|
|
1.IntroductionIn many biomedical applications, tissue pressure is used to reduce index mismatch and increase light penetration.1, 2 This phenomenon enhances the diagnostic and therapeutic capabilities of various optical methods.2 However, in many optical diagnostic applications, external pressure may adversely influence optical properties and skin microcirculation. Often, various optical methods for therapy and diagnostics use optical probes that produce uncontrolled pressure on tissue and may reduce the accuracy of these methods. Optical coherence tomography (OCT), which has been proposed for noninvasive glucose monitoring,3 is subject to error induced by motion artifacts, changes in skin temperature, and other variables, both in vivo 4 and in vitro.5 Recently, we observed that tissue pressure also influenced accuracy during continuous blood glucose monitoring with OCT.6 Compression of soft tissue produces the following effects: reduction of blood and interstitial fluid volume in the compressed site,2 thickness reduction that leads to an increase in light transmittance,7 and vasodilation as a physiological response to an increase of progressive local pressure.8 Under local pressure, the spacing between tissue components and the refractive index mismatch decrease due to water displacement, while closer packing of tissue components reduces scattering due to cooperative (interference) effects.9 These effects decrease average light scattering.2 However, local pressure reduces tissue thickness, which in turn increases the scatterers’ concentration inside the tissue. This effect is considered to be more dominant than the reduction of index mismatch and the scatterers’ packing effects in vitro.1, 2, 7 Local pressure applied to the skin may play an important role in cutaneous microcirculation impairment in vivo. Local stress activates a considerable number of cutaneous receptors, producing local vasodilation that increases skin blood flow. In the absence of the vasodilation effect, skin blood flow gradually decreases because of local pressure. The vasodilation response to local tissue pressure may protect skin from ischemia during mechanical stimulation.8, 10 It was also shown that the local pressure significantly decreases skin blood flow from the baseline in diabetic patients and normal subjects,8 and produces vasodilation10 as well as other changes in tissues.11, 12 Therefore, the pressure-induced effects may change tissue optical properties and influence the performance of OCT glucose monitoring, which is based on the detection of backscattered light. In our recent in vivo experiments, in order to avoid motion artifacts, we placed our OCT probe on the skin and applied a slight pressure to the probe. A gradual drift of the OCT signal slope was noted during blood glucose monitoring, which we hypothesized was due to tissue pressure.6 In this study, we substantially reduced the influence of the pressure effects on continuous blood glucose monitoring with OCT. 2.Materials and MethodsThe in vivo experiments were performed in 19 anesthetized farm pigs (weight 20 to ) placed in a supine position. OCT images were acquired from the abdominal area of the skin during variation in blood glucose concentration induced by an intravenous (i.v.) injection of 50% glucose solution (50% dextrose, Abbot Laboratories, North Chicago, Illinois). We produced one or two peaks of blood glucose concentration using a digital pump at rates of 1.5 to per minute for 20 to . As a result, the blood glucose concentration increased from baseline concentrations of 50 to (in the first peak) or 150 to (in the second peak) up to 400 to . At the end of the experiments, under deep general anesthesia with 5% isofluorane, the pigs were sacrificed with an i.v. injection of saturated KCl solution . The OCT device used in the experiments was designed at the Institute of Applied Physics of the Russian Academy of Sciences (Nizhny Novgorod, Russia) with a central wavelength of and spatial resolution of .6, 13, 14 In this study we employed a 2-D scanning mode to significantly reduce the speckle noise on the OCT blood glucose sensing.6, 14 We varied the acquisition time for a single OCT image to minimize noise associated with motion artifacts. The acquisition time was , , or (Figs. 1, 2, 3 , respectively) depending on the level of pressure exerted by the OCT probe. Each OCT signal was obtained by averaging 300 A-scans that formed the OCT image. The OCT signal slopes were calculated with a least-square linear fit of segments of OCT signals at a fixed depth6, 14 and plotted (along with actual blood glucose concentration) versus time. Fig. 1OCT signal slope and blood glucose concentration versus time when high pressure was applied on the skin by the 20-mm OCT probe. 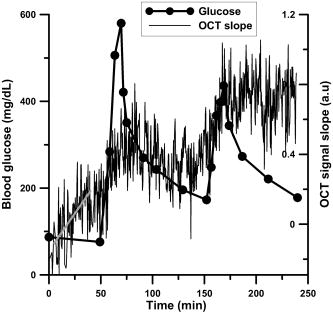 Fig. 2OCT signal slope and blood glucose concentration versus time when low pressure was applied on the skin by the OCT probe with the 50-mm adaptor. 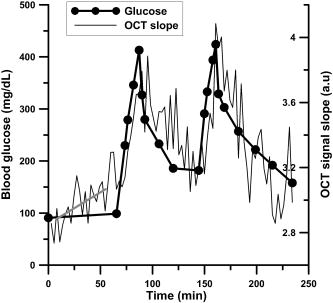 Fig. 3OCT signal slope and blood glucose concentration versus time when high pressure (intervals 1 and 3) and low pressure (intervals 2 and 4) were interchangeably applied on the skin by the OCT probe with the 50-mm adaptor. The measurements in intervals 5 and 6 were performed at low pressure. Glucose was injected during interval 5. 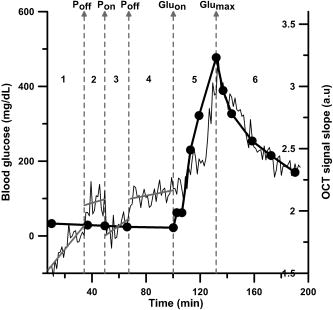 We performed three sets of experiments. In the first set of experiments we used an OCT probe (weight: ) that had a 20-mm-diameter contact area with the skin (the pressure exerted by the probe alone on skin was ), and we applied additional positive pressure that exceeded . Therefore, the total pressure exerted by the probe on the skin exceeded . The additional positive pressure was applied to minimize motion artifacts, which did not allow for accurate measurement of the OCT signal slope. In the second set of experiments, an adapter with a diameter of was used to reduce the pressure applied to the skin by the probe down to (the pressure exerted by the probe alone on skin was and we applied additional negative pressure). The total weight of the OCT probe with the adaptor was . In the third set of experiments, we used the same probe as in the second set of experiments and applied two levels of pressure: high (positive pressure was applied to the probe) and low (negative pressure was applied to the probe). In the first, second, and third sets of experiments, we used 12, 5, and 2 pigs, respectively. The pressure applied to the pig skin was measured using the following procedure. An ex vivo pig skin sample was placed between the OCT probe and a balance plate. The net force applied by the OCT probe was , where (kg) is the reading of the balance display, is the net mass of the probe, is the external force applied to the probe to achieve the desired level of pressure on the tissue, and is the free-fall acceleration. The mass of skin sample (0.5 to ) was negligible compared to the mass of the OCT probe. Thus, the total pressure was , where is the area of the probe (or adaptor) surface, and is the probe (or adaptor) diameter. The OCT probe was attached with a spring system to an arm that could be moved up and down on a stable pole. The moving arm permitted control of the pressure applied to the skin. The vertical position of the arm was marked when the OCT probe was placed on the object without additional pressure (only the probe weight). The shift of the arm from the recorded position in the in vivo experiments was used to calculate the actual, total pressure applied to the skin in vivo. The local and inter-subject variability in abdominal pig skin elasticity and in internal pressure (opposite to the external pressure applied by the probe) resulted in the large variability (about threefold) in the pressure levels needed to achieve the same low level of motion artifacts in OCT glucose sensing in the different sets of experiments. 3.Results and DiscussionThe results of the first two sets of experiments are summarized in Table 1 . For each pig we calculated the drift at the depth where the maximal absolute value of correlation coefficient (R) between the OCT signal slope and blood glucose concentration was observed ( , ). The depth at which the OCT signal slope was calculated in each pig is shown in column 4 of the table. The drift of the OCT signal slope can be positive or negative depending on the layer where the OCT signal slope was measured. As we showed previously,6, 13 the correlation of the OCT signal slope with blood glucose concentration varies between and with depth due to the layered structure of the skin. The long-term drift (for about ) in the OCT signal slope produced by the high pressure level with the 20-mm probe was noted in our earlier studies.6 The absolute value of the drift in the OCT signal slope was measured in this study, and for the high pressure was arbitrary units (a.u.)/min (Table 1). This was greater (4.8 times) than that for the low pressure: . Table 1OCT signal slope drift measured before glucose injection as shown in Figs. 1 and 2 when variation in glucose concentration did not exceed 15mg∕dL . The time interval used for the fit is shown in the third column. Depth refers to the distance from the skin surface at which the correlation between blood glucose concentration and OCT signal slope was greatest.
Figure 1 shows typical OCT signal slopes and blood glucose concentrations over time when the 20-mm OCT probe was used. The estimated pressure applied to the skin was in the range of 13 to . The second peak in the OCT signal slope is shifted up due to the pressure-induced effects. To reduce but not eliminate the pressure of the OCT probe on the skin, we lifted the arm attached to the OCT probe. When we lifted the arm attached to the 20-mm OCT probe, the motion artifacts increased and precluded accurate OCT signal measurement. In the second set of experiments, in which we used the adaptor and lifted the support arm, the level of motion artifacts was acceptable because the amplitude of motion was inversely proportional to the force needed to move the heavier system (the probe with the adaptor) relative to the skin. When the arm was lifted, the estimated residual level of pressure applied to the skin surface was in the range of 0.25 to . Results for one of the pigs in this set of experiments are shown in Fig. 2. No significant pressure-induced drift of the glucose-induced changes in OCT signal slope was observed for 3 to 4 hours in this set of experiments. However, in this particular pig, before the glucose injection (first of the experiment), the drift in the OCT signal slope was This value was higher compared with the average drift value of for this set of experiments. In the third set of experiments, we applied two levels of pressure ( and ) at the same tissue site at different times (Fig. 3). During intervals 1 to 4, the glucose level was stable (no glucose injection). Starting from interval 4, the pressure was . Glucose was injected during interval 5 while the pressure was . We estimated the drift in the OCT signal slope with time at these pressure levels using linear fit of the data. Figure 3 shows that the OCT signal slope drift during application of high pressure on the skin was and (intervals 1 and 3, respectively) and was four- to five-fold greater than drift at low pressure: and (intervals 2 and 4, respectively). In the other pig in this set of experiments, the drift at high pressure ( and for intervals 1 and 3, respectively) was greater than that at low pressure ( and for intervals 2 and 4, respectively). In the third set of experiments, the OCT signal slopes were calculated at depths of and for the first and second pigs, respectively. The results of the third set of experiments are consistent with results from the first and second sets of experiments. It should be noted that the signal-to-noise ratio for glucose monitoring in the second (Fig. 2) and third (Fig. 3) sets of experiments was higher than that in the first set of experiments (Fig. 1). This difference is associated with more efficient removal of the motion artifacts with the 50-mm probe compared to the 20-mm probe. The OCT signal slope in Fig. 1 was much smaller than those in the other figures due to local variations of the OCT signal with depth that were observed in this and previous studies.6, 13 The OCT signal slope in pig skin is sharply positive, then negative from the skin surface to 20 to . The slope becomes positive at the papillary layer at approximately 100 to , then negative at the papillary-reticular junction (250 to ) and the reticular skin (400 to ). At the dermis-hypodermis junction, it becomes sharply negative. One can see from Table 1 and our previous investigations6, 13, 14 that the maximum correlation between the OCT signal slope and glucose concentration is observed near specific morphological regions, namely the papillary-reticular and dermis-hypodermis junctions. The depth of these junctions has inter-subject variability. To predict glucose levels, the OCT system first should be calibrated for each subject using standard invasive glucose measurement methods. ConclusionIn conclusion, using two substantially different pressure levels, we showed in vivo that pressure applied to the skin by an OCT probe reduces motion artifacts but may generate drift of the OCT signal slope during continuous noninvasive blood glucose monitoring. Moreover, we reduced the drift four- to five-fold by increasing the contact area between the OCT probe and tissue, then applying optimal negative external pressure to the probe. This results in more accurate and reproducible glucose monitoring with OCT at minimal long-term drift and motion artifacts. AcknowledgmentsThis work was supported by the National Institutes of Health (grant #R01 EB001467 from the National Institute for Biomedical Imaging and Bioengineering). The authors thank Dr. Donald J. Deyo for assistance with the animal experiments. ReferencesE. K. Chan,
B. Sorg,
D. Protsenko,
M. O‘Neil,
M. Motamedi, and
A. J. Welch,
“Effects of compression on soft tissue optical properties,”
IEEE J. Sel. Top. Quantum Electron., 2 943
–950
(1996). https://doi.org/10.1109/2944.577320 1077-260X Google Scholar
V. V. Tuchin, Optical Clearing of Tissue and Blood, SPIE Press, Bellingham, Washington (2006). Google Scholar
R. O. Esenaliev,
K. V. Larin,
I. V. Larina, and
M. Motamedi,
“Noninvasive monitoring of glucose concentration with optical coherence tomography,”
Opt. Lett., 26 992
–994
(2001). 0146-9592 Google Scholar
K. V. Larin,
M. Motamedi,
T. V. Ashitkov, and
R. O. Esenaliev,
“Specificity of noninvasive blood glucose sensing using optical coherence tomography technique: A pilot study,”
Phys. Med. Biol., 48 1371
–1390
(2003). https://doi.org/10.1088/0031-9155/48/10/310 0031-9155 Google Scholar
M. Kinnunen,
R. Myllyla,
T. Jokela, and
S. Vainio,
“In vitro studies toward noninvasive glucose monitoring with optical coherence tomography,”
Appl. Opt., 45 2251
–2260
(2006). https://doi.org/10.1364/AO.45.002251 0003-6935 Google Scholar
R. V. Kuranov,
V. V. Sapozhnikova,
D. S. Prough,
I. Cicenaite, and
R. O. Esenaliev,
“In vivo study of glucose-induced changes in skin properties assessed with optical coherence tomography,”
Phys. Med. Biol., 51 3885
–3900
(2006). https://doi.org/10.1088/0031-9155/51/16/001 0031-9155 Google Scholar
A. Vogel,
C. Dlugos,
R. Nuffer, and
R. Birngruber,
“Optical-properties of human sclera, and their consequences for transscleral laser applications,”
Lasers Surg. Med., 11 331
–340
(1991). https://doi.org/10.1002/lsm.1900110404 0196-8092 Google Scholar
B. Fromy,
P. Abraham,
C. Bouvet,
B. Bouhanick,
P. Frasinaud, and
J. L. Saumet,
“Early decrease of skin blood flow in response to locally applied pressure in diabetic subjects,”
Diabetes, 51 1214
–1217
(2002). 0012-1797 Google Scholar
N. G. Klebtsov,
I. L. Maksimova,
V. V. Tuchin, and
L. Wang, Handbook of Optical Biomedical Diagnostics, SPIE Press, Bellingham, Washington (2002). Google Scholar
P. Abraham,
B. Fromy,
S. Merzeau,
A. Jardel, and
J.-L. Saumet,
“Dynamics of local pressure-induced cutaneous vasodilation in the human hand,”
Microvasc. Res., 61 122
–129
(2001). 0026-2862 Google Scholar
M. Nitzan,
A. Babchenko,
B. Khanokh, and
H. Taitelbaum,
“Measurement of oxygen saturation in venous blood by dynamic near infrared spectroscopy,”
J. Biomed. Opt., 5 155
–162
(2000). https://doi.org/10.1117/1.429982 1083-3668 Google Scholar
S. A. Carp,
T. Kauffman,
Q. Q. Fang,
E. Rafferty,
R. Moore,
D. Kopans, and
D. Boas,
“Compression-induced changes in the physiological state of the breast as observed through frequency domain photon migration measurements,”
J. Biomed. Opt., 11 064106
(2006). 1083-3668 Google Scholar
V. V. Sapozhnikova,
D. S. Prough,
R. V. Kuranov,
I. Cicenaite, and
R. O. Esenaliev,
“Influence of osmolytes on in vivo glucose monitoring using optical coherence tomography,”
Exp. Biol. Med., 231 1323
–1332
(2006). 0071-3384 Google Scholar
R. V. Kuranov,
V. V. Sapozhnikova,
D. S. Prough,
I. Cicenaite, and
R. O. Esenaliev,
“Prediction capability of optical coherence tomography for blood glucose concentration monitoring,”
J. Diabet. Sci. Technol., 1 164
–171
(2007). Google Scholar
|

