|
|
1.IntroductionHSV- /GCV system, which is the “virus-directed enzyme/prodrug therapy”1 of herpes simplex virus (HSV) thymidine kinase gene/antiviral reagent ganciclovir (GCV),2, 3 is one of the promising approaches in the rapidly growing area of gene therapy. The “bystander effect,” a phenomenon in which HSV- cells exposed to GCV are toxic to adjacent HSV- cells, was reported to play an important role in suicide gene therapy. GCV is a synthetic nucleoside analogue, which is phosphorylated by viral thymidine kinase (TK) produced in HSV- cells. Phosphorylated GCV is further converted to the triphosphate form by cellular kinases and then incorporated into DNA molecules, where it prevents DNA elongation,3 thus killing the cell. However, the therapeutic effect of the HSV- /GCV system in cancer therapy is not restricted to killing cancer cells in this way. Another therapeutic effect has been reported, called the bystander effect.4 The bystander effect can lead to the killing of nontransduced tumor cells in the immediate vicinity of GCV-treated HSV- cells. The magnitude of the bystander effect in vivo is substantial, the number of cells killed being at least comparable to those killed by direct transduction. HSV- /GCV also induces ligand-independent death receptor aggregation and the activation of caspases.5, 6 However, activated caspases were detected by Western blot, which does not distinguish between activated caspases from HSV- cells or HSV- cells. Furthermore, very little is known about caspase activation induced by the bystander effect. Therefore, our purpose in this study is to monitor in real time the activity of caspase-3 induced by the HSV- /GCV system in single cells and to analyze directly whether the activity of caspase-3 is involved in the bystander effect in single cells. The imaging of single cells is essential for understanding the molecular mechanism of HSV- /GCV-induced apoptosis and the bystander effect. In our previous work, we have constructed two fluorescence resonance energy transfer (FRET) probes (CD3 and CD2), which have linkers containing the caspase-3 cleavage sequence (DEVD) and caspase-2 cleavage sequence (VDVAD) fused with enhanced cyan fluorescent protein (ECFP) and DsRed2,7 respectively. With these probes, the dynamics of caspases were monitored in real time in single HeLa cells during cisplatin-induced apoptosis. In the present study, the activation of caspase-3 induced by the HSV- /GCV system in single cells was monitored in real time by using CD3 and TK-GFP (the fusion protein of TK and green fluorescence protein) co-expressed in human adenoid cystic carcinoma (ACC-M-TK-GFP-CD3) cells. By using mixed cultured CD3-expressing ACC-M (ACC-M-CD3) cells and TK-GFP-expressing ACC-M (ACC-M-TK-GFP) cells, we have directly demonstrated that the activation of caspase-3 is not involved in the bystander effect of the HSV- /GCV system. 2.Materials and Methods2.1.Gene ConstructionFor this study, we used a genetically encoded FRET sensor CD3 (CFP-DEVD-DsRed2). The details of construction protocols were described previously,7 in which the enhanced mutant ECFP was referred to using the more concise name CFP throughout, and DsRed was generated from pDsRed2 (Clontech). 2.2.Cell Culture, Transfection, and ScreeningA human adenoid cystic carcinoma (ACC-M) cell line was obtained from the China Center for Type Culture Collections (Wuhan, China).8 The TK-GFP vector was kindly provided by Professor Ariane Söling (Germany).9 ACC-M cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with penicillin G sodium , streptomycin sulfate and 10% fetal bovine serum at in a humidified atmosphere of 5% air. ACC-M cells were transfected with the plasmid TK-GFP, which expresses the herpes simplex virus thymidine kinase gene (HSV- ) and EGFP (enhanced mutant green fluorescence protein) in the same bicistronic message, by FuGENE6 Transfection Reagent (Roche, Switzerland). Stable TK-GFP-expressing ACC-M cell clones (ACC-M-TK-GFP) were screened with G418 (Promega Corp., Madison, Wisconsin). ACC-M-TK-GFP cells and ACC-M cells were transfected with CD3 using the same methods. Stable CD3-expressing ACC-M (ACC-M-CD3) cell clones were screened with G418. 2.3.Imaging system for FRET Measurement and Multicolor Fluorescent ImagingFRET measurements were performed using an FV1000 laser confocal scanning microscope (Olympus, Japan) with a Plan Apo oil immersion objective having 1.42 numerical aperture (NA). The fluorescence images of CD3-expressing ACC-M cells were detected by excitation at (Ar laser), and the emitted fluorescence bands were separated by a grating and detected by photomultiplier tubes (PMT) in a cyan fluorescent protein (CFP) channel (465 to ) and FRET channel (580 to ). For reflecting FRET efficiency of CD3 in living cells, the ratio of FRET was calculated as follows:10, 11 where and are fluorescence intensities from the region of interest detected through the FRET channel and the CFP channel, respectively. Images of were calculated and presented using MATLAB 7.0. We gave the value 1 if the FRET probe CD3 was intact, and normalized other values of relative to the maximum value of .For fluorescent imaging of multicolor labeled cells, the fluorescence images of ACC-M-TK-GFP-CD3 cells were obtained using laser confocal scanning microscopy, in which the CFP channel used excitation at and emission at 465 to , the DsRed channel used excitation at and emission at 580 to , and the GFP channel used excitation at and emission at 500 to . The dead cells induced by GCV were identified by morphological changes in transmission images, such as membrane blebbing and cell shrinkage. 3.Results3.1.Real-Time Imaging of Caspase-3 Activity During HSV- /GCV–Induced Apoptosis in Living ACC-M CellsThe genetically encoded probe CD3 was transfected into ACC-M-TK-GFP cells, giving TK-GFP and CD3 co-expressed ACC-M cells, named ACC-M-TK-GFP-CD3 cells. The fluorescence images of ACC-M-TK-GFP-CD3 cells showed CD3 localized in the cytosol and TK-GFP localized in the nucleus (Fig. 1 ). To monitor in real time GCV-induced apoptosis in HSV- cells, TK-GFP and CD3 co-expressed ACC-M cells were treated with GCV, and then images of the ACC-M-TK-GFP-CD3 cells were obtained at about 15-min intervals. A time series of images, transmission images, and multichannel fluorescence images of ACC-M-TK-GFP-CD3 cells is shown in Fig. 2a . The FRET ratio of CD3 in ACC-M-TK-GFP-CD3 cells obviously decreased as the caspase-3 activity triggered the cleavage of the CD3 probe.7, 12 In the plot of versus time [Fig. 2b] and the plot of diameter of cells versus time [Fig. 2c], it is apparent that caspase-3 was activated before cell morphological changes began. Furthermore, once the activation of caspase-3 had been initiated in the cytosol, the activation process was completed within several minutes.7 Using ACC-M-CD3 cells expressing only CD3, and without TK-GFP, as a negative control, the FRET ratio of CD3 in ACC-M-CD3 cells was stable and the cells still survived and after treatment with GCV. It was especially notable that mitosis of ACC-M-CD3 cells was not affected by GCV (Fig. 3 , arrow). This indicates that GCV did not induce the activation of caspase-3 in ACC-M-CD3 cells without TK expression. The same experiments were repeated at least three times. Fig. 1Fluorescence and transmission images of TK-GFP and CD3 co-expressed ACC-M cells. The fluorescent images of ACC-M-TK-GFP-CD3 cells were detected using a laser confocal scanning microscope in (a) the CFP channel with excitation at and emission at 465 to , (b) the DsRed channel with excitation at and emission at 580 to , and (c) the GFP channel with excitation at and emission at 500 to , respectively. A transmission image of ACC-M-TK-GFP-CD3 cells is also shown (d). The scale bar is . 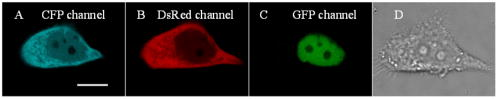 Fig. 2Single-cell imaging FRET changes of CD3 in response to caspase-3 activity during GCV-induced apoptosis in ACC-M-TK-GFP-CD3 cells. (a) A time series of images of the ACC-M-TK-GFP-CD3 cells treated with GCV are shown in the top. The color bar represents the FRET ratio of the CD3 probe. The transmission image (first column) and multichannel fluorescent images (second column: DsRed channel; third column: CFP channel; fourth column: GFP channel) of the ACC-M-TK-GFP-CD3 cells at , and , after GCV treatment are shown in the middle row and bottom row, respectively. (b) The plot of versus time. (c) The plot of the diameter of cells versus time. The diameter of cells is calculated using the dimension of the longest axis from the transmission images. 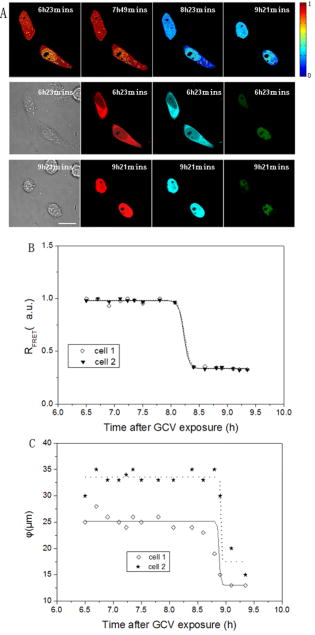 Fig. 3Single-cell imaging FRET ratio of CD3 in living ACC-M-CD3 cells treated with GCV. Bright field images and a time series of images of ACC-M-CD3 cells treated with GCV showed that the FRET ratio of the CD3 probe was stable and the cells still survived. The mitosis of ACC-M-CD3 cells was not affect by GCV (arrow). The scale bar is . 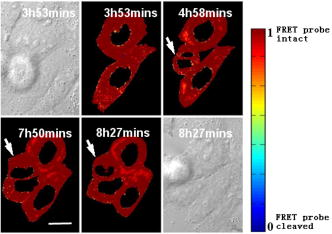 3.2.Real-Time Imaging of the Bystander Effect of the HSV- /GCV System on Living ACC-M CellsTo confirm the relationship of caspase-3 activation and the bystander effect of the HSV- /GCV system in living cells, we mixed ACC-M-TK-GFP cells and ACC-M-CD3 cells in a 1:3 ratio and seeded them onto a 35-mm “coverslip-bottom culture dish.” After the cells were cultured for , GCV was added to induce apoptosis. Mesnil 4, 13 reported that the bystander effect involved a transfer of phosphorylated ganciclovir molecules from HSV- cells to HSV- cells through gap junctions. Therefore, we chose to image the ACC-M-CD3 cells growing adjacent to ACC-M-TK-GFP cells. The FRET images of the ACC-M-CD3 cells adjacent to ACC-M-TK-GFP cells showed that the FRET ratio of the CD3 probe remained stable even as the cells died [Fig. 4a ]. The same experiment was repeated at least three times. In Fig. 4b, the plots of FRET ratio versus time were calculated from four individual ACC-M-CD3 cells in each repeat experiment. Error bars indicate SD, and the values of did not normalize. Three approximately parallel lines showed that the FRET ratio of CD3 in ACC-M-CD3 cells was stable. This indicated that the bystander effect of the HSV- /GCV system can kill the adjacent HSV- cells via a caspase-3-independent pathway. Fig. 4Single-cell imaging FRET ratio of CD3 in living ACC-M-CD3 cells mixed with ACC-M-TK-GFP cells after GCV treatment. (a) Bright field images and a time series of images of ACC-M-CD3 cells adjacent with ACC-M-TK-GFP cells treated with GCV showed that the FRET ratio of the CD3 probe in ACC-M-CD3 cells remained stable until the cells’ death. The scale bar is . (b) The plots of the FRET ratio versus time were calculated from four individual ACC-M-CD3 cells in three repeated experiments. 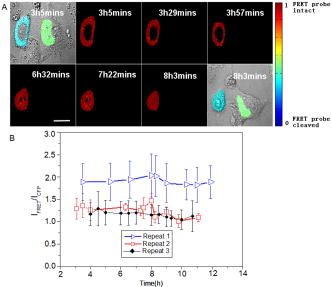 4.ConclusionFluorescence resonance energy transfer can be utilized to make highly sensitive detectors of molecular activity. Here we introduce FRET-based sensors, which utilize variants of enhanced GFPs fused by a caspase-3-specific peptide linker to detect caspase-3 activity, to the study of the bystander effect of the HSV- /GCV system. HSV- gene therapy has been extensively studied on different kinds of tumors and largely relies on the bystander effect. Using fluorescent protein (FP) based multicolor cellular labeling combined with multichannel fluorescence imaging and FRET imaging techniques, the activation of caspase-3 in the direct and the bystander killing effects of HSV- /GCV system were visualized in real time. The advantages of this approach are: (1) CD3 was expressed in the cytosol and TK-GFP was expressed in the nucleus (Figs. 1 and 2), which results in less cross-talk between fluorescence signals from each kind of fluorescent protein: and (2) CD3 was insensitive to changes of during apoptosis and was a sensitive indicator of the activity of caspase-3.7, 12 In order to monitor HSV- /GCV system-induced apoptosis and its bystander effect, ACC-M-TK-GFP, ACC-M-CD3, and ACC-M-TK-GFP-CD3 tumor cells were treated with GCV and imaged with the FRET imaging technique. The results showed that GCV could induce the activation of caspase-3 in ACC-M cells co-expressed with HSV- and CD3 (Fig. 2) but could not induce the activation of caspase-3 in ACC-M cells without HSV- being expressed (Fig. 3). Although the bystander effect of the HSV- /GCV system could induce apoptosis in cells adjacent to HSV- expressing cells, apoptosis did not occur via a caspase-3 activation pathway. That is to say, the activation of caspase-3 is not involved in the bystander effect. In summary, the combination with FP-based multicolor cellular labeling and FRET imaging directly confirmed that caspase-3 activation was involved in HSV- /GCV-induced apoptosis in HSV- cells but was not involved in the bystander effect. This work has provided an improved method of studying the molecular mechanism of the HSV- /GCV system. AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Grant Nos. 90508003, 30770525, and 60440420131). ReferencesB. E. Huber,
C. A. Richards, and
T. A. Krenitsky,
“Retroviral-mediated gene therapy for the treatment of hepatocellular carcinoma: an innovative approach for cancer therapy,”
Proc. Natl. Acad. Sci. U.S.A., 88
(18), 8039
–8043
(1991). 0027-8424 Google Scholar
F. L. Moolten and
J. M. Wells,
“Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors,”
J. Natl. Cancer Inst., 82
(4), 297
–300
(1990). 0027-8874 Google Scholar
E. H. Oldfield,
Z. Ram,
K. W. Culver,
R. M. Blaese,
H. L. DeVroom, and
W. F. Anderson,
“Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir,”
Hum. Gene Ther., 4 39
–69
(1993). 1043-0342 Google Scholar
M. Mesnil and
H. Yamasaki,
“Bystander effect in herpes simplex virus-thymidine kinase/ganciclovier cancer gene therapy: role of gap-junctional intercellular communication,”
Cancer Res., 60
(15), 3989
–3999
(2000). 0008-5472 Google Scholar
C. Beltinger,
S. Fulda,
T. Kammertöns,
E. Meyer,
W. Uckert, and
K. M. Debatin,
“Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involveslig and-independent death receptor aggregation and activation of caspases,”
Proc. Natl. Acad. Sci. U.S.A., 96 8699
–8704
(1999). 0027-8424 Google Scholar
C. C. Chiu,
Y. L. Kang,
T. H. Yang,
C. H. Huang, and
K. Fang,
“Ectopic expression of herpes simplex virus-thymidine kinase gene in human non-small cell lung cancer cells conferred caspase-activated apoptosis sensitized by ganciclovir,”
Int. J. Cancer, 102 328
–333
(2002). 0020-7136 Google Scholar
J. Q. Lin,
Z. H. Zhang,
J. Yang,
S. Q. Zeng,
B. F. Liu, and
Q. M. Luo,
“Real-time detection of caspase-2 activation in a single living HeLa cell during cisplatin-induced apoptosis,”
J. Biomed. Opt., 11
(2), 024011
(2006). https://doi.org/10.1117/1.2187013 1083-3668 Google Scholar
T. Xiong,
Z. H. Zhang,
B. F. Liu,
S. Q. Zeng,
Y. P. Chen,
J. Chu and
Q. M. Luo,
“In vivo optical imaging of human adenoid cystic carcinoma cell metastasis,”
Oral Oncol., 41
(7), 709
–715
(2005). 0964-1955 Google Scholar
A. Soling,
A. Simm, and
N. Rainov,
“Intracellular localization of herpes simplex virus type 1 thymidine kinase fused to different fluorescent proteins depends on choice of fluorescent tag,”
FEBS Lett., 527
(1–3), 153
–158
(2002). 0014-5793 Google Scholar
M. Rehm,
H. Dussmann,
R. U. Janicke,
J. M. Tavare,
D. Kogel, and
J. H. Prehn,
“Single-cell fluorescence resonance energy transfer analysis demonstrates that caspase activation during apoptosis is a rapid process. Role of caspase-3,”
J. Biol. Chem., 277
(27), 24506
–24514
(2002). https://doi.org/10.1074/jbc.M110789200 0021-9258 Google Scholar
H. Kawai,
T. Suzuki,
T. Kobayashi,
“Simultaneous real-time detection of initiator- and effector-caspase activation by double fluorescence resonance energy transfer analysis,”
J. Pharm. Sci., 97
(3), 361
–368
(2006). 0022-3549 Google Scholar
J. Q. Lin,
Z. H. Zhang,
S. Q. Zeng,
S. X. Zhuo,
B. F. Liu,
Q. Liu,
J. Yang, and
Q. M. Luo,
“TRAIL-induced apoptosis proceeding from caspase-3-dependent and -independent pathways in distinct HeLa cells,”
Biochem. Biophys. Res. Commun., 346 1136
–1141
(2006). 0006-291X Google Scholar
M. Mesnil,
C. Piccoli,
G. Tiraby,
K. Willecke, and
H. Yamasaki,
“Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins,”
Proc. Natl. Acad. Sci. U.S.A., 93
(5), 1831
–1835
(1996). 0027-8424 Google Scholar
|


