|
|
|
Noninvasive optical reflectance measurements have been used to extract diagnostic information from a variety of tissue types, in vivo, such as colon,1 skin,2 brain,3 cervix,4 esophagus,5 prostate,6 and bronchial mucosa.7 The typical experimental setup of these systems often consists of a fiber probe with a diameter of a few millimeters or less, with one or more fibers that transmit light to and from the tissue. An earlier ex vivo study reported that the amount of pressure applied on a sample of tissue affects its absorption and reduced scattering coefficients.8 It has also been reported that probe pressures with forces of less than do not affect the fluorescence intensity or lineshape of measurements obtained in vivo on cervical tissues.9 This paper reports on a study of the influence of probe pressure on spectral reflectance measurements of biological tissue in vivo. We used the thigh muscle of a mouse as a tissue model because the volume of the tissue is large enough to be considered semiinfinite for the optical geometry of our probe (source-detector separation of approximately ), and because the muscle is relatively homogeneous. The experimental setup consists of a pulsed xenon arc lamp (Perkin Elmer, LS1130-3) as a light source; a spectrometer with a linear CCD array detector (Ocean Optics, S2000); a control computer; and a fiber optic probe, which transmits light to and from the tissue. The fiber probe contains two optical fibers with core diameters of , a numerical aperture of 0.22, and a center-to-center separation of approximately . The optical fibers are encapsulated within a 2.7-mm-diam metal tube. The probe has a handle where different weights can be attached, as shown in Fig. 1 . The weights, added to the weight of the probe itself, resulted in applied forces of 0.24, 0.53, 0.78, 0.98, and over a probe area of , which correspond to pressures of 0.04, 0.09, 0.13, 0.17, and . Ten mice were anesthetized with pentobarbital, and the skin over both the left and right thigh muscles was removed, such that the muscles were exposed. The probe was placed perpendicular to the tissue surface, on the muscle, for the optical measurements. A measurement consisted of an average of five spectra, each spanning the wavelength range of 340 to . Optical contact between the fiber probe and the tissue was assisted by applying water to the surface of the muscle. The experiment was performed on the thigh muscle of each leg. We were not able to collect data from one muscle; therefore, experimental data from 19 thigh muscles (9 left and 10 right) are presented. Each experiment was comprised of five sets, and a set consisted of two measurements. The first measurement was obtained when the fiber probe was placed in gentle contact with the surface of the tissue such that there was no significant pressure applied to the muscle. The probe was held by a fixture, as presented in Fig. 1. The second measurement was obtained by attaching one of the weights to the probe, and loosening the probe fixture, enabling the probe to slide vertically while maintaining a vertical orientation, such that the pressure was applied normal to the tissue surface. Each spectrum was acquired within of the application of pressure. Subsequently, the probe was removed from the muscle, and at least a 30-s time delay was allowed for the tissue to return to its normal state prior to a subsequent measurement. The set was then repeated at a different location on the muscle, with a different pressure until all five weights were used. The order of the pressure applied was randomized on each muscle; therefore, it is assumed that the influence of the previously applied pressure on each measurement is negligible. In total, there were 19 measurements per specified pressure, and 95 measurements with no pressure applied. The repeatability of the amount of pressure applied to the tissue was tested by placing the setup over a scale (in place of the tissue) and measuring the weight. The pressure applied by each weight was measured five times, and the variation was determined to be less than 5%. Each tissue measurement was analyzed10 by using Eq. 1. Briefly, the relative reflectance spectrum is obtained from the ratio of the tissue spectrum over the spectrum obtained with a spectrally flat diffuse reflector (Labsphere, Spectralon), denoted as . The amplitude of is dependent on the distance between the probe and the diffuse reflector. This distance is difficult to control with submillimeter resolution; therefore, the spectrum is normalized at so that its amplitude is independent of the distance between the probe and the diffuse reflector. Finally, the spectrum is referenced by the spectrum obtained from a liquid calibration phantom with known optical properties at . The calibration phantom was a suspension of of titanium dioxide powder (J.T. Baker) in of deionized water. The absorption and reduced scattering coefficients of the tissue are denoted by and , respectively, and the values of , , and were determined to be 0.11, 0.22, and 0.2, respectively, as described in Reif 10 Given that the primary absorbers, oxy- and deoxyhemoglobin, are compartmentalized in blood vessels, a correction factor for the inhomogeneous distribution of the absorption was applied, as derived by other groups.7, 11, 12 The correction factor, given by Eq. 2, assumes that hemoglobin is concentrated in blood vessels that are modeled as infinitely long cylinders. The mean value of the blood vessel radius is given by and the absorption coefficient of whole blood is given7, 11, 12 by . The reduced scattering and absorption coefficients were modeled by where and are the extinction coefficients of oxy- and deoxyhemoglobin, respectively.Five coefficients are obtained from the model: blood volume fraction ; hemoglobin oxygen saturation ; blood vessel radius ; and the reduced scattering coefficient , which is described by the exponent of the power law or Mie-theory slope . Since the value has no physical meaning, and its units depend directly on such that the units of are in inverse length (i.e., inverse centimeters), we describe the value of at , as defined by Eq. 3. The Mie slope is a constant, which can be related to the mean size of the scattering particles, and can have13 a value between 0.37 (particles much larger than the wavelength of the light) and 4 (particles much smaller than the wavelength of the light; also known as Rayleigh scattering). The blood volume fraction is calculated by assuming a blood hemoglobin concentration of , which is typical for whole blood. The model described by Eq. 1 was fit to the relative reflectance spectrum where , , , , and are fitting parameters. A least-squares fit with a Levenberg-Marquardt algorithm was implemented with Matlab, and the parameters had the following constraints: ; ; and . The starting point for all the parameters was the lowest value of the constraint. Other starting points were tested and the values for the parameters obtained converged; therefore, we can assume that the solution is unique. Figure 2a shows an example of the relative reflectance and of the model fit at two different probe pressures. Note that a degree of desaturation is exhibited in the trace taken at , compared with the trace at zero pressure. Fig. 2(a) Example of the relative reflectance and the respective fits for a measurement obtained with a pressure of 0 and and mean and standard deviations of (b) blood volume fraction, (c) oxygen saturation, (d) mean blood vessel radius, (e) Mie slope, and (f) reduced scattering coefficient at as a function of the probe pressure. The solid line is the least-squared fit of Eq. 5 to the mean values of each parameter. 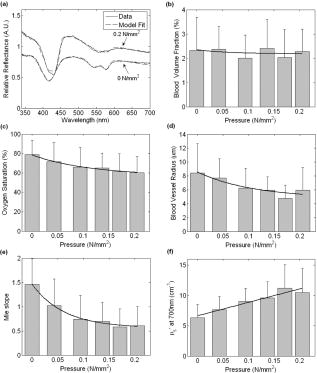 The mean and standard deviations of the five parameters extracted from the model are presented in Figs. 2b through 2f. By observation, the trend of the data seems to follow a linear or exponential relationship with pressure; therefore, the mean value of each parameter was modeled as a function of the probe pressure with an exponential expression given where , , and are fitting coefficients, and is the probe pressure. The results from the exponential fits are plotted as the solid lines in Figs. 2b through 2f.The blood vessel radius, oxygen saturation, and Mie-theory slope decrease with pressure, while the reduced scattering coefficient at increases as a function of pressure. We hypothesize that the pressure applied by the probe compresses the blood vessels, consequently reducing the blood flow. This would explain the decrease in the blood vessel radius and the desaturation of the blood due to the tissue oxygen consumption and disruption of the flow of oxygenated blood arriving to the tissue. The probe pressure might also increase the density of scatterers per unit volume, which would be consistent with the increase in the reduced scattering coefficient. The decrease in Mie slope is an indication that the scattering is mostly due to larger particles, which could be a consequence of increasing the density of large organelles per unit volume. The mean blood volume fraction varies less than 20% for the range of pressures applied and does not seem to follow a trend; therefore, it can be assumed that the blood volume fraction is not dependent on the probe pressure. Note that the dominant contributors to the optical absorption in muscle come from hemoglobin and myoglobin. The absorption spectrum of myoglobin is very similar to that of hemoglobin and its concentration in muscles is typically lower than that of hemoglobin,14, 15 therefore its contribution to the optical absorption was neglected in this paper. Although the blood volume is reduced when the blood vessels are compressed, the concentration of myoglobin might increase, counteracting the decrease in hemoglobin absorption. If so, the model would fit the absorption spectra of hemoglobin to myoglobin, which would explain the lack of change in the blood volume fraction. We are currently working on understanding this effect. In conclusion, the amount of probe pressure applied on the thigh muscle affects the reflectance spectrum in a predictable manner. Therefore, we hypothesize that by limiting or controlling the probe pressure it would be possible to reduce the spectral variability in reflectance measurements and increase the sensitivity and specificity of several studies for early cancer diagnosis. An analytical model was fit to the reflectance spectra to extract five parameters, which provide a basic physiological description for the change in the relative reflectance spectra. All these parameters, with the exception of the blood volume fraction, vary as a function of the probe pressure. We expect to further expand this work to study the changes in reflectance spectra as a function of pressure in more relevant tissues for optical diagnosis, such as colon polyps, and also to study the temporal changes in the reflectance spectra under constant pressures. AcknowledgmentsWe would like to thank Sara Gillers for her assistance throughout the development of the study. The project was supported in part by the National Institutes of Health (NIH) through Grants F31-CA119916 and U54-CA104677. ReferencesG. Zonios,
L. T. Perelman,
V. Backman,
R. Manoharan,
M. Fitzmaurice,
J. Van Dam, and
M. S. Feld,
“Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo,”
Appl. Opt., 38
(31), 6628
–6637
(1999). 0003-6935 Google Scholar
G. Zonios and
A. Dimou,
“Modeling diffuse reflectance from semi-infinite turbid media: application to the study of skin optical properties,”
Opt. Express, 14 8661
–8674
(2006). https://doi.org/10.1364/OE.14.008661 1094-4087 Google Scholar
M. Johns,
C. Giller,
D. German, and
H. Liu,
“Determination of reduced scattering coefficient of biological tissue from a needle-like probe,”
Opt. Express, 13 4828
–4842
(2005). https://doi.org/10.1364/OPEX.13.004828 1094-4087 Google Scholar
Y. N. Mirabal,
S. K. Chang,
E. N. Atkinson,
A. Malpica,
M. Follen, and
R. Richards-Kortum,
“Reflectance spectroscopy for in vivo detection of cervical precancer,”
J. Biomed. Opt., 7
(4), 587
–594
(2002). https://doi.org/10.1117/1.1502675 1083-3668 Google Scholar
L. B. Lovat,
K. Johnson,
G. D. Mackenzie,
B. R. Clark,
M. R. Novelli,
S. Davies,
M. O’Donovan,
C. Selvasekar,
S. M. Thorpe,
D. Pickard,
R. Fitzgerald,
T. Feam,
I. Bigio, and
S. G. Bown,
“Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett’s oesophagus,”
Gut, 55 1078
–1083
(2006). 0017-5749 Google Scholar
I. J. Bigio,
O. A’Amar, and
M. S. Hirsch,
“Elastic scattering spectroscopy for detection of prostate cancer: preliminary feasibility study,”
Proc. SPIE, 5141 142
–146
(2003). https://doi.org/10.1117/12.500947 0277-786X Google Scholar
A. Amelink,
H. J. C. M. Sterenborg,
M. P. L. Bard, and
S. A. Burgers,
“In vivo measurements of the local optical properties of tissue by use of differential path-length spectroscopy,”
Opt. Lett., 29
(10), 1087
–1089
(2004). https://doi.org/10.1364/OL.29.001087 0146-9592 Google Scholar
H. Shangguan,
S. A. Prahl,
S. L. Jacques, and
L. W. Casperson,
“Pressure effects on soft tissues monitored by changes in tissue optical properties,”
Proc. SPIE, 3254 366
–371
(1998). https://doi.org/10.1117/12.308187 0277-786X Google Scholar
A. Nath,
K. Rivoire,
S. Chang,
D. Cox,
E. N. Atkinson,
M. Follen, and
R. Richards-Kortum,
“Effect of probe pressure on cervical fluorescence spectroscopy measurements,”
J. Biomed. Opt., 9
(3), 523
–533
(2004). https://doi.org/10.1117/1.1695562 1083-3668 Google Scholar
R. Reif,
O. A’Amar, and
I. J. Bigio,
“Analytical model of light reflectance for extraction of the optical properties in small volumes of turbid media,”
Appl. Opt., 46
(29), 7317
–7328
(2007). https://doi.org/10.1364/AO.46.007317 0003-6935 Google Scholar
R. L. P. van Veen,
W. Verkruysse, and
H. J. C. M. Sterenborg,
“Diffuse-reflectance spectroscopy from 500 to by correction of inhomogeneously distributed absorbers,”
Opt. Lett., 27 246
–248
(2002). https://doi.org/10.1038/nature01260 0146-9592 Google Scholar
L. O. Svaasand,
E. J. Fiskerstrand,
G. Kopstad,
L. T. Norvang,
E. K. Svaasand,
J. S. Nelson, and
M. W. Berns,
“Therapeutic response during pulsed laser treatment of port-wine stains: dependence on vessel diameter and depth in dermis,”
Lasers Med. Sci., 10 235
–243
(1995). 0268-8921 Google Scholar
J. R. Mourant,
T. Fuselier,
J. Boyer,
T. M. Johnson, and
I. J. Bigio,
“Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms,”
Appl. Opt., 36 949
–957
(1997). 0003-6935 Google Scholar
P. J. O’Brien,
H. Shen,
L. J. McCutcheon,
M. O’Grady,
P. J. Byrne,
H. W. Ferguson,
M. S. Mirsalimi,
R. J. Julian,
J. M. Sargeant,
R. R. M. Tremblay, and
T. E. Blackwell,
“Rapid, simple and sensitive microassay for skeletal and cardiac muscle myoglobin and hemoglobin: use in various animals indicates functional role of myohemoproteins,”
Mol. Cell. Biochem., 112 45
–52
(1992). 0300-8177 Google Scholar
C. Casavola,
L. A. Paunescu,
S. Fantini, and
E. Gratton,
“Blood flow and oxygen consumption with near-infrared spectroscopy and venous occlusion: spatial maps and the effect of time and pressure inflation,”
J. Biomed. Opt., 5 269
–276
(2000). https://doi.org/10.1117/1.429995 1083-3668 Google Scholar
|