|
|
1.IntroductionThe ultraviolet A (UVA) component of solar radiation has been shown to produce deleterious biological effects in which singlet oxygen plays a major role.1 Therefore, exposure to UVA irradiation has been recognized as a cause of aging in eye lens proteins and as a risk factor for cataract formation.2 In revisiting the photochemistry of solar UVA in human skin, it was stated recently that the importance of UVA in skin cancer is lively discussed.3, 4 At the same time, over 1 million cases of basal cell and squamous cell skin cancer are expected to be diagnosed in 2007.5 Recently, Mouret found that cyclobutane pyrimidine dimers were produced at a significant yield in whole human skin exposed to UVA radiation.6 UVA irradiation is also responsible for the most frequent photo-dermatosis of the skin.7 However, the mechanisms by which UVA irradiation-induced photo damage occurs are not fully understood.8 UVA radiation is absorbed by a variety of molecules in tissue. After light absorption, some of the molecules can cross over to a long-lived state (triplet state), which allows the transfer of either energy or charge to substrate or molecular oxygen. This process generates radicals or singlet oxygen. Some of the endogenous photosensitizers in cells or tissue are identified, such as flavins,9 NADH/NADPH,10 urocanic acid,8, 9 sterols,11 and anthraquinones.12 If light-absorbing molecules are not able to form such a triplet state (e.g., linear-shaped molecules), the generation of singlet oxygen should fail.13 However, the generation of singlet oxygen may take place assisted by chemical reactions.1, 14 Recently, we showed that singlet oxygen was produced by phosphatidylcholine under UVA irradiation.15 Phospholipids and the respective fatty acids are major constituents of many cellular membranes. In cells, a molecule such as ceramide is a key component of stress responses. UVA radiation and singlet oxygen both generated ceramide in protein-free, sphingomyelin-containing liposomes.16 Furthermore, human skin, especially the stratum corneum, contains free saturated and unsaturated fatty acids with mostly chain lengths of to atoms.17, 18 Therefore, we applied our highly sensitive IR-photomultiplier technology to detect UVA-induced singlet oxygen directly by its luminescence for different lipids and fatty acids. 2.Material and Methods2.1.Preparation of SolutionsThe following substances were dissolve in EtOH: L- -phosphatidylcholine (purity , from egg yolk); sphingomyelin (purity , from egg yolk); 1,2-distearoyl-sn-glycero-3-phosphocholine (purity ); 1,2-dioleoyl-sn-glycero-3-phosphocholine ; 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (purity ); palmitic acid (purity ); stearic acid (purity ); oleic acid (purity ) linoleic acid (purity ); linolenic acid (purity ); arachidonic acid (purity ); and docosahexaenoic acid (purity ). All substances were purchased from Sigma-Aldrich (Steinheim, Germany). 2.2.Absorption SpectraTransmission spectra of each probe were recorded at room temperature with a Beckman DU640 spectrophotometer (Beckman Instruments GmbH, Munich, Germany) using a quartz cuvette with an optical path of (QS-101, Hellma Optik, Jena, Germany). The absorption values were calculated as , where is the transmission value. 2.3.Oxygen Concentration in SolutionThe oxygen concentration in solution was measured in the cuvette with a needle sensor that was placed in the cuvette (MICROX TX, PreSens GmbH, Regensburg, Germany). During the measurement, the cuvette was sealed and the oxygen concentration of the solution was determined and referred to solution with an air saturation (100%). 2.4.Luminescence ExperimentsFatty acids or lipids in solution were transferred into a cuvette (QS-101, Hellma Optik, Jena, Germany). They were excited using a frequency-tripled Nd:YAG laser (PhotonEnergy, Ottensoos, Germany) with a repetition rate of (wavelength , pulse duration ). The laser pulse energy for luminescence experiments was . The singlet oxygen luminescence at was detected in a near-backward direction with respect to the excitation beam using an IR-sensitive photomultiplier (R5509-42, Hamamatsu Photonics Deutschland GmbH, Herrsching, Germany) with a rise time of about . Additional details of the setup were described elsewhere.19. The number of laser pulses for excitation was 40,000. The luminescence signal was detected at wavelengths of 1150, 1200, 1250, 1270 (emission maximum of singlet oxygen luminescence), 1300, 1350, or using appropriate interference filters in front of the photomultiplier.20 2.5.Determination of Singlet Oxygen Luminescence Decay and Rise TimeAs shown in Ref. 21 the luminescence intensity is given by The constant C was used to fit the luminescence signal. and are the decay and rise times, respectively. To determine the rise and decay times of singlet oxygen, we used the least square fit routine of Mathematica 5.1 (Wolfram Research, Berlin, Germany). The experimental error of the fit was estimated to be between 15% and 25% of the values that were determined by the fit. The low signal level in some samples yielded a higher error of 25%. The integral of Eq. 1 from yields the luminescence energy. For a better comparison, the integral value was calculated per mol of the respective substance.3.Results and DiscussionUVA irradiation comprises about 95% of incident midday solar UV irradiation.22. It penetrates skin much deeper than UVB irradiation. The absorption of UVA irradiation in endogenous chromophores frequently leads to the generation of reactive oxygen species such as singlet oxygen. Singlet oxygen is an important biochemical intermediate in multiple biological processes, including UVA radiation-induced gene expression in human keratinocytes and fibroblasts. It has been stated that direct detection of singlet oxygen cells does not exist. Such a detection method, however, is essential to elucidate the role of singlet oxygen in gene induction.16 Recently, we provided evidence to directly detect singlet oxygen by its weak luminescence signal at in living cells under UVA irradiation at .15 Lipids and fatty acids usually are the targets of singlet oxygen that has been generated by any photosensitizer.23, 24, 25 However, we recently showed that singlet oxygen is generated in suspensions of egg yolk phosphatidylcholine during irradiation with UVA. This generation process seemed to be provoked by chemical reactions involving oxygen radicals.15 This was confirmed by using a quencher of a superoxide anion or hydroxyl radicals such as superoxide dismutase (SOD) or mannitol, respectively. To investigate this important phenomenon in depth, we irradiated different fatty acids or lipids under normal oxygen conditions in a solvent at and measured singlet oxygen luminescence at . 3.1.Singlet Oxygen Generation in Fatty Acid Solutions3.1.1.Absorption atThe major prerequisite of singlet oxygen generation is the absorption of light or radiation, which is laser radiation at in this study. After dissolving the fatty acid in ethanol, the absorption at , was measured. The fatty acids without double bonds (palmitic acid, stearic acid) and oleic acid (one double bond) showed a rather low absorption cross section at , and less than 0.5% of light was absorbed at a concentration of . For fatty acids with more than two double bonds, the absorption of radiation was in the range of 3.8% to 12.4% (Table 1 ). Table 1Singlet oxygen produced by fatty acids.
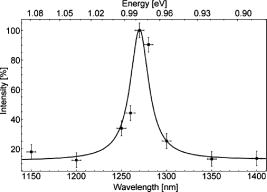
The radiation absorption of fatty acids should fail for wavelengths longer than . Nevertheless, auto-oxidation can occur, in particular under normal oxygen conditions. Subsequently, the absorption spectrum of fatty acids such as linoleic acid exhibit another absorption maximum at about due to peroxidation of the fatty acid and a broad absorption at due to decomposition products (e.g., aldehydes).26 Auto-oxidation can be considered an omnipresent process, in particular in living cells that contain a sufficient amount of oxygen. 3.1.2.Singlet oxygen luminescenceWe measured the luminescence signal at for the seven fatty acids. In solutions with palmitic, stearic, and oleic acids, we detected a clear but weak signal, and the respective integrated luminescence signal was in the range from 1 to 4 arbitrary units (a.u.). This is well above the noise of the detector . Despite the very low absorption coefficient of these fatty acids at , the detection of singlet oxygen by its luminescence was feasible (Table 1). The irradiation of solutions of linoleic, linolenic, arachidonic, or docosahexaenoic acids yielded a noticeable luminescence signal at , which is shown for arachidonic acid in Fig. 1 . The decay time of the luminescence signal was , which is equivalent to the decay time of singlet oxygen in ethanol.27 These results are astonishing because fatty acids should not act as a photosensitizer due to the following reasons. First, a typical photosensitizer absorbs light and populates its triplet state. Then the energy is transferred from the triplet state to oxygen, leading to the generation of singlet oxygen (type 2 mechanism). However, the fatty acids and their oxidative derivatives are rather linear molecules, and the transition to such a triplet state is not very likely. Second, in the case of a type 2 mechanism, the luminescence signal contains the triplet state deactivation of the photosensitizer (usually the rising part of the signal) and the singlet oxygen luminescence (usually the decaying part of the signal), and both instances are on a microsecond time scale.9 However, Fig. 1 shows a luminescence signal with an extremely fast rise time less than a microsecond. For comparison, a luminescence signal of singlet oxygen is included that shows a typical shape for an exogenous photosensitizer. It shows a rising part of the signal and a clear maximum before decaying. In the case of fatty acids, we did not assume an energy transfer from a photosensitizer (e.g., fatty acid), but instead the involvement of a fast chemical process such as radical formation by charge transfer (type 1 mechanism). The latter was recently proven when phospholipids were investigated under UVA irradiation at .20 Fig. 1Time-resolved singlet oxygen luminescence. The time-resolved luminescence signal of singlet oxygen at for arachidonic acid in ethanol showing a decay time of . For comparison, the insert shows a typical luminescence signal of singlet oxygen that has been generated by a photosensitizer via energy transfer from its triplet state (Riboflavin, , excited with light).9 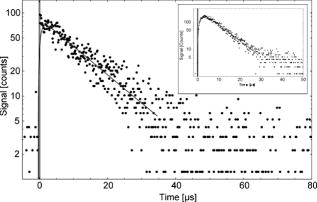 To double check singlet oxygen generation in all fatty acids, the luminescence signal was detected at different wavelengths in the range from . Figure 2 shows a characteristic spectrally resolved curve of arachidonic acid that reveals a clear maximum at . This corresponds to the transition of singlet oxygen at the energy of (Fig. 2). 3.2.Singlet Oxygen Generation in Lipid Solutions3.2.1.Light absorption atIn a solution of normal egg yolk phosphatidylcholine in EtOH, 14.6% of the radiation at was absorbed, whereas 18.7% was absorbed in a solution of sphingomyelin (SM) in EtOH (Table 2 ). Out of the three different phosphatidylcholines in solution, only 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (LI-PC) showed a clear absorption (13.4%) at . The lipids 1,2-distearoyl-sn-glycero-3-phophocholine (ST-PC) and 1,2-dioleoyl-sn-glycero-3-phophocholine (OL-PC) absorbed less than 2% each, which is comparable to the low absorption of stearic or oleic solutions. Table 2Singlet oxygen produced by phospholipids.
1Percentage and length of chain:number of double bonds. The sum of constituents is not complete and therefore less than 100%. Similar to fatty acid solutions, the fatty acids in phospholipids can undergo auto-oxidation, producing oxidative products in the solution. This has been also shown for phospholipids containing linoleic acid.26 3.2.2.Singlet oxygen luminescenceSinglet oxygen generation in aqueous or ethanol solutions of egg yolk was recently shown.20 When comparing and sphingomyelin, yielded twice the signal in sphingomyelin. Interestingly, the integrated luminescence signals (251 or ) were significantly higher than that of the linolenic acid solution, which showed the highest luminescence signal of fatty acids (see Table 1). 3.2.3.and sphingomyelinIn , there should be only a small contribution of palmitic, stearic, or oleic acid (and its oxidative products) to either the absorption of radiation or to singlet oxygen generation (see Table 1). However, these molecules represent 77% of the fatty acids in used in our experiments. In addition, in the linoleic acid solution, which counted for 15% of the constituents, there was no strong signal of singlet oxygen luminescence (Table 1). Only linolenic and arachidonic acid (less than 8% constituent) effectively contributed to the generation of singlet oxygen by this phospholipid. Based upon the results of the fatty acid solutions, the small fraction of fatty acids (8%) in should yield about for . However, we measured (Table 2), which is a factor 35 higher than expected. This includes the two fatty acids that are present in one lipid molecule. Thus, the presence of linolenic and arachidonic acids and their oxidative products might be responsible for the absorption of radiation (Fig. 1), but their direct contribution to singlet oxygen generation in should be minor. To interpret the increased efficacy of singlet oxygen generation by , we assumed the following two mechanisms. First, different oxidative products were present in than in the respective fatty acids in solution. Second, there was an unknown synergistic effect of the polar head of the lipid molecules, leading to an enhanced generation of superoxide anions. In either case, these radicals can generate singlet oxygen by chemical reactions, as proposed by Khan, 14 and we added a scheme for illustration (Fig. 3 ). Fig. 3Scheme of mechanisms. The scheme shows the possible mechanisms that take place during irradiation of a lipid or fatty acids with UVA. The light could be absorbed in fatty acids, in particular in its oxidized forms. Electrons are released from the molecules that lead to the formation of superoxide anions. These oxygen radicals can generate singlet oxygen.14, 20 The same mechanism also may occur in pure fatty acids, but would obviously be less effective. The singlet oxygen generated causes peroxidation of fatty acids and lipids (vicious circle). In addition, singlet oxygen could be generated by the Russell mechanism—that is, two peroxyl radicals would form a linear tetraoxide intermediate and decompose to the corresponding products alcohol, ketone, and singlet oxygen.1 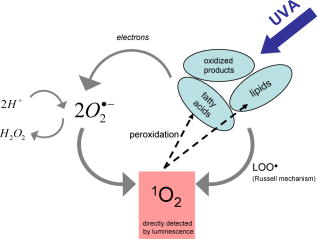 Lipidhydroperoxidation occurs during UVA irradiation as a common process. Despite their relative stability, lipid hydroperoxides can be further decomposed by generating fatty acid-peroxyl radicals and/or alkoxyl radicals, which are responsible for the propagation of lipid peroxidation and ultimately the generation of toxic compounds.28 The decomposition of lipid hydroperoxides into peroxyl radicals has been shown to be a potential source of singlet oxygen in biological systems.1, 29 In 1957, Russell proposed a self-reaction mechanism of such peroxyl radicals involving the formation of a cyclic mechanism from a linear tetraoxide intermediate that decomposed to give different products as well as singlet oxygen.30 Mendenhall and colleagues demonstrated that such a self-reaction formation of peroxyl radicals deriving from fatty acids generated predominantly singlet oxygen.31 Therefore, generated fatty acid peroxyl radicals participate in reactions that lead to the formation of lipid fragmentation compounds. Consequently, UVA radiation starts to cause lipidperoxidation, and the resulting products generate singlet oxygen, as shown by our luminescence experiments. These additional singlet oxygen molecules in turn cause the peroxidation of lipids, and cycles of singlet oxygen generation arise (see scheme in Fig. 3). This process leads to an enhancement of UVA-induced lipid peroxidation that should clearly affect cellular membranes, and therefore skin cell integrity. These considerations are supported by the following results. When the irradiation of a lipid solution was continued for about , the singlet oxygen signal did not disappear, although the oxygen concentration reached the value of zero after this time span (closed cuvette). In contrast to that, in protein solutions32 and bacteria,33 oxygen molecules and singlet oxygen luminescence disappeared very rapidly during irradiation. When oxygen was removed from the closed cuvette by adding nitrogen gas prior to UV irradiation, the luminescence signal was zero (within the experimental accuracy). This again confirms that oxygen is required right from the start to generate singlet oxygen. During the short time of singlet oxygen detection , the oxygen concentration was constant. The conditions for singlet oxygen generation in sphingomyelin solution were comparable to that of . About 76% of the fatty acids were palmitic or stearic acid, which hardly absorb at . The luminescence energy per mol was smaller than in , which might be partially due to a smaller percentage of unsaturated fatty acids. This effect should be even more pronounced, since the absorption of radiation was higher in sphingomyelin (18.7% absorption) than in phosphatidylcholine (14.6% absorption). 3.2.4.Other phosphatidylcholinesTo prove the role of fatty acids and their oxidized products in singlet oxygen generation, three different phospholipids were investigated that contained only ST-PC, OL-PC, or LI-PC. The luminescence energy per mol was weak for ST-PC and OL-PC, but again clearly higher compared to the respective fatty acid in solution (Table 1). In the case of LI-PC, the signal was high and at a level comparable to that of . The signal for LI-PC was about 14-fold higher than that of the free linoleic acid . These results support the discussions above. 4.ConclusionUsually, lipids and proteins in the skin are attacked by singlet oxygen, leading to peroxidation, which may cause deleterious effects in cellular systems. We have shown that fatty acids and lipids themselves, together with their oxidized products, generate singlet oxygen to a significant extent when irradiated with UVA. Saturated fatty acids generated singlet oxygen under UVA irradiation at to some extent, the reason for which remains unknown. For unsaturated fatty acids with more than two double bonds, the absorption of radiation and singlet oxygen generation increased, possibly due to oxidized products of the fatty acids (auto-oxidation). Linolenic and arachidonic acid yielded the highest luminescence signal for the fatty acids investigated. When the fatty acids were constituents of sphingomyelin or phosphatidylcholine, the luminescence signal increased significantly. This may indicate an unknown or a synergistic effect of fatty acids and their backbones inside the lipids. These mechanisms should be elucidated in the near future. The singlet oxygen molecules generated in such solutions can then oxidize other fatty acids, leading to an enhancement of UVA-induced damage of fatty acids and lipids. ReferencesS. Miyamoto,
G. R. Martinez,
M. H. Medeiros, and
P. Di Mascio,
“Singlet molecular oxygen generated from lipid hydroperoxides by the Russell mechanism: studies using 18(O)-labeled linoleic acid hydroperoxide and monomol light emission measurements,”
J. Am. Chem. Soc., 125
(20), 6172
–6179
(2003). 0002-7863 Google Scholar
C. A. McCarty and
H. R. Taylor,
“Recent developments in vision research: light damage in cataract,”
Invest. Ophthalmol. Visual Sci., 37
(9), 1720
–1723
(1996). 0146-0404 Google Scholar
D. Mitchell,
“Revisiting the photochemistry of solar UVA in human skin,”
Proc. Natl. Acad. Sci. U.S.A., 103
(37), 13567
–13568
(2006). 0027-8424 Google Scholar
K. Wertz,
P. B. Hunziker,
N. Seifert,
G. Riss,
M. Neeb,
G. Steiner,
W. Hunziker, and
R. Goralczyk,
“Beta-carotene interferes with ultraviolet light A-induced gene expression by multiple pathways,”
J. Invest. Dermatol., 124
(2), 428
–434
(2005). https://doi.org/10.1111/j.0022-202X.2004.23593.x 0022-202X Google Scholar
A. Jemal,
R. Siegel,
E. Ward,
T. Murray,
J. Xu, and
M. J. Thun,
“Cancer statistics, 2007,”
Ca-Cancer J. Clin., 57
(1), 43
–66
(2007). 0007-9235 Google Scholar
S. Mouret,
C. Baudouin,
M. Charveron,
A. Favier,
J. Cadet, and
T. Douki,
“Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation,”
Proc. Natl. Acad. Sci. U.S.A., 103
(37), 13765
–13770
(2006). https://doi.org/10.1073/pnas.0604213103 0027-8424 Google Scholar
T. Hasan,
F. Nyberg,
E. Stephansson,
P. Puska,
M. Hakkinen,
S. Sarna,
A. M. Ros, and
A. Ranki,
“Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings,”
Br. J. Dermatol., 136
(5), 699
–705
(1997). 0007-0963 Google Scholar
K. M. Hanson and
J. D. Simon,
“Epidermal trans-urocanic acid and the UV-A-induced photoaging of the skin,”
Proc. Natl. Acad. Sci. U.S.A., 95
(18), 10576
–10578
(1998). 0027-8424 Google Scholar
J. Baier,
T. Maisch,
M. Maier,
E. Engel,
M. Landthaler, and
W. Baumler,
“Singlet oxygen generation by UVA light exposure of endogenous photosensitizers,”
Biophys. J., 91
(4), 1452
–1459
(2006). https://doi.org/10.1529/biophysj.106.082388 0006-3495 Google Scholar
R. S. Sohal and
R. Weindruch,
“Oxidative stress, caloric restriction, and aging,”
Science, 273
(5271), 59
–63
(1996). 0036-8075 Google Scholar
P. W. Albro,
P. Bilski,
J. T. Corbett,
J. L. Schroeder, and
C. F. Chignell,
“Photochemical reactions and phototoxicity of sterols: novel self-perpetuating mechanisms for lipid photooxidation,”
Photochem. Photobiol., 66
(3), 316
–325
(1997). 0031-8655 Google Scholar
Q. Xia,
J. J. Yin,
P. P. Fu, and
M. D. Boudreau,
“Photo-irradiation of aloe vera by UVA—formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation,”
Toxicol. Lett., 168
(2), 165
–175
(2007). 0378-4274 Google Scholar
K. Drexhage,
“Structure and properties of laser dyes,”
Dye Lasers, 155
–185 Springer, New York
(1989). Google Scholar
A. U. Khan and
M. Kasha,
“Singlet molecular oxygen in the Haber-Weiss reaction,”
Proc. Natl. Acad. Sci. U.S.A., 91
(26), 12365
–12367
(1994). 0027-8424 Google Scholar
J. Baier,
T. Maisch,
M. Maier,
M. Landthaler, and
W. Baumler,
“Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin,”
J. Invest. Dermatol., 127
(6), 1498
–1506
(2007). https://doi.org/10.1038/sj.jid.5700741 0022-202X Google Scholar
S. Grether-Beck,
G. Bonizzi,
H. Schmitt-Brenden,
I. Felsner,
A. Timmer,
H. Sies,
J. P. Johnson,
J. Piette, and
J. Krutmann,
“Non-enzymatic triggering of the ceramide signalling cascade by solar UVA radiation,”
EMBO J., 19
(21), 5793
–5800
(2000). 0261-4189 Google Scholar
M. A. Lampe,
A. L. Burlingame,
J. Whitney,
M. L. Williams,
B. E. Brown,
E. Roitman, and
P. M. Elias,
“Human stratum corneum lipids: characterization and regional variations,”
J. Lipid Res., 24
(2), 120
–130
(1983). 0022-2275 Google Scholar
M. A. Lampe,
M. L. Williams, and
P. M. Elias,
“Human epidermal lipids: characterization and modulations during differentiation,”
J. Lipid Res., 24
(2), 131
–140
(1983). 0022-2275 Google Scholar
J. Baier,
M. Maier,
R. Engl,
M. Landthaler, and
W. Baumler,
“Time-resolved investigations of singlet oxygen luminescence in water, in phosphatidylcholine, and in aqueous suspensions of phosphatidylcholine or HT29 cells,”
J. Phys. Chem. B, 109
(7), 3041
–3046
(2005). https://doi.org/10.1021/jp0455531 1089-5647 Google Scholar
J. Baier,
T. Fuss,
C. Pöllmann,
C. Wiesmann,
K. Pindl,
R. Engl,
D. Baumer,
M. Maier,
M. Landthaler, and
W. Bäumler,
“Theoretical and experimental analysis of the luminescence signal of singlet oxygen for different photosensitizers,”
J. Photochem. Photobiol., B, 87
(3), 163
–173
(2007). https://doi.org/10.1016/j.jphotobiol.2007.02.006 1011-1344 Google Scholar
D. Baumer,
M. Maier,
R. Engl,
R. M. Szeimies, and
W. Baumler,
“Singlet oxygen generation by 9-acetoxy-2,7,12,17-tetrakis-(Beta-methoxyethyl)-porphycene (ATMPn) in solution,”
Chem. Phys., 285 309
–318
(2002). https://doi.org/10.1016/S0301-0104(02)00806-6 0301-0104 Google Scholar
U. Feister,
E. Jakel, and
K. Gericke,
“Parameterization of daily solar global ultraviolet irradiation,”
Photochem. Photobiol., 76
(3), 281
–293
(2002). 0031-8655 Google Scholar
N. Bando,
H. Hayashi,
S. Wakamatsu,
T. Inakuma,
M. Miyoshi,
A. Nagao,
R. Yamauchi, and
J. Terao,
“Participation of singlet oxygen in ultraviolet-a-induced lipid peroxidation in mouse skin and its inhibition by dietary beta-carotene: an ex vivo study,”
Free Radic Biol. Med., 37
(11), 1854
–1863
(2004). 0891-5849 Google Scholar
T. Kriska,
W. Korytowski, and
A. W. Girotti,
“Hyperresistance to photosensitized lipid peroxidation and apoptotic killing in 5-aminolevulinate-treated tumor cells overexpressing mitochondrial GPX4,”
Free Radic Biol. Med., 33
(10), 1389
–1402
(2002). 0891-5849 Google Scholar
M. Wrona,
W. Korytowski,
M. Rozanowska,
T. Sarna, and
T. G. Truscott,
“Cooperation of antioxidants in protection against photosensitized oxidation,”
Free Radic Biol. Med., 35
(10), 1319
–1329
(2003). https://doi.org/10.1016/j.freeradbiomed.2003.07.005 0891-5849 Google Scholar
K. P. Coolbear and
K. M. Keough,
“Lipid oxidation and gel to liquid-crystalline transition temperatures of synthetic polyunsaturated mixed-acid phosphatidylcholines,”
Biochim. Biophys. Acta, 732
(3), 531
–540
(1983). 0006-3002 Google Scholar
F. Wilkinson,
W. P. Helman, and
A. B. Ross,
“Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation,”
J. Phys. Chem. Ref. Data, 24 663
–1021
(1995). 0047-2689 Google Scholar
H. W. Gardner,
“Oxygen radical chemistry of polyunsaturated fatty acids,”
Free Radic Biol. Med., 7
(1), 65
–86
(1989). 0891-5849 Google Scholar
S. Miyamoto,
G. E. Ronsein,
F. M. Prado,
M. Uemi,
T. C. Correa,
I. N. Toma,
A. Bertolucci,
M. C. Oliveira,
F. D. Motta,
M. H. Medeiros, and
P. D. Mascio,
“Biological hydroperoxides and singlet molecular oxygen generation,”
IUBMB Life, 59
(4), 322
–331
(2007). 1521-6543 Google Scholar
G. A. Russell,
“Deuterium isotope effects in the autooxidation of aralkyl hydrocarbons. Mechanisms of the interaction of peroxy radicals.,”
J. Am. Chem. Soc., 79 3871
–3877
(1957). https://doi.org/10.1021/ja01571a068 0002-7863 Google Scholar
D. G. Mendenhall,
C. Sheng, and
T. Wilson,
“Yields of excited carbonyl species from alkoxyl and from alkylperoxyl radical dismutations,”
J. Am. Chem. Soc., 113 8976
–8977
(1991). 0002-7863 Google Scholar
J. Baier,
T. Maisch,
J. Regensburger,
M. Loibl,
R. Vasold, and
W. Baumler,
“Time dependence of singlet oxygen luminescence provides an indication of oxygen concentration during oxygen consumption,”
J. Biomed. Opt., 12
(6), 064008
(2007). https://doi.org/10.1117/1.2821153 1083-3668 Google Scholar
T. Maisch,
J. Baier,
B. Franz,
M. Maier,
M. Landthaler,
R. M. Szeimies, and
W. Bäumler,
“The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria,”
Proc. Natl. Acad. Sci. U.S.A., 104
(17), 7223
–7228
(2007). https://doi.org/10.1073/pnas.0611328104 0027-8424 Google Scholar
|