|
|
|
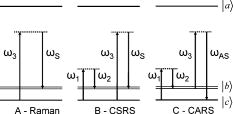
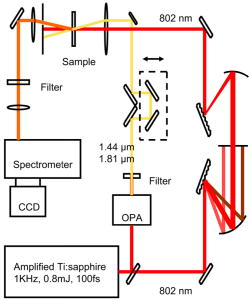
Optical spectroscopy as a method of monitoring blood analytes is an attractive alternative to traditional chemical assays. There are several recognized drawbacks, however; interfering signals from different analytes, background noise, for instance, has precluded the implementation in the medical practice. The most popular technique, fluorescence spectroscopy, relies on markers for efficient detection of analytes and uses optical filters to control the spectra of excitation and emission of light. Raman spectroscopy, on the other hand, has evolved into a flexible and sophisticated technology that is sensitive to scattering of light by molecular vibrations in real time. Becaue molecular vibrations are quite sensitive to composition, detailed molecular information can be extracted. A common technique for molecular identification, Raman spectroscopy has been extensively used in chemistry, physics, biology, medicine, etc., for the last century. On the basis of inelastic scattering of photons off molecular vibrations, the Raman effect is very well suited for chemical recognition and is especially useful in biology.1 However, because of the low conversion efficiency of pump photons into Stokes-shifted photons, spontaneous Raman measurements are time consuming and often difficult to separate from associated broadband fluorescence. This often prohibits real-time identification of molecular vibrations. Coherent Raman spectroscopy, on the other hand, shows much higher conversion efficiencies. Since its first demonstration by Terhune2 and Maker and Terhune,3 coherent anti-Stokes Raman scattering (CARS) has proved to be a powerful spectroscopic technique of probing Raman active resonances in gases, liquids, and solids. In CARS, one coherently excites the vibrational levels using two beams, a pump beam and a Stokes shifted beam, which greatly enhance the probability of Raman scattering for a probe beam.4, 5 Figure 1 shows, schematically, the difference between spontaneous and coherent Raman: If only the probe beam is sent to the sample (case A), we can record the spontaneous Raman spectrum as the difference between the input probe beam and the detected Stokes-shifted beam. The Stokes shifts correspond to the ground-state vibrational modes that are excited (the difference between levels and ). However, we can drive the vibrational modes of the molecule by sending the pump and Stokes beams just before sending the probe beam. In this way, we coherently excite the ground-state vibrations (for example the and levels shown here) and can detect the coherent Stokes Raman scattering (CSRS) signal to level when probing (case B), or the CARS signal to level when probing (case C). If spontaneous Raman suffers from low signals and large undesired backgrounds, such as fluorescence, CARS has its own drawbacks, most notably the nonresonant four-wave mixing electronic contributions either from solvents, substrates, or other modes of the target molecule. Depending on the particulars of a specific experiment, one can make a decision about which method is appropriate.6, 7, 8 In spontaneous Raman measurements (case A of Fig. 1), one is limited to signal processing and data analysis when one tries to recover the vibrational features from a strong fluorescence background signal. In CARS measurements (case C of Fig. 1), there are several ways to deal with the background: using polarization sensitive, interferometric, heterodyne, or time-resolved techniques. All these techniques are aimed at increasing the ratio between the resonant CARS signal and the nonresonant background noise. Using several of these techniques, CARS has been successfully used for chemically selective microscopic biological imaging.9, 10, 11, 12, 13 Time-resolved CARS is a very sensitive technique for investigating molecular vibrations and also has the advantage of measuring another parameter: decoherence time, or the lifetime of the coherent molecular vibrations. Moreover, when more than one vibrational level is present (which is the case in most molecular systems, including all the blood components), quantum beats will appear between different vibrational modes. For example, we have shown that a time-resolved CARS measurement can easily distinguish between two very similar molecules: 2,6 pyridinedicarboxylic acid and 3,5 pyridinedicarboxylic acid.14 Although the chemical composition is the same, the vibrational levels in the fingerprint region around have slightly different separation, leading to different quantum beat frequencies in the time-resolved CARS signals. The small shift of the vibrational energies for the respective modes (and, hence, shifts in the Raman spectra) is exactly the shift recently reported between Raman lines of oxygenated and deoxygenated red blood cells.15, 16 Broadband (or multiplex) CARS uses a combination of broadband and narrowband laser pulses to excite a wide spectral region of vibrational modes, while still having a good spectral resolution in order to resolve each individual Raman mode. If the time-resolved CARS provides for high-sensitivity measurements and can measure the decoherence times for the vibrational levels, the broadband CARS provides the spectral information about the vibrational modes. In particular, coherent Raman using properly shaped and timed pulses has been proposed for molecular identification of certain biological samples.5 A recently demonstrated technique combines the temporal and spectral resolution of these two approaches,17, 18, 19 allowing for identification of chemical fingerprints in real time.20, 21 In the quest for real-time blood analysis, Raman spectroscopy has been proven to reveal the vibrational lines of red blood cells,15, 16 unprocessed whole blood,22, 23 while multiplex CARS has been demonstrated in hemoglobin.24 Whole blood is a fluidized gel in an aqueous plasma solution. The cell matter makes up to 46% of the volume in human blood and consists primarily of red blood cells or erythrocytes, white blood cells, or leukocytes, and platelets . Here we report on CARS measurements performed in real time on whole blood and demonstrate that we can identify the Raman lines associated with the red blood cells in milliseconds from picoliters of unprocessed blood, without the need of data processing. The experimental setup is shown in Fig. 2 . The laser source is a Spectra-Physics amplified femtosecond system, which delivers 100 femtosecond pulses at with repetition rate. Part of the laser output is used to pump an optical parametric amplifier, which generates signal and idler pulses with their frequency tunable in the near infrared. The detection system is comprised of an Acton spectrometer and a liquid-nitrogen-cooled CCD camera from Princeton Instruments . In order to excite the ground-state vibrational levels in the midinfrared fingerprint region, we tune the signal and idler to be centered at 1.44 and for the pump and Stokes beams, respectively. The signal and idler beams are perpendicularly polarized, collinear, and temporally overlapped. The broadband pump and Stokes pulses ensure a broad excitation of the vibrational lines between 1000 and . For the probe pulse, we send the other part of the amplified broadband near-infrared pulses through a pulse shaper. The pulse shaper spatially separates the frequency components of the pulse before recombining them. Using a slit, as shown in Fig. 2, we select a narrow pulse ( , providing our spectral resolution) at , corresponding to an pulsewidth. The probe pulse is time delayed from the pump and Stokes pulses in order to ensure a good resonant (CARS) signal in the presence of a reduced nonresonant background resulting from the four-wave mixing signal generated by the three beams. The delay is limited by the fast vibrational decoherence of the molecules. The energies delivered to the sample are for the pump and Stokes beams, and for the probe beam. The total average power is comparable to the powers used in the literature for Raman investigation of blood, which range from ,15, 16, 22, 23, 24 and is low enough to ensure no damage due to laser exposure while acquiring spectra. Using the experimental setup shown in Fig. 2, we have recorded spectra from a drop of whole blood (a few microliters) placed in a 100-μm cell. With a probe beam of , we estimate the volume of blood investigated to be 1250 μm3 (or 125 pL). The results are shown in Fig. 3 . The CARS spectra shown on the top of Fig. 3 are two separate raw data recorded by our spectrometer in from of blood. The two curves are not averaged; they are taken from different spots on the cell. Hence, they show the repeatability of the spectra. The lower spectra show the vibrational lines obtained after extracting the modest nonresonant background. Because the CARS spectra are usually dominated by a nonresonant broadband background, retrieval of the spontaneous Raman spectrum, in general, requires the extraction of the imaginary part of the third-order susceptibility from the complex signal aquired.25, 26 However, by delaying the probe pulse and reducing the broadband contribution, the simple subtraction can yield lines with minimal dispersive shape, such as the lower curves shown in Fig. 3. Given our spectral resolution of , this method gives us enough accuracy, while allowing for fast, real-time data processing. The vibrational modes that clearly identify the oxygenated blood are in very good agreement with recently published data.15, 16, 22, 23, 24 We note that, while preserving the wavelengths for the Raman lines, the relative intensities in our CARS spectra differ from the spontaneous Raman spectra. This is because the magnitude of the CARS signals is given by the spectral shape of the pump and Stokes pulses. This apparent disadvantage of CARS becomes very useful if one is interested in selectively exciting vibrational lines. The real advantage of coherent pumping, however, is the magnitude of the signals measured, which allows for very short acquisition times. In our experiments, there was no need for microscopy setups, isolation of red blood cells, chemical preparation, and long integration times necessary for spontaneous Raman experiments. For example, Wood and McNaughton15 obtained Raman spectra from red blood cells in ; Ward 22 reported resonance Raman spectra of blood in ; and Enejder 23 demonstrated unprocessed blood spectra recorded in . Using a CARS setup, Rinia 24 reported acquisition times for recording hemoglobin spectra. In our case, using the coherent enhancement with background subtraction provided by our CARS setup, we are able to obtain the vibrational information on the millisecond time scale from unprocessed blood, using laser beams focused to , and detecting the signal from the sample. All these results suggest CARS as a very appealing technique for real-time in vivo blood monitoring. Fig. 3CARS spectra obtained in from oxygenated blood probed at shown as raw data (top curves) and after subtraction of the nonresonant background (lower curves). 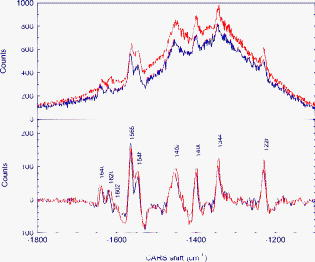 In the following, we list a few of the advantages of CARS over spontaneous Raman in the present experiment and envisioned extensions:
ConclusionWe have demonstrated a real-time measurement of Raman spectrum of whole blood using a hybrid CARS technique, which combines the benefits of temporal and spectral CARS and provides strong enhancement over spontaneous Raman and traditional CARS measurements. We have clearly identified, in milliseconds, all the Raman lines associated with the red blood cells from picoliters of oxygenated blood. The coherent enhancement demonstrated here makes CARS suitable for real-time monitoring of blood: the same vibrational spectrum that otherwise (using spontaneous Raman) would take minutes, it can now be obtained in milliseconds. AcknowledgmentsWe thank Tom Spiro and Aristide Dogariu for their help and stimulating discussions and gratefully acknowledge the support from the Office for Naval Research and the Robert A. Welch Foundation (Grant No. A-1261). ReferencesT. G. Spiro, Biological Applications of Raman Spectroscopy, Wiley, Hoboken, NJ
(1987). Google Scholar
R. W. Terhune,
“Non-linear optics,”
Bull. Am. Phys. Soc., 8 359
(1963). 0003-0503 Google Scholar
P. D. Maker and
R. W. Terhune,
“Study of optical effects due to an induced polarization third order in the electric field strength,”
Phys. Rev., 137 A801
(1965). https://doi.org/10.1103/PhysRev.137.A801 0031-899X Google Scholar
J. W. Nibler and
G. V. Knighten, Raman Spectroscopy of Gases and Liquids, Springer-Verlag, New York
(1979). Google Scholar
M. O. Scully,
G. W. Kattawar,
R. P. Lucht,
T. Opatrny,
H. Pilloff,
A. Rebane,
A. V. Sokolov, and
M. S. Zubairy,
“FAST CARS: Engineering a laser spectroscopic technique for rapid identification of bacterial spores,”
Proc. Natl. Acad. Sci. U.S.A., 99 10994
–11001
(2002). https://doi.org/10.1073/pnas.172290899 0027-8424 Google Scholar
M. D. Levenson, Introduction to Nonlinear Laser Spectroscopy, Academic Press, Boston
(1988). Google Scholar
W. M. Tolles and
R. D. Turner,
“A comparative analysis of the analytical capabilities of coherent anti-Stokes Raman spectroscopy (CARS) relative to Raman scattering and absorption spectroscopy,”
Appl. Spectrosc., 31 96
(1977). https://doi.org/10.1366/000370277774463968 0003-7028 Google Scholar
W. M. Tolles,
J. W. Nebler,
J. R. McDonald, and
A. B. Harvey,
“A review of the theory and application of coherent anti-Stokes Raman spectroscopy (CARS),”
Appl. Spectrosc., 31 253
(1977). https://doi.org/10.1366/000370277774463625 0003-7028 Google Scholar
M. D. Duncan,
J. Reintjes, and
T. J. Manuccia,
“Scanning coherent anti-Stokes Raman microscope,”
Opt. Lett., 7 350
(1982). 0146-9592 Google Scholar
A. Zumbusch,
G. R. Holton, and
X. S. Xie,
“Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering,”
Phys. Rev. Lett., 82 4142
(1999). https://doi.org/10.1103/PhysRevLett.82.4142 0031-9007 Google Scholar
M. Hashimoto and
T. Araki,
“Molecular vibration imaging in the fingerprint region by use of coherent anti-Stokes Raman scattering microscopy with a collinear configuration,”
Opt. Lett., 25 1768
(2000). https://doi.org/10.1364/OL.25.001768 0146-9592 Google Scholar
E. O. Potma,
W. P. de Boeij,
P. J. M. van Haastert, and
D. A. Wiersma,
“Real-time visualization of intracellular hydrodynamics in single living cells,”
Proc. Natl. Acad. Sci. U.S.A., 98 1577
(2001). https://doi.org/10.1073/pnas.031575698 0027-8424 Google Scholar
J.-X. Cheng and
X. S. Xie,
“Coherent anti-Stokes Raman scattering microscopy: instrumentation, theory, and applications,”
J. Phys. Chem. B, 108 827
(2004). https://doi.org/10.1021/jp035693v 1089-5647 Google Scholar
A. Dogariu,
Y. Huang,
Y. Avitzour,
R. K. Murawski, and
M. O. Scully,
“Sensitive femtosecond CARS discrimination between 2,6 dipicolinic acid and 3,5 dipicolinic acid,”
Opt. Lett., 31
(21), 3176
(2006). https://doi.org/10.1364/OL.31.003176 0146-9592 Google Scholar
B. R. Wood and
D. McNaughton,
“Raman excitation wavelength investigation of single red blood cells in vivo,”
J. Raman Spectrosc., 33 517
(2002). https://doi.org/10.1002/jrs.870 0377-0486 Google Scholar
B. R. Wood,
P. Caspers,
G. J. Puppels,
S. Pandiancherri, and
D. McNaughton,
“Resonance Raman spectroscopy of red blood cells using near-infrared laser excitation,”
Anal. Bioanal. Chem., 387 1691
(2007). https://doi.org/10.1007/s00216-006-0881-8 1618-2642 Google Scholar
B. D. Prince,
A. Chakraborty,
B. M. Prince, and
H. A. Stauffer,
“Development of simultaneous frequency- and time-resolved coherent anti-Stokes Raman scattering for ultrafast detection of molecular Raman spectra,”
J. Chem. Phys., 125 044502
(2006). https://doi.org/10.1063/1.2219439 0021-9606 Google Scholar
H. Kano and
H. Hamaguchi,
“Dispersion-compensated supercontinuum generation for ultrabroadband multiplex coherent anti-Stokes Raman scattering spectroscopy,”
J. Raman Spectrosc., 37 411
(2006). https://doi.org/10.1002/jrs.1436 0377-0486 Google Scholar
D. Pestov,
R. K. Murawski,
G. O. Ariunbold,
X. Wang,
M. Chi,
A. V. Sokolov,
V. Sautenkov,
Y. Rostovtsev,
A. Dogariu,
Y. Huang, and
M. O. Scully,
“Optimizing the laser pulse cConfiguration for coherent Raman spectroscopy,”
Science, 316 265
(2007). https://doi.org/10.1126/science.1139055 0036-8075 Google Scholar
A. Dogariu,
A. Goltsov,
D. Pestov,
A. V. Sokolov, and
M. O. Scully,
“Real-time detection of bacterial spores using coherent anti-Stokes Raman spectroscopy,”
J. Appl. Phys., 103 036103
(2008). https://doi.org/10.1063/1.2837108 0021-8979 Google Scholar
D. Pestov,
X. Wang,
G. O. Ariunbold,
R. K. Murawski,
V. A. Sautenkov,
A. Dogariu,
A. V. Sokolov, and
Marlan O. Scully,
“Single-shot detection of bacterial endospores via coherent Raman spectroscopy,”
Proc. Natl. Acad. Sci. U.S.A., 105 422
(2008). https://doi.org/10.1073/pnas.0710427105 0027-8424 Google Scholar
K. R. Ward,
R. W. Barbee,
P. S. Reynolds,
I. P. Torres Filho,
M. H. Tiba,
L. Torres,
R. N. Pittman, and
J. Terner,
“"Oxygenation monitoring of tissue vasculature by resonance Raman Spectroscopy,”
Anal. Chem., 79 1514
(2007). https://doi.org/10.1021/ac061072x 0003-2700 Google Scholar
A. M. K. Enejder,
T. W. Koo,
J. Oh,
M. Hunder,
S. Sasic,
M. Feld, and
G. L. Horowitz,
“Blood analysis by Raman spectroscopy,”
Opt. Lett., 27 2004
(2002). https://doi.org/10.1364/OL.27.002004 0146-9592 Google Scholar
H. A. Rinia,
M. Bonn,
E. M. Vartiainen,
C. B. Schaffer, and
M. Muller,
“Spectroscopic analysis of the oxygenation state of hemoglobin using coherent anti-Stokes Raman scattering,”
J. Biomed. Opt., 11 050502
(2006). https://doi.org/10.1117/1.2355671 1083-3668 Google Scholar
E. M. Vartiainen,
H. A. Rinia,
M. Bonn, and
M. Muller,
“Direct extraction of Raman line-shapes from congested CARS spectra,”
Opt. Express, 14 3622
(2006). https://doi.org/10.1364/OE.14.003622 1094-4087 Google Scholar
E. M. Vartiainen,
“Phase retrieval approach for coherent anti-Stokes Raman scattering spectrum analysis,”
J. Opt. Soc. Am. B, 9 1209
(1992). 0740-3224 Google Scholar
A. Dogariu,
Y. Huang,
G. Balakrishnan,
T. G. Spiro, and
M. O. Scully,
“Direct measurements of coherent enhancement in Raman spectroscopy,”
J. Raman Spectrosc.,
(0377-0486) Google Scholar
|