|
|
|
Photodynamic therapy (PDT) is a viable treatment option for a variety of applications, including oncology, dermatology, and ophthalmology.1 In particular, 5-aminolevulinic acid (ALA)-PDT is widely used to treat a range of dermatologic conditions.2 PDT is based on the interaction of a photosensitizer (PS), light, and oxygen, in which photoactivation of PS generates cytotoxic molecular species. Customized dosimetry could, in principle, impact the efficacy of treatment outcome and of the effective use of resources. Dosimetry in PDT is complex, as the treatment effect is generated by an interaction of multiple components. A number of dose metrics have been evaluated to monitor the outcome of ALA-PDT in dermatological treatment.3, 4 Since singlet oxygen , which phosphoresces to the ground triplet state, is believed to be a key cytotoxic species in PDT,1 its direct monitoring is of great interest. Until now, in-vivo optical detection of luminescence at remained elusive because of its low signal yield. In recent pioneering works, Niedre 5 and Yamamoto 6 measured PDT-generated luminescence in vivo in mice, and demonstrated its correlation with treatment outcome. Recently, we have developed a fiber-based monitoring device7 that is compatible with human studies. We present the first clinical trial measuring PDT-generated levels in skin of healthy volunteers before and after ALA application, and correlating the luminescence signal with the photobiological effects of ALA-PDT, possibly indicating treatment outcome. A total of 18 healthy subjects (14 males and 4 females) were enrolled with written, informed consent in the clinical study approved by our internal Institutional Review Board to ensure adherence to the Declaration of Helsinki protocols. Power analysis indicated that the sample size of 18 subjects provided 80% power ( , ) to detect a significant linear relationship between the change in luminescence signal and photobiological skin response following PDT using repeated-measures mixed model regression analysis (version 6.0, nQuery Advisor, Statistical Solutions, Saugus, Massachusetts). A newly developed, entirely fiber-based dosimeter was used to detect luminescence in vivo.7 The device uses a low power, time-resolved diode laser of ( repetition rate, pulse duration, less than /pulse) for PS activation. signal was measured at the end of each laser pulse to reduce background fluorescence signal. Simultaneously, three optical signal strengths at 1.22, 1.27, and are recorded using narrow bandpass filters and a photon multiplier tube (PMT) (Hamamatsu H9170-45) with a fast photon counter (model MSA-300, Becker and Hickl, Berlin). The average signal from 1.22 and 1.315 is used to estimate the background signal and then subtracted from the signal at to eliminate background noise. Measurements typically take only a few seconds. Each subject had two treatment sites on the right upper arm outlined and randomly assigned for ALA-PDT (Levulan Kerastick, DUSA Pharmaceuticals, Wilmington, Massachusetts) with either one-hour or three-hour incubation periods. ALA incubation time was varied to control PS accumulation, keeping in mind that longer incubation times result in increased PpIX levels,8 leading to different production and treatment outcomes. measurements were done before ALA application (pre-ALA) when no exogenous PS was administered and immediately before the therapeutic light dose (pre-PDT) following PpIX production from ALA application. Following measurements, each treatment site was irradiated with a therapeutic light dose of (irradiance ) with a separate fiber-based continuous diode laser at (HPD 7401, High Power Devices, North Brunswick, New Jersey). Standardized digital photographs of the treatment sites were taken within and at after this treatment and used for the evaluation of phototoxic reactions by four blinded investigators. Each investigator rated the degree of erythema and edema between a scale of 0 (no response) to 10 (most pronounced). Median of the four readings is reported to minimize any potential interobserver variability. The photobiological response to the treatment sites were more pronounced and more clinically significant compared to treatment sites (Fig. 1 ). Consistent with Ref. 9, edema and erythema response peaked at and following PDT, respectively. Subsequent analysis was performed using the maximal photobiological skin response: acute edema at post-PDT and erythema at follow-up post-PDT. PS-induced signal was defined by change in luminescence between pre-ALA (time of minimum PpIX concentration) and pre-PDT (time of maximum PpIX concentration) time points. Fig. 1Representative images of treatment sites with or of ALA application time. The phototoxic reaction in sites (edema immediately after PDT and erythema afterward) is more intense than in sites. 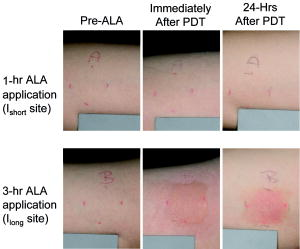 Several researchers have shown that the degree of erythema and edema following ALA-PDT is predictive of clinical outcome in various dermatologic conditions.4, 10 To evaluate the relationship between signal and photobiological skin responses (edema and erythema), we applied repeated-measures linear mixed model analysis.11 Using this model, the luminescence signal significantly correlated with acute edema in sites and follow-up erythema for and combined (Table 1 ). Table 1Effect of O21 signal on photobiological responses based on a linear mixed model regression analysis of the data. Slope of the linear correlation between O21 signal and photobiological response in either Ishort sites, Ilong sites, or the combined data are reported. Edema is measured at post-PDT; erythema is measured at follow-up. The slope test shows the comparison of slopes estimated from Ishort sites and Ilong sites using an F-test. ST is statistically significant. CI=confidence interval.
Equality of slopes for and sites was assessed with an F-test to decide whether a common slope parameter could be modeled to describe this correlation.12 The relationship between erythema and signal showed no significant difference in slopes between and sites . As a result, correlation between follow-up erythema and signal was investigated with a common slope fitted to the combined dataset for the and sites (Fig. 2 ). The interpretation is that the percent change in signal from baseline is positively correlated for both sites in a similar way, and thus can be described using a common slope. Fig. 2Scatter plots illustrating the relationship between luminescence signal versus (a) acute edema rating and (b) erythema rating after PDT. Regression-based fits to the , , and combined data are shown by the red dashed line, the blue dotted line, and the solid green line, respectively. (Color online only.) 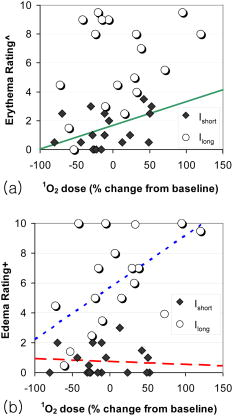 On the other hand, the correlation between acute edema and signal was modeled separately for and sites because they had unequal slopes (Fig. 2). In fact, greater edema was significantly correlated with greater percent change from baseline in signal but only for the sites , not sites (Table 1). Interestingly, only sites showed a significant correlation between signal and acute edema (Fig. 2). It is possible that the minor acute edema at the sites (median score of 0.25 out of 10) may not have been clearly discernable by the blinded investigators in a 2-D photograph, and thus may not have correlated significantly with the quantitative signal. Unexpectedly, a decrease in signal after the incubation period was observed in some treatment sites. This might be related to modification of skin optical properties by ALA and its carrier.13 A carrier-only control treatment site could potentially provide additional information when incorporated in future studies. This is the first study showing the feasibility of monitoring PS-generated signal in vivo in human subjects. signal measured immediately prior to PDT light irradiation correlated significantly with the photobiological response to ALA-PDT in normal skin. As a result, monitoring production in the skin is predictive of the clinical ALA-PDT outcome and is a helpful tool for customizing the clinical ALA-PDT treatment. AcknowledgmentsThis work was supported by NIH SBIR Phase II grant R44 CA096243 and in part by NIH grant P01 CA84203. We also thank Oleg Akilov, Mehron Puoris’haag, Fernanda Sakamoto, and Kana Watanabe for evaluating the phototoxic response from the clinical photographs. ReferencesT. Hasan,
B. Ortel,
N. Solban, and
B. Pogue,
“Photodynamic therapy of cancer,”
Cancer Medicine, 537
–548 7th ed.B.C. Decker, Inc., Hamilton, Ontario
(2006). Google Scholar
P. Babilas,
M. Landthaler, and
R. M. Szeimies,
“Photodynamic therapy in dermatology,”
Eur. J. Dermatol., 16
(4), 340
–348
(2006). 1167-1122 Google Scholar
M. B. Ericson,
C. Sandberg,
B. Stenquist,
F. Gudmundson,
M. Karlsson,
A. M. Ros,
A. Rosen,
O. Larko,
A. M. Wennberg, and
I. Rosdahl,
“Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome,”
Br. J. Dermatol., 151
(6), 1204
–1212
(2004). 0007-0963 Google Scholar
E. W. Jeffes,
J. L. McCullough,
G. D. Weinstein,
P. E. Fergin,
J. S. Nelson,
T. F. Shull,
K. R. Simpson,
L. M. Bukaty,
W. L. Hoffman, and
N. L. Fong,
“Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study,”
Arch. Dermatol., 133
(6), 727
–732
(1997). 0003-987X Google Scholar
M. J. Niedre,
C. S. Yu,
M. S. Patterson, and
B. C. Wilson,
“Singlet oxygen luminescence as an in vivo photodynamic therapy dose metric: validation in normal mouse skin with topical amino-levulinic acid,”
Br. J. Cancer, 92
(2), 298
–304
(2005). 0007-0920 Google Scholar
J. Yamamoto,
S. Yamamoto,
T. Hirano,
S. Li,
M. Koide,
E. Kohno,
M. Okada,
C. Inenaga,
T. Tokuyama,
N. Yokota,
S. Terakawa, and
H. Namba,
“Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma,”
Clin. Cancer Res., 12
(23), 7132
–7139
(2006). 1078-0432 Google Scholar
S. Lee,
L. Zhu,
A. M. Minhaj,
M. F. Hinds,
D. H. Vu,
D. I. Rosen,
S. J. Davis, and
T. Hasan,
“Pulsed diode laser-based monitor for singlet molecular oxygen,”
J. Biomed. Opt., 13
(3), 034010
(2008). https://doi.org/10.1117/1.2927465 1083-3668 Google Scholar
A. Juzeniene,
P. Juzenas,
L. W. Ma,
V. Iani, and
J. Moan,
“Topical application of 5-aminolaevulinic acid, methyl 5-aminolaevulinate and hexyl 5-aminolaevulinate on normal human skin,”
Br. J. Dermatol., 155
(4), 791
–799
(2006). 0007-0963 Google Scholar
R. C. Brooke,
A. Sinha,
M. K. Sidhu,
R. E. Watson,
M. K. Church,
P. S. Friedmann,
G. F. Clough, and
L. E. Rhodes,
“Histamine is released following aminolevulinic acid-photodynamic therapy of human skin and mediates an aminolevulinic acid dose-related immediate inflammatory response,”
J. Invest. Dermatol., 126
(10), 2296
–2301
(2006). https://doi.org/10.1038/sj.jid.5700449 0022-202X Google Scholar
A. D. Tosca,
C. J. Balas,
M. P. Stefanidou,
J. C. Katsantonis,
S. K. Georgiou, and
M. N. Tzardi,
“Photodynamic treatment of skin malignancies with aminolevulinic acid. Emphasis on anatomical observations and in vivo erythema visual assessment,”
Dermatol. Surg., 22
(11), 929
–934
(1996). 1076-0512 Google Scholar
E. Vittinghoff,
D. V. Glidden,
S. C. Shiboski, and
C. E. McCulloch, Regression Methods in Biostatistics. Linear, Logistic, Survival, and Repeated Measures Models, Springer, Berlin
(2005). Google Scholar
P. J. Diggle,
K. Liang, and
S. L. Zeger, Analysis of Longitudinal Data, Oxford University Press, Oxford, UK Google Scholar
J. Welzel,
C. Reinhardt,
E. Lankenau,
C. Winter, and
H. H. Wolff,
“Changes in function and morphology of normal human skin: evaluation using optical coherence tomography,”
Br. J. Dermatol., 150
(2), 220
–225
(2004). https://doi.org/10.1111/j.1365-2133.2004.05810.x 0007-0963 Google Scholar
|
|||||||||||||||||||||||||||||

