|
|
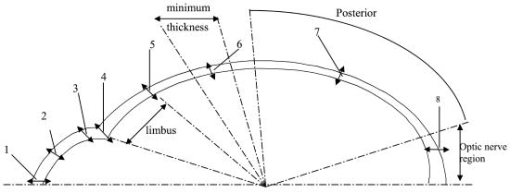
1.IntroductionThe thickness of the outer coats of the eye is an important biometric measure for a number of research and clinical purposes: for studies on the growth of the eyeball, for investigating changes in disease states, and for ascertaining response to induced experimental conditions required in the measurement of material properties. In the clinic and in studies conducted in vivo, it is only possible to study the anterior sections of the eye. Measurement of scleral thickness has thus far required slicing the eyeball after freezing or fixation. Temperature changes and/or use of chemicals will have an effect on the tissue, and it is not certain how much error this introduces to the parameter being measured. Similarly, measurements of rheology of the cornea and/or sclera have been conducted using methods that may alter the properties being measured (slicing, freezing, and thawing), and this may explain the lack of consistency in findings.1, 2, 3 Ideally, material properties of the cornea and sclera should be measured on fresh whole eyeballs. Such approaches have been used previously.4, 5 These studies have approximated the eyeball to a pressure vessel capable of radial expansion with progressive injections of fluid to measure elasticity and rigidity. The theory of pressure vessels requires that certain assumptions about the expansion of the vessel and the thickness of its walls are met.6 The thickness must be less than 20 times the mean diameter of the eyeball (a condition that does apply to the eye), and the stress is assumed to be constant over the wall thickness.6 Although the thickness of the outer coat of the eyeball is not constant, if the variations are small enough not to cause differences in stress and hence disproportionate changes in thickness with expansion, the pressure vessel model can be applied to the eye to estimate elasticity of the tissues that constitute the outer coat of the eyeball. The thickness of the cornea and sclera can be measured using optical coherence tomography (OCT). OCT was developed for imaging the retina using interferometry to compare the delay and intensity of light backscattered from a sample to a reference beam that has traversed a known path length with specified time delay. The first tomographic image of a sample was obtained by Huang, 7 who used a fiber optic interferometer with a reference mirror that moved at the requisite high speed to perform low-coherence interferometric scans. The signals from these scans were used to produce the tomographic image. This technique was further developed to incorporate a dual-beam system and to image, for the first time, the in vivo optic disc.8 The technique continued to improve and systems for imaging not just features of the retina and its supporting structures, but also those of the anterior eye were developed9 and are now commonly used in the clinical setting.10 The Visante OCT uses light at to produce high-resolution images of the anterior segment and to calculate the biometric parameters of the cornea and anterior chamber.11 The method of OCT for imaging various features of the eye is not restricted to just the clinic; a prototype instrument was shown to be effective in imaging the anterior chamber angle and parts of the ciliary body in enucleated porcine eyes.12 This study uses the Visante OCT to measure the thickness of the cornea and sclera in fresh whole porcine eyeballs and to determine changes in thickness of the outer coats of the eye and the dimensional changes of the anterior chamber in response to incremental injections of saline. 2.MethodsTwenty-four porcine eyes, used in this study, were obtained from the local abattoir; collection was within of death. All eyes were from animals aged between 5 and . Twelve eyeballs were used to measure thickness of the cornea, the diameter, depth, and angle of the anterior chamber; the other twelve were used to measure the thickness of the sclera. Experiments were completed between 3 and postmortem; each experiment lasted no longer than . During the experimental procedure, samples were kept moist with saline solution to prevent dehydration. Extraocular musculature and extraneous fat were removed from each eyeball before OCT imaging. Eyeballs were placed on a specially designed holder5 fixed onto the chin rest of the Visante OCT system (Carl Zeiss Meditec, Inc). The instrument has an optical axial resolution of (in the tissue), a lateral resolution of , and a scan field. The holder ensured that eyeballs were maintained in a secure position during imaging and measurement,5 allowing only rotation of the eyeball in an axis perpendicular to the longitudinal plane. Positions at which measurements were made are shown in Fig. 1 . This diagram shows positions of measurements made on the combined sets of samples: the twelve eyeballs on which corneal and anterior chamber measurements were made and the twelve eyeballs used for measuring the thickness of the sclera (from the corneal limbus to the optic nerve). A syringe clamped to the side of the tube was used for injecting fluid into the optic nerve without disturbing eyeball position,5 and this also ensured that measurements were made around the same plane (the longitudinal plane). The changes in corneal thickness and anterior chamber angle, depth, and diameter in response to intraocular fluid increases were measured on the twelve eyeballs used to determine baseline corneal thickness and anterior chamber dimensions. Similarly, the changes in scleral thickness, in response to fluid increase, were measured on the twelve eyeballs for which baseline scleral thickness had been determined. Images were acquired after each of five consecutive injections of of saline. The biometric parameters were evaluated using the analysis mode of the Visante OCT system software. Experiments were performed at an ambient temperature of . Eyeballs were weighed before and after experimentation. The final eyeball weights were within 8% of their baseline weights, as determined by subtracting the weight of the injected intraocular fluid from the weight of the eyeball at the end of the experiment. 3.ResultsThe baseline thicknesses of the cornea and sclera in fresh porcine eyes are shown in Table 1 (corresponding locations of measurement are shown in Fig. 1). The baseline thickness of the cornea is relatively constant: at the center (point 1, Fig. 1), at the midpoint (point 2, Fig. 1), and in the periphery (point 3, Fig. 1) [Table 1, Fig. 2a ]. At the limbus, the corneal thickness increases slightly to an average of (Table 1) decreasing on the scleral side of the limbus to an average of (Table 1). The thickness of the anterior sclera gradually decreases over an arc length of to an average minimum thickness of [Figs. 1 and 2b]. This region of marked decrease in scleral thickness covers 7% of the total sclera. The posterior section of the sclera is more constant in thickness than the anterior section and has an average thickness of over 45% of the total sclera. At a distance of from the edge of the optic nerve, the posterior scleral thickness increases to a maximum of . This scleral thickness is maintained to the closest measurable point of from the optic nerve [Fig. 2c]. Fig. 2Tomographic images of (a) the anterior chamber, (b) anterior and posterior sclera, and (c) sclera near the optic nerve. 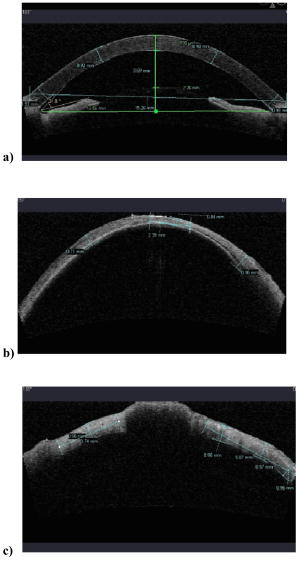 Table 1Baseline OCT measurements, mean±SD (standard deviation). At baseline, the anterior chamber angle is and the average anterior chamber diameter is . The depth of the anterior chamber, measured as the distance from the posterior cornea to the anterior lens, is [Fig. 2a]. After incremental injections of saline, the thicknesses of the central, mid, and peripheral cornea decrease slightly (Fig. 3 ). Standard deviations for all points range from 0.04 to 0.06 (the error bars are not shown in Fig. 3 because the superimposition of three sets of error bars on the same points along the axis makes it difficult to distinguish one set from another). The incremental decrease in corneal thickness (central, mid, and peripheral) is around after the first and last incremental injections and is greatest after the third and fourth injections. The overall decrease in central and midcorneal thickness values, after the final injection of is 10% of the initial thickness value. The overall peripheral thickness decreases to 9% of the initial peripheral thickness. These changes are statistically significant . Fig. 3Corneal thickness (in millimeters) plotted against the incremental change in volume (in microliters). 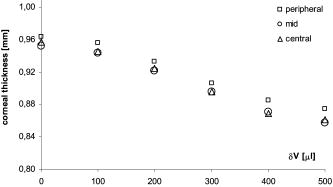 The limbal and scleral thicknesses show smaller changes than the cornea after injections of saline. Thickness changes after injections were measured at all points except point 8 (near the optic nerve) because the presence of the injecting device made imaging of this point difficult. Any decreases in scleral thickness between baseline and after final injection of of fluid, are less than the standard deviations and vary from 1 to 3% from baseline thickness (Table 2 ). Table 2The mean±SD of corneal and scleral thickness at baseline and after incremental intraocular injections of saline. The locations of points 4 to 7 are shown in Fig. 1.
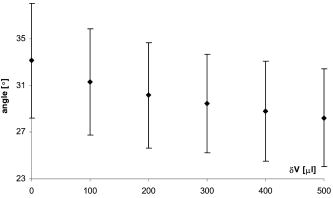
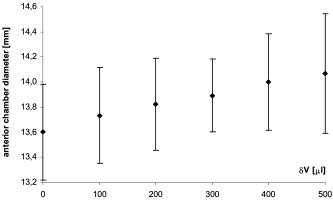
The anterior chamber angle decreases with incremental increase in fluid (Fig. 4 ). The difference between the average value of the angle at baseline and after maximum injection of and , respectively) is statistically significant . The change in diameter of the anterior chamber (Fig. 5 ) from its baseline of , following the maximum fluid injection, was not statistically significant . There was a statistically significant decrease in anterior chamber depth from baseline of after the maximum injection of . 4.DiscussionThe thickness of the outer coats of the porcine eye, as indeed those for eyes of other species, has thus far been measured using histological methods such as freezing or fixation. These methods alter the material properties of tissues and this may have an effect on tissue size. The OCT system provides highly resolved measurements of anterior segment structures, including thickness of the cornea and anterior sclera as well as depth, angle, and diameter of the anterior chamber of the in vivo human eye. This study has further exploited the imaging capacity of this instrument by measuring the thickness of the entire outer coat of the eye as well as features of the anterior chamber in fresh porcine eyes in vitro. The results show that the variations in thicknesses of the porcine cornea and sclera are less than those observed in human13, 14, 15 and monkey eyes.16 Unlike human and monkey corneae, the thickness of the porcine cornea does not show significant variations. The average OCT thickness at the center of the porcine corneae measured in this study is comparable to in vitro ultrasound biomicroscopic (UBM) measurements of corneae from fresh porcine eyes .17 The posterior sclera, which comprises almost half of the total sclera, has a fairly constant average thickness to within from the optic nerve, where scleral thickness increases to approximately . Anterior scleral thickness varies significantly more than the rest of the sclera; however, the variations are smaller than those measured following formalin fixation.18 Fixation has been shown19 to increase the variations in thickness in different parts of the sclera in canine and equine eyes by over 50%. The baseline porcine anterior chamber depth was recorded as . Note that the OCT uses the optical path length and converts this to its geometrical counterpart, dividing the optical path length by the refractive index value of 1.376. As the anterior chamber is filled with aqueous, which has a refractive index of 1.333, the more accurate value for the anterior chamber depth is . This is very close to the value of obtained from in vitro measurements of fresh porcine eyes using Scheimpflug photography20 but slightly smaller than the measurement of on fresh porcine eyes found17 using UBM. The latter study also reported an anterior chamber angle of , which is within the range of values found in this study . Incremental intraocular injections of did not cause significant changes in the thicknesses of either cornea or sclera. The maximum volume of of fluid resulted in a significant decrease of 9 to 10% in the thickness of the cornea and smaller, nonsignificant decreases in scleral thickness (between 1 and 3% of the initial thickness). Following intraocular fluid injections, neither the cornea nor the sclera showed any significantly varying or disproportionate changes in thickness in any sections along their respective lengths. In view of these findings, and the fact that the baseline cornea and posterior sclera have relatively constant thickness values, the pressure vessel model can be applied to the eyeball to estimate corneal and scleral elasticity.6 The cornea and posterior sclera should be treated as separate tissues in the calculations. With incremental injection of fluid, the anterior chamber angle and depth decreased slightly, while the diameter of the anterior chamber increased, indicating that with increase in fluid volume in the eyeball, the cornea is stretched in the plane of the limbus. An increase in intraocular fluid will result in a rise in intraocular pressure (IOP). From a previous study, in which the change in IOP with fluid increase was measured in porcine eyes,5 the injection of increments of fluid caused the IOP to rise by about (from a baseline of ): the final value after five incremental injections reached just under . The pressure/volume relationship of the globe was found to be approximately linear over the range of fluid volume injected intraocularly.5 A more recent study considered the effect of increasing pressure on exised human corneae.21 Samples were mounted in a sealed artificial anterior chamber and saline injected at a steady rate. Using time-domain OCT, the measured decrease in corneal thickness was found to be between 112 and in response to a pressure increase of , while the radius of curvature increased by . Approximately the same decrease in central corneal thickness was observed in this study, in response to a volume increase of (which was found to correspond to an IOP value of around ). The comparable decrease in corneal thickness between the two studies demonstrates that IOP has only a minimal effect on corneal thickness. These observations21, 22 add further support to the concept that the cornea acts to buffer and protect the eye from sharp increases in IOP. 5.ConclusionThe anterior chamber OCT can be exploited to measure the biometry of the whole eyeball in fresh samples, alleviating the need for fixation, freezing, and cutting. The eyeball can tolerate an increase of of fluid without causing disproportionate changes in thickness along the lengths of both cornea and sclera. This finding together with the relatively constant thickness of the cornea and posterior sclera render the eyeball an appropriate system for application of the pressure vessel model for estimating corneal and scleral elasticity. ReferencesG. W. Nyquist,
“Rheology of the cornea: experimental techniques and results,”
Exp. Eye Res., 7 183
–188
(1968). 0014-4835 Google Scholar
J. Kampmeier,
B. Radt,
R. Birngruber, and
R. Brinkmann,
“Thermal and biomechanical parameters of porcine cornea,”
Cornea, 19 355
–363
(2000). 0277-3740 Google Scholar
K. Anderson,
A. El-Sheikh, and
T. Newson,
“Application of structural analysis to the mechanical behaviour of the cornea,”
J. R. Soc. Lon. Interface., 1 1
–13
(2004). Google Scholar
P. Purslow and
W. Karwatowski,
“Ocular elasticity. Is engineering stiffness a more useful characterization parameter than ocular rigidity?,”
Ophthalmology, 103 1686
–1692
(1996). 0161-6420 Google Scholar
B. K. Pierscionek,
M. Asejczyk-Widlicka, and
R. A. Schachar,
“The effect of changing intraocular pressure on the corneal and scleral curvatures in the fresh porcine eye,”
Br. J. Ophthamol., 91 801
–803
(2006)
(0007-1161) https://doi.org/10.1136/bjo.2006.110221 Google Scholar
D. W. A. Rees,
“Elasticity,”
Basic Solid Mechanics, 57
–71 Macmillan Press, New York
(1997). Google Scholar
D. Huang,
E. A. Swanson,
C. P. Lin,
J. S. Schuman,
W. G. Stinson,
W. Chang,
M. R. Hee,
T. Flotte,
K. Gregory,
C. A. Puliafito, and
J. G. Fujimoto,
“Optical coherence tomography,”
Science, 254 1178
–1181
(1991). https://doi.org/10.1126/science.1957169 0036-8075 Google Scholar
A. F. Fercher,
C. K. Hitzenberger,
W. Drexler,
G. Kamp, and
H. Sattman,
“In vivo optical coherence tomography,”
Am. J. Ophthalmol., 116 113
–114
(1993). 0002-9394 Google Scholar
J. A. Izatt,
M. R. Hee,
E. A. Swanson,
C. P. Lin,
D. Huang,
J. S. Schuman,
C. A. Puliafito, and
J. G. Fujimoto,
“Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography,”
Arch. Ophthalmol. (Chicago), 112 1584
–1589
(1994). 0003-9950 Google Scholar
G. Baikoff,
E. Lutun,
C. Ferraz, and
J. Wei,
“Static and dynamic analysis of the anterior segment with optical coherence tomography,”
J. Cataract Refractive Surg., 30 1843
–1850
(2004). 0886-3350 Google Scholar
A. Konstantopoulos,
P. Hossain, and
D. F. Anderson,
“Recent advances in ophthalmic anterior segment imaging: a new era for ophthalmic diagnosis?,”
Br. J. Ophthamol., 91 551
–557
(2007). 0007-1161 Google Scholar
H. Hoerauf,
R. S. Gordes,
C. Scholz,
C. Wirbelauer,
P. Koch,
R. Engelhardt,
J. Winkler,
H. Laqua, and
R. Birngruber,
“First experimental and clinical results with transscleral optical coherence tomography,”
Ophthalmic Surg. Lasers, 31 218
–222
(2000). 1082-3069 Google Scholar
T. W. Olsen,
S. Y. Aaberg,
D. H. Geroski, and
H. F. Edelhauser,
“Human sclera: thickness and surface area,”
Am. J. Ophthalmol., 125 237
–241
(1998). https://doi.org/10.1016/S0002-9394(99)80096-8 0002-9394 Google Scholar
P. Cho and
S.-W. Cheung,
“Central and peripheral corneal thickness measured with the TOPCON specular microscope SP-2000P,”
Curr. Eye Res., 21 799
–807
(2000). 0271-3683 Google Scholar
R. Rufer,
A. Schroder,
C. Bader, and
C. Erb,
“Age-related changes in central and peripheral corneal thickness: determination of normal values with the Orbscan II topography system,”
Cornea, 26 1
–5
(2007). 0277-3740 Google Scholar
J. C. Downs,
R. A. Bildner,
A. J. Bellezza,
H. W. Thompson,
R. T. Hart, and
C. F. Burgoyne,
“Peripapillary scleral thickness in perfusion-fixed normal monkey eyes,”
Invest. Ophthalmol. Visual Sci., 43 2229
–2235
(2002). 0146-0404 Google Scholar
L. R. Bartholomew,
D. X. Pang,
D. A. Sam, and
J. C. Cavender,
“Ultrasound biomicroscopy of globes from young adult pigs,”
Am. J. Vet. Res., 58 942
–948
(1997). 0002-9645 Google Scholar
T. W. Olsen,
S. Sanderson,
X. Feng, and
W. C. Hubbard,
“Porcine Sclera: Thickness and Surface Area,”
Invest. Ophthalmol. Visual Sci., 43 2529
–2532
(2002). 0146-0404 Google Scholar
B. C. Gilger,
K.-A. Reeves, and
J. H. Salmon,
“Ocular parameters related to drug delivery in the canine and equine eye: aqueous and vitreous humor volume and scleral surface area and thickness,”
Vet. Ophthalmol., 8 265
–269
(2005). Google Scholar
J. B. Holmén,
B. Ekesten, and
B. Lundgren,
“Anterior chamber depth estimation by Scheimpflug photography,”
Acta Ophthalmol. Scand., 79 576
–579
(2001). 1395-3907 Google Scholar
C. S. Johnson,
S. I. Mian,
S. Morot,
D. Epstein,
J. Izatt, and
N. A. Afshart,
“Role of corneal elasticity in damping of intraocular pressure,”
Invest. Ophthalmol. Visual Sci., 48 2540
–2544
(2007). 0146-0404 Google Scholar
M. Asejczyk-Widlicka and
B. K. Pierscionek,
“Fluctuations in intraocular pressure and the potential effect on aberrations of the eye,”
Br. J. Ophthamol., 91 1054
–1058
(2007). 0007-1161 Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||