|
|
|
Confocal laser scanning microscopy (CLSM) is a valuable tool for obtaining high-resolution images and 3-D reconstructions.1 Vertebrate rod photoreceptors, the neurons responsible for visual phototransduction, mediate vision at low-light intensities using a modified cilium (called the outer segment) for the detection of light. The rod outer segment (rod OS) is packed with hundreds of membrane sacs, called disks, on the surface of which the initial biochemical reactions of visual transduction take place.2, 3 The disks, with dimensions of about ,4, 5 are regularly stacked on top of each other with a repeat distance of .4 The visual pigment rhodopsin (Rh), an integral membrane protein, accounts for about 90%6 of total disk protein content. Photoreceptor disks are continuously renewed through formation of new disks at the base of the outer segment, displaced distally along the length of the outer segment, and eventual detachment and phagocytosis occurs by adjacent pigment epithelial cells.7 Several microscopy techniques have been used to analyze the morphological organization of rods and to identify the membrane proteins in rod OS. For example, measurements of the ellipsoid mitochondria density, sizes, and shapes of inner segments were conducted by electron microscopy and Nomads differential interference contrast (NDIC) imaging.8 Studies on the stability of Rh in native membranes were done by single-molecule force spectroscopy.9 Moreover, a recent progress in modeling the native conformation of Rh was based on topography of the disk membranes recorded by an atomic force microscope (AFM).9 CLSM was only used on whole retina or rod OS to observe the protein distribution in the disk incisures.10 Therefore, even though rod imaging has been achieved in many ways, to the best of our knowledge, until now no study has been reported regarding the application of immunofluorescence for confocal optical imaging of isolated intact disks. In fact, even though fluorescent optical imaging is among the most widely used approach for studying cells in vivo, it is not easily applicable for subcellular fractions. Apart from the classic inclusion methods, it is difficult to isolate and image a single subcellular fraction. A significant limitation in this case is the attachment to glasses. We verified that isolated disk populations do not adhere to plastic or glass. Use of either scarified glasses or synthetic molecules, like poly-D-Lysine, as a coating to enhance cell attachment was not effective to obtain an effective binding of disks to the glass surface. The novelty of our technique lays in the preparation of the sample for immunofluorescence confocal analysis. Namely, disks are incubated with primary and secondary antibodies directly in solution, in Eppendorf tubes. Only at the end of the procedure is the sample put on the glasses and sealed. Osmotically intact disks were obtained from intact rod OS. Rod OSs were isolated under dim red light from 20 bovine retinas (from a local slaughterhouse), by sucrose gradient centrifugation11 in the presence of a protease inhibitor cocktail, under sterile conditions. After bursting isolated rod OS for in 5% Ficoll (Sigma-Aldrich, Saint Louis, Missouri) solution in distilled water with 5-mM DTT and leupeptin, osmotically intact disks were collected on the Ficoll solution surface after centrifuging for at in a Beckman FW-27 rotor.12 Ampicillin was added to all solutions. Protein concentration was determined by the Bradford method.13 The disk suspensions were characterized: Rh concentration was . ratio was , as determined spectrophotometrically14 by measuring the difference in 500-nm absorption between spectra recorded before and after exhaustive bleaching. Disks were stained with a mouse monoclonal antibody (Ab) raised against bovine Rh and a Cy3-conjugated secondary antimouse Ab. Treatments on intact disks were conducted in solution in Eppendorf vials at room temperature. Disks ( protein in ) were washed with 10-mM phosphate-buffered saline (PBS), pH 7.3 plus 150-mM , and collected by centrifugation at for in an Eppendorf centrifuge (Eppendorf, Fremont, California). This step was performed after each treatment. After fixation in 3% paraformaldehyde , disks were resuspended and incubated with 30-mM . Then disks were incubated with mouse monoclonal Ab against bovine Rh diluted 1:500 in PBS . To eliminate unbound primary antibodies, samples were washed two times with PBS. Disks were then incubated with the secondary antibody (goat antimouse IgG Ab conjugated with Cy3 fluorochrome, Molecular Probes), diluted 1:800 in PBS . After PBS washings to eliminate unbound secondary Ab, the disk pellet was resuspended in of Milli-Q water. This volume was put onto glass slides, covered, and sealed with MOVIOL resin at in a dry place. In controls, disks were treated with the secondary Cy3-conjugated Ab only. In this study, immunofluorescence CLSM imaging was performed on an inverted Leica TCS SP5 AOBS confocal laser scanning microscope (Leica Microsystems CMS, Mannheim, Germany) equipped with a set of lasers covering the 458-, 476-, 488-, 496-, 514-, 543-, and 633-nm lines. Confocal fluorescence imaging was done on these samples at . Images were collected using a Leica APO NA 1.40 oil immersion objective (Leica Microsystems CMS, Mannheim, Germany). Images were obtained using the 543-nm line of a ion laser (laser power 20%, ). Under this imaging configuration, typical confocal resolution is of the order of in the lateral and in the axial direction. To detect the presence/absence of the red dye (CY3), the images were collected in the spectral region 550 to accordingly to reported emission spectra.1 To verify that the signal acquired is really due to CY3, we performed spectral analysis by exciting the red dye at and acquiring the fluorescence spectrum from 550 to with a band width of . Figures 1a, 1b, 1c, 1d show the protein stained by indirect immunocytochemistry (in red). These show that Rh is uniformly distributed on the surface of disks. The technique revealed that freshly prepared disks are mostly aggregated [Fig. 1a]. The disk aggregates are in numbers of 2 to 4 disks, as judged by their mean diameter. Larger aggregates may not be found either, because they do not form or because they may not flotate up to the Ficoll surface during disk purification. To eliminate the larger disk aggregates, disks were subjected to 15 passages through a needle (25 gauge) during each resuspension [Fig. 1b]. Such shearing of disks adds negative charges to the disks membrane, causing repulsion between the organelles. Fig. 1Confocal fluorescence imaging of rhodopsin on disks. Confocal fluorescence image of intact disks stained with a Cy3-labeled secondary antibody (Ab) bound to a primary Ab against rhodopsin (1:500). (a) Freshly prepared aggregated disks are shown. (b) Smaller aggregates were represented. (c) An overview of a single disk. In (d), the object is an enlarged single disk, observed in the , , and planes. Its dimensions are estimated to be about . The images were acquired with the Leica confocal software (LCS) (color online only). 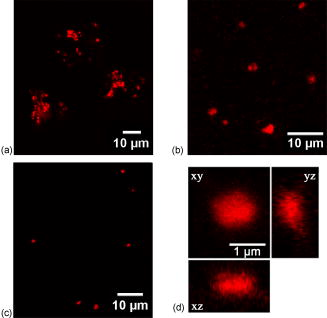 Fig. 2Spectral analysis of the red Cy3 dye. The figure shows the spectral analysis, elaborated from several spectral acquisitions within the 560- to 660-nm range, with a wavelength resolution of , of the fluorescence intensity of the red Cy3 dye (exc. 543.5/em. ) (color online only). 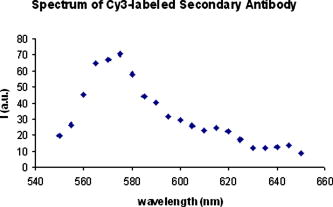 However, a lower protein concentration is necessary for better disaggregating of the aggregates. When protein concentration in the sample was lowered to , intact single disks were observed after shearing, as shown in Fig. 1c. The technique described is an improvement of a protocol that we utilized to demonstrate the presence of ryanodine receptors on the rod disk.15 In the cited paper large aggregates were present. The object shown in the orthogonal projection [Fig. 1d] is an isolated osmotically intact disk, of approximately , as expected for bovine rods.16 Moreover, recent studies on murine rod OS show that the disks’ diameter is about ,4 similar to the dimensions of the object visualized in Fig. 1d, considering the similarity among all mammalian retinas.17 The technique reveals that the method of Smith and Litman12 isolates only those disks that are osmotically active, as judged by their swollen round shape [Fig. 1d]. As shown by the spectral analysis (plotted graph in Fig. 2 ), the fluorescence intensity of the sample is only due to the red fluorochrome Cy3 (exc. . peak ). Incubation of disks with the secondary Ab only yielded negligible immunoreactivity, confirming the specificity of the interaction of the antibody with Rh on the surface of disks (data not shown). No differences are observed in rhodopsin imaging when samples are prepared either in dim red or in room light. However, as the technique can be applied to observe proteins embedded or the peripheral of the disk surface, a protein that can be modified by light exposure might be looked for. Considering the similarities among mammalian retinas and disks in particular,17 the technique described here may also be applied to imaging of human disks, even though a great number of retinas would be necessary to obtain a sufficient quantity of disks. Moreover, the procedure would need to be adjusted in case of human specimens, as retinas must be extracted immediately after death to prepare the disks. We anticipate that this procedure, allowing us to observe isolated osmotically intact disks12 without prior embedding of the sample, may become an instrument to study the function and structure of OS disks. AcknowledgmentsWe thank Isidoro M. Pepe and Alessandro Morelli (DIBIO, University of Genova, Italy) for their invaluable contribution. ReferencesJ. B. Pawley, Handbook of Biological Confocal Microscopy, 3rd ed.Plenum Press-Springer, New York (2006). Google Scholar
L. Stryer,
“Vision: from photon to perception,”
Proc. Natl. Acad. Sci. U.S.A., 93 557
–559
(1996). https://doi.org/10.1073/pnas.93.2.557 0027-8424 Google Scholar
K. D. Ridge,
N. G. Abdulaev,
M. Sousa, and
K. Palczewski,
“Photo transduction: crystal clear,”
Trends Biochem. Sci., 28 479
–487
(2003). https://doi.org/10.1016/S0968-0004(03)00172-5 0167-7640 Google Scholar
S. Nickell,
P. S. Park,
W. Baumeister, and
K. Palczewski,
“Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography,”
J. Cell Biol., 177 917
–925
(2007). 0021-9525 Google Scholar
A. E. Blaurock and
M. H. Wilkins,
“Structure of frog photoreceptor membranes,”
Nature (London), 223 906
–909
(1969). https://doi.org/10.1038/223906a0 0028-0836 Google Scholar
S. Filipek,
R. E. Stenkamp,
D. C. Teller, and
K. Palczewski,
“G protein-coupled receptor rhodopsin: a prospectus,”
Annu. Rev. Physiol., 65 851
–879
(2003). https://doi.org/10.1146/annurev.physiol.65.092101.142611 0066-4278 Google Scholar
D. H. Anderson and
S. K. Fisher,
“Disk shedding in rodlike and conelike photoreceptors of tree squirrels,”
Science, 197 953
–955
(1975). https://doi.org/10.1126/science.1145180 0036-8075 Google Scholar
Q. V. Hoang,
R. A. Linssenmeier,
C. K. Chung, and
C. A. Curcio,
“Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation,”
Visual Neurosci., 19 395
–407
(2002). https://doi.org/10.1017/S0952523802194028 0952-5238 Google Scholar
D. Fotiadis,
B. Jastrzebska,
A. Philippsen,
D. J. Muller, K. Palczewsky, A. Engel,
“Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors,”
Curr. Opin. Struct. Biol., 16 252
–259
(2006). https://doi.org/10.1016/j.sbi.2006.03.013 0959-440X Google Scholar
B. M. Tam,
O. L. Moritz, and
D. S. Papermaster,
“The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures,”
Mol. Biol. Cell, 15 2027
–2037
(2004). https://doi.org/10.1091/mbc.E03-09-0650 1059-1524 Google Scholar
P. P. Schnetkamp and
F. J. Daemen,
“Isolation and characterization of osmotically sealed bovine rod outer segments,”
Methods Enzymol., 81 110
–116
(1982). https://doi.org/10.1016/S0076-6879(82)81019-7 0076-6879 Google Scholar
G. Smith and
B. J. Litman,
“Preparation of osmotically intact rod outer segment disks by Ficoll flotation,”
Methods Enzymol., 81 57
–61
(1982). https://doi.org/10.1016/S0076-6879(82)81012-4 0076-6879 Google Scholar
M. M. Bradford,
“A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,”
Anal. Biochem., 72 248
–256
(1976). https://doi.org/10.1006/abio.1976.9999 0003-2697 Google Scholar
G. Wald and
P. K. Brown,
“The molar extinction of rhodopsin,”
J. Gen. Physiol., 37 189
–200
(1953). 0022-1295 Google Scholar
I. Panfoli,
S. Ravera,
A. Fabiano,
R. Magrassi,
A. Diaspro, A. Morelli, I. M. Pepe,
“Localization of the cyclic ADP-ribose-dependent calcium signaling pathway in bovine rod outer segments,”
Invest. Ophthalmol. Visual Sci., 48 978
–984
(2007). 0146-0404 Google Scholar
T. Norisuye,
W. F. Hoffman, and
H. Yu,
“Intact photoreceptor membrane from bovine rod outer segment: size and shape in bleached state,”
Biochemistry, 15 5678
–5682
(1976). https://doi.org/10.1021/bi00670a038 0006-2960 Google Scholar
H. Wassle and
B. B. Boycott,
“Functional architecture of the mammalian retina,”
Physiol. Rev., 71 447
–480
(1991). 0031-9333 Google Scholar
|


