescence. 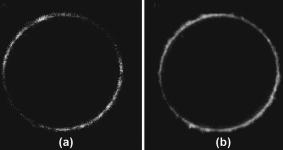 Table 1 shows the data from one-photon, two-photon, and SHG experiments for all dyes studied in these series of experiments. The molecular structures are shown in the rightmost column to illustrate the progressive alteration of certain chemical moieties, showing the effects on kinetics, signal sensitivity, and absorption wavelength of different dye segments. The left-most data columns of Table 1 are the absorption and emission maxima for one-photon fluorescence in soybean multilamellar lipid vesicles used to mimic the membrane environment. The column indicating relative signal change for one-photon fluorescence also lists the excitation light source used and the lower bounds of the long-pass emission detected. The remaining columns of Table 1 list the nonlinear optical responses of the dyes to a voltage change on a patch-clamped neuroblastoma cell. Excitation for both SHG and 2PF is at . The transmitted SHG signal was collected at , and the two-photon excited emission was measured with two different wavelength bands, as indicated in the respective column headings. We show that maximal SHG sensitivity is observed in PY-1282, at . The maximum 2PF sensitivities were observed in the tri-thiophene PY-1268 with a change of . This was the largest sensitivity observed for all dyes and imaging modalities. Importantly, 2PF changes were instantaneous, i.e., within the 5-ms time resolution of our measurements, for PY-1268 and all other dyes, while the SHG responses varied in their kinetics (rightmost column in Table 1). The kinetics data for SHG, 2PF detected between 615 to , and 2PF detected between 750 to for PY-1268 are shown in Fig. 3 . Here, the SHG sensitivity per is less than 2%, so the response to the voltage change, occurring at line number 254, is hard to distinguish from noise. However, the exponential fit to this data suggests that SHG occurs instantaneously. Figure 3b shows signal change kinetics for 2PF detected over a range of emission wavelengths between and . This signal sensitivity is consistent with the data indicated in the table (which was measured using a slow voltage clamp protocol) and indicates an instantaneous signal change. Curve fitting for this data set resulted in two zero-slope baselines before and after the signal change and a signal change that lasted less than one row (i.e., ) during the scan. The final graph here, Fig. 3c, shows a similar kinetics pattern for a smaller signal sensitivity of detected between 750 and . Fig. 3Kinetics data for PY-1268 showing the change in signal intensity at a location halfway through the page scan for a voltage switch of : (a) SHG relative signal change; (b) 2PF relative signal change detected between 615 to ; (c) 2PF signal change detected between 750 to . 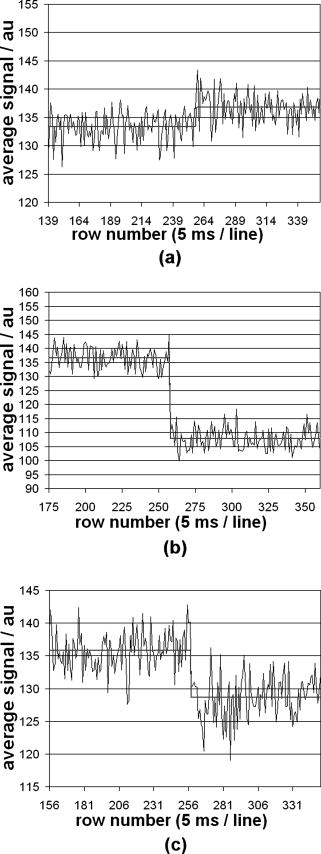 In contrast, the data for PY-1261 in Fig. 4 displays instantaneous kinetics for all imaging modalities, but with the largest signal sensitivity occurring in the SHG signal. This dye has a shorter chromophore than PY-1268, which is consistent with the relationship between their 2PF signal sensitivities. Interestingly, PY-1261 shows nearly a SHG signal change, which is more than five times larger than its tri-thiophene counterpart, but a maximum 2PF sensitivity that is close to three times smaller. Moreover, the larger 2PF change occurs in the wavelength range between 750 and since PY-1261 exhibits a spectral shift toward the red. Dyes such as PY-1261 and PY-1278 are of particular interest since they have both instantaneous SHG kinetics and a large (close to ) signal sensitivity. Fig. 4Kinetics data for PY-1261 showing the change in signal intensity at a location halfway through the page scan for a voltage switch of : (a) SHG relative signal change; (b) 2PF relative signal change detected between 615 to ; (c) 2PF relative signal change detected between 750 to . 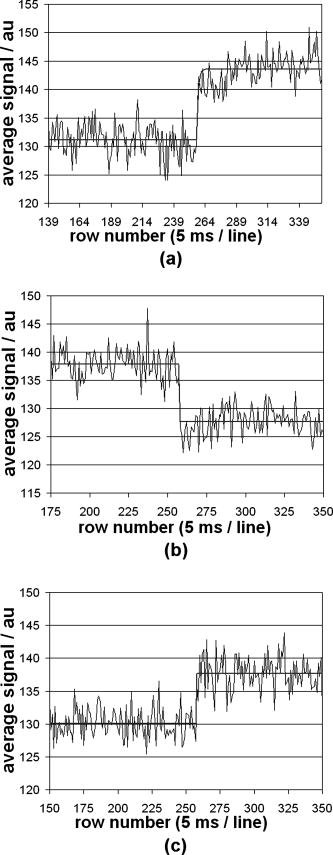 The dyes in Table 1 were designed so as to be able to compare the voltage-dependent nonlinear responses as a function of specific molecular features. Figure 5 shows the results obtained from altering the number of double bonds in the chromophore in the quinolinium propyl trimethyl ammonium series. The two vertical axes show both the nonlinear signal sensitivity to a 50-mV switch and the SHG signal kinetics to a switch. The latter is indicated by the time constant, , as the light gray bar on the graph. It should be emphasized that the signal speed for the 2PF responses of all the dyes are as fast as our measurement allows, . These data show that while the SHG sensitivity is the same for all dyes within a margin of error, the signal speed is different for the different chromophores. Specifically, optimal dye kinetics occurs with the two double-bond dye, di-4-ANBDQPQ. The amount of time for the dye to react to a given voltage switch is prolonged in the case of the three double-bond di-4-ANHTQPQ, illustrated in the last data set. Interestingly, the time for the smallest molecule, di-4-ANEQPQ, to react to voltage is the longest. Last, there is a distinguishable spectral shift exhibited by the longer chromophore dyes when compared to di-4-ANEQPQ. This is most noticeable in the range of wavelengths between 615 and , labeled 2PFr in the graph. While di-4-ANEQPQ exhibits a positive signal change in both the 615 to and the 750 to ranges of wavelengths, the two longer dyes exhibit a spectral shift as evidenced by the larger negative signal sensitivity in the 615 to range of wavelengths. The larger signal sensitivity is consistent with the idea that a longer chromophore should show a more significant electrochromic effect. This is because the positive charge redistribution upon transition to the excited state from the quinolinium to the naphthyl amine moieties has a greater distance to traverse and therefore is more sensitive to the intramembrane electric field. However, we did not attempt to systematically scan the excitation and emission wavelengths to try to determine the maximal electrochromic effect for these dyes. Fig. 5Comparison chart showing signal sensitivities for both SHG and 2PF and SHG kinetic response for the QPQ dye series. The left vertical axis shows the percentage for signal sensitivity, and the right vertical axis shows kinetics in milliseconds, represented by the last bar of each series. 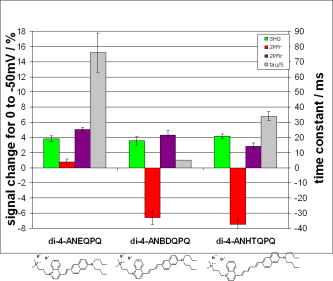 Figure 6 compares results for a set of three dyes in which the head group changes. The most significant of the results here is that of the dye on the leftmost portion of the graph, PY-1280, showing a significantly slower signal response to a change in voltage. It is important to note that both PY-1261 and PY-1278 have a “fast” kinetic response, which in Fig. 6 is illustrated as being , the temporal resolution of these experiments. Fig. 6Comparison chart showing signal sensitivities for both SHG and 2PF and SHG kinetic response for the head group variation. The left vertical axis shows the percentage for signal sensitivity, and the right vertical axis shows kinetics in milliseconds, represented by the last bar of each series. 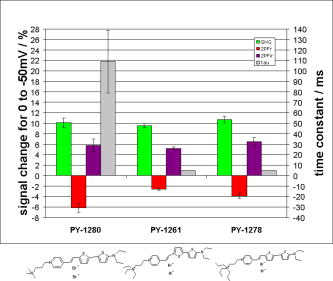 The remaining variable in the dye synthesis permutations is the length of the two hydrocarbon chains in the hydrophobic tail of the dye molecules; this is shown in Fig. 7 . These data show a significant decrease in both SHG and 2PFir signal sensitivity with increasing tail length. In both cases, the largest signal sensitivity is observed with the one-carbon tail group, PY-1282. The signal decreases with each added pair of carbons. In this set of dyes, PY-1261 has an “instantaneous” SHG voltage switch, depicted by the smallest experimental time unit of . Fig. 7Comparison chart showing signal sensitivities for both SHG and 2PF and SHG kinetic response for the tail group variation. The left vertical axis shows the percentage for signal sensitivity, and the right vertical axis shows kinetics in milliseconds, represented by the last bar of each series. 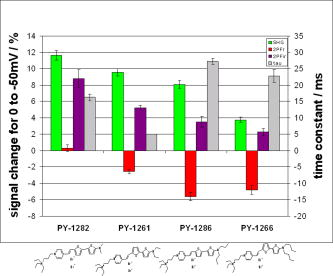 Table 1Series of dyes. 4.DiscussionThe data presented here show the results of a systematic screening of new nonlinear optical dyes designed to respond to membrane potential. Both dye kinetics and average signal change to TMP are presented. We searched for an optimal combination of “speed and sensitivity” to identify the ideal dye for monitoring cellular membrane electrical activity. Several such dyes tested satisfy this description. Moreover, a methodical alteration of chemical moieties, such as that described in this work, can further elucidate the molecular mechanism by which these potentiometric dyes respond to voltage. A total of 12 dyes were tested for 2PF/SHG sensitivity and kinetics, as well as one-photon absorption and emission peaks and sensitivity. The dyes were grouped according to common chemical characteristics; the results between common dye variations were compared. All data acquired in these series of experiments are shown in Table 1. Although kinetics for both 2PF and SHG were tested, the former is consistently instantaneous across all dyes within a temporal resolution of . However, the SHG response to TMP is not always instantaneous. Figures 3 and 4 highlight two dyes with strong intensities indicating heavy staining levels, showing large signal changes as well as fast kinetics. PY-1261 in Fig. 4a shows one of the first thiophene-based potentiometric dyes synthesized in our laboratory with nearly a SHG change and instantaneous kinetics. PY-1268 shows a similarly instantaneous SHG change, albeit with a much lower amplitude, but a more remarkable result is its 2PF sensitivity. It has generally been presumed that SHG signal sensitivity would be larger than 2PF given the same change in voltage, but our data show a nearly change in PY-1268 2PF. Considering that this change occurs quickly and given the abundance of 2PF detection systems, PY-1268 is a prime candidate for physiological applications requiring the ability to detect action potentials. For comparison, previously published data on the SHG kinetics of di-4-ANEPPS show a slow response to the same voltage change, which occurred with an exponential time constant of .10 FM4-64 shows an SHG change that is fast, but the response is .7, 11 We have not yet tested the linearity of the voltage-dependent responses of our dyes, but the SHG of PY-1261 and the 2PF of PY-1268 already show greater rapid responses to a potential step than is observed for the FM4-64 SHG response to . The chromophore variations are shown in the QPQ dye series (Fig. 5). Here, the length of chromophore does not profoundly alter the maximum signal sensitivity. The average absolute SHG signal sensitivity for this dye series is , and the average maximal absolute 2PF signal sensitivity is . Deviations all range within 2% of the mean. The most significant result seen here is the SHG kinetics of di-4-ANBDQPQ. While the maximal signal sensitivity of this dye is essentially identical to the other two, its kinetic curve statistically resembles a step function. This is in vast contrast to di-4-ANEQPQ and di-4-ANHTQPQ, where the exponential time constant exceeds , making them unusable for electrophysiological applications. The effect of the hydrophobicity of the quaternary ammonium head group on voltage sensitivity and kinetics has not been systematically studied previously. The results of experiments with a series of dyes with different head groups are shown in Table 1 and compared in Fig. 6. Since this portion of the dye is outside the chromophore, changes do not significantly affect the shift of electrons in the excited state. This removes the possibility of the head group affecting the electronic mechanism of dye activity. It has been previously documented that it is the hydrophobic tail group that interacts with the membrane,19 and it is possible that the interaction of the head group with water could affect the dyes’ sensitivities, as the least hydrophobic of these should have the strongest anchoring interactions at the aqueous interface. The results in Fig. 6 indicate that this cannot be an important consideration. SHG signal sensitivity is identical for all three dyes, within an experimental margin of error. Any variations in 2PF signal sensitivity most likely arise from the emission spectra shifting through the detected wavelength bands. However, the SHG kinetics is significantly slower for the trimethylammonium head group compared to the “instantaneous” changes observed for the other two dyes, and this could reflect some rigidification of the dye in the membrane due to strong anchoring. A systematically significant decrease in SHG signal sensitivity is observed in a series of four dyes with varied tail groups. This data is shown in Fig. 7. PY-1282, with two methyl groups at the amino tail, shows a threefold increase in signal sensitivity when compared with its four-carbon counterpart, PY-1266. Moreover, we observe a statistically significant decrease in SHG sensitivity with each additional carbon added to the tail hydrocarbon chains. The result here suggests that the molecular interaction of the tail group with the membrane dominates the mechanism by which the SHG signal changes in response to TMP. SHG signal change could occur by way of a molecular rearrangement occurring in response to a change in TMP. This rearrangement may change the chromophore alignment in the electric field and thus vary the output signal. Such mechanisms have been described for the fluorescence responses of voltage-sensitive dyes.20 A probe with a longer hydrophobic tail will bind to the membrane more strongly and rigidly than probes with shorter tails. If the signal change results from an on-off or reorientation mechanism, then the hydrophobic dyes will be less likely to exhibit a large change. This theory is supported by the kinetics data shown in Fig. 7, where the more hydrophobic head groups of PY-1266 and PY-1286 display a slower kinetic response. Importantly, the fluorescence kinetics of all of these dyes were fast , suggesting that the mechanisms for the fluorescence responses to membrane potential changes are different from the mechanisms of the SHG responses. The rapid responses to voltage-clamp steps of all the dyes in both SHG and 2PF modalities argue against these responses being due to a change in membrane composition or a change in their intracellular location. These dyes are very sensitive to their environment and are likely to be excellent reporters of lipid composition. However, changes in lipid composition occur from processes such as endocytosis, exocytosis, or lipid trafficking, which are on time scales of many seconds to minutes. Thus, the millisecond response times of the dyes are most likely a reflection of the rapid change in transmembrane potential. Our data suggest that the mechanism behind SHG signal sensitivity may not be purely electrochromic, that is, dependent only on the electronic interaction of the dye chromophore with the electric field, but may additionally involve some very local voltage-dependent change in how the dye orients in or associates with the membrane. Moreover, this work also shows that we are able to synthesize a series of dyes excitable at , exploiting the utility of relatively inexpensive turnkey fiber-based femtosecond lasers. Several of these dyes respond quickly to voltage change, with large signal sensitivity to TMP without any apparent effects on cellular activity. PY-1261 and PY-1278 exhibit both a large SHG signal change and instantaneous kinetics. PY-1268 shows a large and instantaneous 2PF signal change in a visible emission band. These dyes thus significantly enhance and expand the range of optical approaches available to experimentalists interested in mapping electrical activity in complex excitable cells and tissue. Importantly, the availability of a choice of dyes will allow investigators to choose probes based not only on sensitivity to voltage but also on such additional factors as solubility, excitation and emission wavelengths, tissue penetration, staining persistence, and resistance to cell internalization. ReferencesL. B. Cohen,
B. M. Salzberg,
H. V. Davila,
W. N. Ross,
D. Landowne,
A. S. Waggoner, and
C. H. Wang,
“Changes in axon fluorescence during activity: molecular probes of membrane potential,”
J. Membr. Biol., 19 1
–36
(1974). https://doi.org/10.1007/BF01869968 0022-2631 Google Scholar
I. Tasaki,
“Energy transduction in the nerve membrane and studies of excitation processes with extrinsic fluorescence probes,”
Ann. N.Y. Acad. Sci., 227 247
–267
(1974). https://doi.org/10.1111/j.1749-6632.1974.tb14390.x 0077-8923 Google Scholar
R. Araya,
J. Jiang,
K. B. Eisenthal, and
R. Yuste,
“The spine neck filters membrane potentials,”
Proc. Natl. Acad. Sci. U.S.A., 103 17961
–17966
(2006). https://doi.org/10.1073/pnas.0608755103 0027-8424 Google Scholar
I. Ben-Oren,
G. Peleg,
A. Lewis,
B. Minke, and
L. M. Loew,
“Infrared nonlinear optical measurements of membrane potential in photoreceptor cells,”
Biophys. J., 71 1616
–1620
(1996). 0006-3495 Google Scholar
O. Bouevitch,
A. Lewis,
I. Pinevsky,
J. P. Wuskell, and
L. M. Loew,
“Probing membrane potential with non-linear optics,”
Biophys. J., 65 672
–679
(1993). 0006-3495 Google Scholar
P. J. Campagnola,
M.-D. Wei,
A. Lewis, and
L. M. Loew,
“High resolution optical imaging of live cells by second harmonic generation,”
Biophys. J., 77 3341
–3349
(1999). 0006-3495 Google Scholar
D. A. Dombeck,
L. Sacconi,
M. Blanchard-Desce, and
W. W. Webb,
“Optical recording of fast neuronal membrane potential transients in acute mammalian brain slices by second-harmonic generation microscopy,”
J. Neurophysiol., 94 3628
–3636
(2005). https://doi.org/10.1152/jn.00416.2005 0022-3077 Google Scholar
A. C. Millard,
L. Jin,
A. Lewis, and
L. M. Loew,
“Direct measurement of the voltage sensitivity of second-harmonic generation from a membrane dye in patch-clamped cells,”
Opt. Lett., 28 1221
–1223
(2003). 0146-9592 Google Scholar
A. C. Millard,
L. Jin,
M.-D. Wei,
J. P. Wuskell,
A. Lewis, and
L. M. Loew,
“Sensitivity of second harmonic generation from styryl dyes to trans-membrane potential,”
Biophys. J., 86 1169
–1176
(2004). 0006-3495 Google Scholar
A. C. Millard,
L. Jin,
J. P. Wuskell,
D. M. Boudreau,
A. Lewis, and
L. M. Loew,
“Wavelength- and time-dependence of potentiometric non-linear optical signals from styryl dyes,”
J. Membr. Biol., 208 103
–111
(2005). https://doi.org/10.1007/s00232-005-0823-y 0022-2631 Google Scholar
M. Nuriya,
J. Jiang,
B. Nemet,
K. B. Eisenthal, and
R. Yuste,
“Imaging membrane potential in dendritic spines,”
Proc. Natl. Acad. Sci. U.S.A., 103 786
–790
(2006). https://doi.org/10.1073/pnas.0510092103 0027-8424 Google Scholar
P. Yan,
A. C. Millard,
M. Wei, and
L. M. Loew,
“Unique contrast patterns from resonance-enhanced chiral SHG of cell membranes,”
J. Am. Chem. Soc., 128 11030
–11031
(2006). https://doi.org/10.1021/ja0635534 0002-7863 Google Scholar
A. C. Millard,
P. J. Campagnola,
W. Mohler,
A. Lewis, and
L. M. Loew,
“Second harmonic imaging microscopy,”
Methods Enzymol., 361 47
–69
(2003). https://doi.org/10.1016/S0076-6879(03)61005-0 0076-6879 Google Scholar
A. C. Millard,
L. Jin, and
L. M. Loew,
“Second harmonic generation imaging microscopy with a high power ultrafast fiber laser,”
Proc. SPIE, 5714 92
–98
(2005). https://doi.org/10.1117/12.590999 0277-786X Google Scholar
A. C. Millard,
L. Jin,
M.-D. Wei,
J. P. Wuskell,
A. Lewis, and
L. M. Loew,
“Sensitivity of second harmonic generation from styryl dyes to transmembrane potential,”
Biophys. J., 86 1169
–1176
(2004). 0006-3495 Google Scholar
L. Moreaux,
O. Sandre,
S. Charpak,
M. Blanchard-Desce, and
J. Mertz,
“Coherent scattering in multi-harmonic light microscopy,”
Biophys. J., 80 1568
–1574
(2001). 0006-3495 Google Scholar
L. Moreaux,
O. Sandre, and
J. Mertz,
“Membrane imaging by second harmonic generation microscopy,”
J. Opt. Soc. Am. B, 17 1685
–1694
(2000). 0740-3224 Google Scholar
P. J. Campagnola and
L. M. Loew,
“Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues, organisms,”
Nat. Biotechnol., 21 1356
–1360
(2003). https://doi.org/10.1038/nbt894 1087-0156 Google Scholar
E. Fluhler,
V. G. Burnham, and
L. M. Loew,
“Spectra, membrane binding, and potentiometric responses of new charge shift probes,”
Biochemistry, 24 5749
–5755
(1985). https://doi.org/10.1021/bi00342a010 0006-2960 Google Scholar
L. M. Loew,
“How to choose a potentiometric membrane probe,”
Spectroscopic Membrane Probes, 139
–152 CRC Press, Boca Raton, Florida (1988). Google Scholar
|