|
|
1.IntroductionThere is an increasing demand for alternative models of analysis for laser bioeffects. Although assessment of ocular damage in nonhuman primate (NHP) models (in vivo) remains critical for providing guidelines for eye-safe exposures in humans, cost, availability, and ethical constraints on their use have hampered advances in the field of laser-tissue interaction. In vitro models must be validated by showing that they follow or predict the same basic trends between dosimetry and cell damage described in the NHP model. Computer simulation programs and cell culture systems (in vitro) are models that can provide important information about laser bioeffects. The simple and flexible nature of cell culture systems is ideal for providing rapid feedback to the modeling community regarding basic cellular response to lasers. In this unified experimental and mathematical model scenario, testing of novel lasers and discovery of new concepts (physics and biology) could initially be performed using an iterative approach between cell exposures and simulations before final validation in the NHP model, thus minimizing the use of animals. Data from in vivo studies have shown that laser damage in the retina depends on wavelength, power density, and duration of the exposure.1 Damage has been defined as the minimum irradiance leading to a visible lesion,1, 2 or by the statistical method of Probit,3, 4 which correlates laser dose and the probability of achieving a damaging event. The Probit estimated dose for 50% lethality is often defined as the damage threshold, which can be compared over a range of wavelengths (action spectrum) or exposure durations (temporal action profile) to identify trends in damage efficacy. We have previously described and reported damage thresholds in a human retinal pigment epithelial (RPE) cell model in which the sensitivity of the cells depends on the number of intracellular melanosome particles (MPs).5, 6, 7 Here, we provide additional in vitro data that, when combined with our previous work, are presented as action spectra and temporal action profiles over four wavelengths and four exposure durations. Further, we compare our in vitro threshold data to those reported in the literature for minimum visible lesions in the NHP model. 2.ExperimentalThe materials and methods used to generate values in this report (514 and ), including the use of the same batch of isolated melanosomes, were identical to those used previously,6 and thus, all data described here are comparable. Artificial pigmentation of hTERT-RPE1 cells (in 96-well microtiter plates) was carried out using volumes of stock MPs corresponding to equivalents of either 160 or 1600 particles per cell. Laser delivery to the RPE cells is shown in Fig. 1 . For our experiments, the Millennia Pro (Spectra Physics, Irvine, California) supplied the light, and the Innova large-frame argon (Coherent Inc., Santa Clara, California) laser provided light of 514 and . For exposures at , the Sabre large-frame krypton laser (Coherent) was used. All beams were co-aligned to a common optical path using apertures and a flip-up mirror (F). Attenuation of laser power was achieved by the combination of a half-wave plate and polarizing beamsplitter (Pol). The optical path included a telescope (T), a beam shaper (BSh, model GBS-AR14, Newport Corp., Irvine, California), a computer-controlled shutter (S), and a single lens (L) imaging system ( focal length) generating a beam diameter of about at the cells. The telescope allowed for collimated beam expansion to prior to entry into the beam shaper, which converted the beam to a flat-top profile. The imaging system was designed to image the beam at the near-field output of the beam shaper ( diameter) via magnification. The effect of the column of Hank's balanced salt solution above the cells during exposure was taken into account when identifying the laser beam diameter (knife-edge method). Fig. 1Laser delivery for in vitro damage threshold experiments. M, mirror; F, flip-up mirror; Pol, polarizing cube; T, optical telescope; BSh, beam shaper; S, mechanical shutter; L, lens; PM, power meter; ND, neutral density filter; CCD, charge-coupled device camera; Obj, microscope objective. Details are found in Ref. 6. 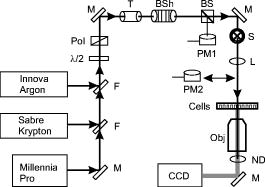 Uncertainty in our irradiance values was determined from calculated combined standard uncertainties (types A and B) for measuring both laser power and diameter at the sample.6 Damage threshold irradiance values were determined using the Probit4 method. The Probit output includes additional uncertainty intervals (fiducial limits) related to the value, for which 95% confidence levels were used. 3.Results and DiscussionIn vitro damage threshold values for all four wavelengths are summarized in Tables 1, 2 . Although experimental uncertainty in irradiance varied depending upon the power detector used, the maximum extended uncertainty of our values was 20% with an overall average of 14%. The Probit slope reported is the first derivative of the Probit curve at the value. Table 1Threshold ED50 values for laser exposures to the in vitro retinal model using 160MPs∕cell .
Lower and upper fiducial limits (LFL and UFL) correspond to 95% confidence intervals. Probit slope represents the first derivative of the probit curve at the
ED50
value. The total number of exposures
(n)
for each data set is provided. Table 2Threshold ED50 values for laser exposures to the in vitro retinal model using 1600MPs∕cell .
Lower and upper fiducial limits (LFL and UFL) correspond to 95% confidence intervals. Probit slope represents the first derivative of the probit curve at the
ED50
value. The total number of exposures
(n)
for each data set is provided. Trends in the data found in Tables 1, 2 are summarized as action spectra (Fig. 2 ) and temporal action profiles (Fig. 3 ). It is apparent from both types of analyses that there was a pigment-dependent effect on the trends for the data. The consequence of having approximately 10 times the number of melanosomes within each cell was an enhanced sensitivity to laser exposure. The effect, reported as fold reduction in (Table 3 of Ref. 6), was most pronounced for exposures presumed to produce damage by photothermal mechanisms, namely exposure at . The values for (new data) follow the same pigment-dependent trend as 532 and , falling between the 532 and ratios. Fig. 2Action spectra (inverse irradiance thresholds) for laser damage in the in vitro retinal model using (a) /cell or (b) /cell, and in (c) the rhesus animal model (data taken from Ref. 2).  Fig. 3Temporal action profiles for laser damage in (a) the in vitro retinal model, (b) the rhesus model (data taken from Ref. 2). (c) Comparison of in vitro and in vivo temporal action profiles for laser damage at . Rhesus data taken from Ref. 2 ( beam diameter and post-exposure damage assessment) and Ref. 8 ( retinal beam diameter and either or post-exposure damage assessment). (d) Rhesus data for post-exposure damage using a beam diameter from Ref. 10 (solid line, thresholds; dashed line, thresholds).  Table 3Power function equations describing the “linear” portions (log-log plot) of the temporal action profiles found in Fig. 3.
The irradiance action spectra for the /cell thresholds [Fig. 2a] show a substantial increase in cellular sensitivity (larger inverse irradiance) for cells exposed for at , relative to the other wavelengths and exposure durations. Each of the lines generated from the data had a negative slope, indicating increased damage sensitivity at the shorter wavelengths. Additionally, for each of the wavelengths tested, there was increased damage sensitivity as exposure duration was increased. Both of these trends were also apparent in the in vivo data from Ham, 2 which have been summarized in Fig. 2c. Notice the increasing sensitivity of the rhesus retina at the shorter wavelengths and the longer exposure durations within a given wavelength. The threshold data for cells with the higher pigmentation [Fig. 2b] also showed increased damage sensitivity as exposure duration was lengthened for each wavelength, but the magnitude of this effect was very minimal for the data [compare in Figs. 2a and 2b the and thresholds at ]. The slopes of the lines generated in the action spectra in Fig. 2b were positive, indicating a different trend relative to the in vivo data. Thus, even though the irradiance values for cells with the higher pigmentation were closer to the absolute values of the thresholds from Ham, 2 the wavelength dependent trend in thresholds was lost. Figure 3a relates our in vitro damage thresholds (radiant exposure) to exposure duration for each of the wavelengths using temporal action profiles. Although it was apparent that cells with greater pigmentation were more sensitive to the laser exposure, there was little distinction between the trend lines within each pigmentation data set when plotted on log-log axes. The in vitro trends at 514 and followed the trends previously shown for 532 and ,6 respectively. The trend for both 532 and continued beyond exposure duration as a power function (Table 3). The values for exposure of cells containing at 458 and trended together with the 532 and data between 0.1 and (Table 3) but then deviated to a trend that was more horizontal. This transition appears to approximate the transitions seen in Fig. 3b, where there was nearly constant threshold radiant exposure over time (irradiance reciprocity). Due to both a shift downward in thresholds relative to the longer wavelengths and a different break-off point , the data in Fig. 3b deviate from the trends just discussed. In general, this biphasic response to changing laser exposure duration has been observed for damage at wavelengths shorter than [see Fig. 3b] and has been used as an indicator of the transition from photothermal to nonthermal (photochemical) damage mechanisms. As shown in Fig. 3a, this biphasic response had not occurred within the duration limit of our experiment when the RPE cells contained the higher density of MPs. A comparison of Figs. 3a, 3b, 3c, 3d provides support to the validity of the in vitro model when using /cell. The transition to irradiance reciprocity, and thus the presumed transition from photothermal to photochemical damage mechanisms, occurred in both the in vivo and in vitro models at exposure durations of about in the data for wavelengths of and shorter. Of interest is the comparison for exposure to . The in vitro data suggest that damage from continues to be predominately thermal even at , whereas the data from Ham 2 indicate irradiance reciprocity. Gibbons and Allen8 have shown that, using Probit analysis in the rhesus model, is a pivotal wavelength for achieving photochemical damage. Figure 3c shows that Gibbons and Allen8 found reciprocity when assessing for damage at post exposure (latency), but not after post exposure. We interpret these results to indicate that the energy of photons at is sufficient to achieve photochemical damage, but the mechanism of injury is dictated by the time interval chosen for damage assessment. Even though our spot size differs from that in the Gibbons and Allen study (estimated from Ref. 8 to be ), our post-exposure data (trend) for damage from exposure to appear to fall in line with the animal model. Even though differences in spot size and damage assessment led to disparate damage thresholds in both of the in vivo methods summarized in Fig. 3c, the overall trends in their temporal action profile remain similar. Another means of comparing threshold trends is curve fitting of the data in temporal action profiles. The slope (exponent) of the “linear” portion (log-log plot) of pertinent curves in Fig. 3 compared favorably across the in vivo and in vitro ( /cell) methods (Table 3). We have included in our Fig. 3d the laser diameter data from the in vivo study of Lund, 10 which represent exposure durations of and 0.1 to 100 at a spot size similar to that used both here and in Ham’s studies1, 2, 11 . One of the conclusions made by Lund 10 was that, even though their thresholds were in good agreement to those of Ham, 2 their data differed at exposure durations shorter than . The newer study, which used a HeCd laser with greater power than was available when Ham 2 did their study, now provides trusted data for the wavelength and shows the expected temporal action profile trend (the break point for reciprocity and higher threshold values). The trends in the temporal action profile of Fig. 3d are similar to the trends seen for 458 and in Fig. 3a. Not only is there an apparent transition from photothermal to photochemical damage at around the exposure time, the transitions are not as smooth, as depicted in Figs. 3b and 3c for and longer. The interesting drop from the radiant exposure threshold at to that at in the in vitro model shown in Fig. 3a is similar to the data seen in Fig. 3d. It should be noted, however, that Fig. 3d represents thresholds for damage assessed post exposure, and the threshold results for post exposure reported by Lund 10 did not indicate irradiance reciprocity. This delayed appearance of damage at reduced irradiances is one characteristic used to distinguish thermal from nonthermal damage mechanisms (reviewed in Ref. 12). So it was interesting to find that irradiance reciprocity was shown after only in the in vitro model. Pigmentation could play a role in this difference between the two models. Figure 3a shows how increasing pigmentation 10-fold in our RPE cells caused a loss of irradiance reciprocity at for exposures. Although the number per cell of MPs is about the same for the cell and animal models, the shape and spacing of cells differ in culture and the retina, making the MPs per unit area different. Perhaps lesion formation at post exposure at in the eye has a major photochemical component. Maybe the environment of cultured cells (lack of tissue above and below) allows for more efficient dissipation of heat. We have addressed the issue of heat dissipation during exposures of cultured RPE at using computer simulations of irradiances and found significant heating during exposures of , but not .13 Another plausible explanation for the lack of latency in vitro is one that accounts for the type of damage detected. The in vitro model, as described, uses an overt form of damage (plasmalemma breach) as the end point. Although one would expect the response to photo-damage in the eye to be a complex physiological process involving multiple tissue types, the delayed onset of a visible lesion is indicative of an apoptotic pathway. At present, issues of cell viability and overcrowding preclude our use of analyses for laser damage beyond post exposure, so an analysis for delayed onset of damage (apoptosis) of is not feasible. We are currently implementing methods of detecting biochemical traits that manifest at different times during the early stages of programmed cell death, which would make detection of apoptosis at post exposure unnecessary. We would look for irradiance reciprocity at each of those times post exposure ( exposure at ) and determine if increasing pigmentation in our cultured cells would generate the effect required to cause latency in the appearance of damage. Although latency in damage detection is indicative of photochemical processes, we believe that the underlying principle of irradiance reciprocity is an equally definitive indicator. We do expect, therefore, a greater burst of photochemical oxidation in vitro ( assessment of membrane breach) than the animal model ( assessment of apoptosis). The significance of the similar trends (wavelength and exposure duration) for irradiance reciprocity in the cell and animal models was the motivation for additional studies. We have recently completed an analysis (with computer simulations) at with varying exposure durations between 10 and that has begun to address some of the questions surrounding the transition in damage mechanisms from photothermal to photochemical.13 One inference from our analyses is that comparisons of threshold trends with respect to wavelength and exposure duration are useful for studying laser bioeffects, provided the method of damage assessment is consistent within the data set. For example, Payne 9 used an artificial retina model (in vitro) to replicate the in vivo trends in damage (minimum visible lesions in rhesus) from ultrashort laser pulses when a melanosome-based (in vitro) or ex vivo (RPE and choroid explant) model could not. In this case, because the artificial retina included a depth of water approximating the length of a rhesus eye, the same degree of nonlinear self-focusing of the ultrashort pulses occurred in both. We are presently using methods for in vitro laser exposure where temperature and humidity are controlled. This has led to a level of consistency in damage assessment necessary for accurate mathematical modeling of rate processes for both photothermal and photochemical damage. Using our in vitro data, we are beginning to incorporate photochemical rate processes into traditional thermal models in the hopes of generating a universal laser damage model. 4.ConclusionsThe described in vitro retinal model, with about /cell, accurately depicts how wavelength and exposure duration influence ocular laser damage in an animal model. The ranges of exposure duration and wavelength in which the apparent transition from photothermal to photochemical damage occurs ( and , respectively) are consistent between the in vitro and in vivo models. The pigment dependence of these trends for photochemical damage in vitro corroborates the conclusions of Ham 11 regarding the involvement of melanin in actinic damage processes in the retina. Although the current description is provided as an example of how the model can be used in studying dose response, we do not presume to present a complete mapping of the parameter space available. Additional wavelengths and exposure durations, as well as the effects of laser diameter on thermal damage, are all interesting parameters to pursue in future studies of the in vitro model. Our current results support the idea that the in vitro RPE cell model can be used to study the effects of pigmentation, lipofuscin, and a host of environmental factors as they relate to the described baseline response behavior. The cell model will facilitate studies on cellular response to lasers, such as oxidation, effects of aqueous interfaces, exact inflections between damage mechanisms, and molecular profiling (transcriptomics and proteomics). AcknowledgmentsAny opinions, interpretations, conclusions, and recommendations are not necessarily endorsed by the U.S. Air Force. We thank H. Hodnett and D. Stolarski for technical assistance. This work was supported by the Air Force Office of Scientific Research (Grant No. 92HE04COR). ReferencesW. T. Ham Jr., J. J. Ruffolo Jr., H. Mueller, D. Guerry III,
“The nature of retinal radiation damage: dependence on wavelength, power level and exposure time,”
Vision Res., 20 1105
–1111
(1980). https://doi.org/10.1016/0042-6989(80)90047-4 0042-6989 Google Scholar
W. T. Ham Jr., H. A. Mueller, M. J. Ruffolo Jr., A. M. Clarke,
“Sensitivity of the retina to radiation damage as a function of wavelength,”
Photochem. Photobiol., 29 735
–743
(1979). 0031-8655 Google Scholar
D. J. Finney, Probit Analysis, Cambridge University Press, New York
(1971). Google Scholar
C. P. Cain,
G. D. Noojin, and
L. Manning,
“A comparison of various probit methods for analyzing yes/no data on a log scale,”
(1996). Google Scholar
M. L. Denton,
M. S. Foltz,
L. E. Estlack,
D. J. Stolarski,
G. D. Noojin,
R. J. Thomas,
D. Eikum, and
B. A. Rockwell,
“Damage threshold for exposure to NIR and blue lasers in an in vitro RPE cell system,”
Invest. Ophthalmol. Visual Sci., 47 3065
–3073
(2006). https://doi.org/10.1167/iovs.05-1066 0146-0404 Google Scholar
M. L. Denton,
M. S. Foltz,
K. J. Schuster,
L. E. Estlack, and
T. J. Thomas,
“Damage thresholds for cultured retinal pigment epithelial cells exposed to lasers at and ,”
J. Biomed. Opt., 12 034030
(2007). https://doi.org/10.1117/1.2737394 1083-3668 Google Scholar
M. L. Denton,
M. S. Foltz,
K. J. Schuster,
L. E. Estlack,
H. M. Hodnett,
G. D. Noojin, and
R. J. Thomas,
“An in vitro model for retinal laser damage,”
Optical Interactions with Tissue and Cells XVIIProc. SPIE, 6435 643514
(2007)
(0277-786X) Google Scholar
W. D. Gibbons and
R. G. Allen,
“Retinal damage from long-term exposure to laser radiation,”
Invest. Ophthalmol. Visual Sci., 16 521
–529
(1977). 0146-0404 Google Scholar
D. J. Payne,
“Comparative study of laser damage threshold energies in the artificial retina,”
J. Biomed. Opt., 4 337
–344
(1999). https://doi.org/10.1117/1.429935 1083-3668 Google Scholar
D. J. Lund,
B. E. Stuck, and
P. Edsall,
“Retinal injury thresholds for blue wavelength lasers,”
Health Phys., 90 477
–484
(2006). https://doi.org/10.1097/01.HP.0000190115.83416.cb 0017-9078 Google Scholar
W. T. Ham Jr., J. J. Ruffolo Jr., H. A. Meuller,
A. M. Clarke, and
M. E. Moon,
“Histologic analysis of photochemical lesions produced in rhesus retina by short-wavelength light,”
Invest. Ophthalmol. Visual Sci., 17 1029
–1035
(1978). 0146-0404 Google Scholar
B. E. Stuck,
“The retina action spectrum for photoretinitis (“blue-light hazard”),”
Measurements of Optical Radiation Hazards, 193
–208 Maerkl-Druck, München
(1998). Google Scholar
M. L. Denton,
M. S. Foltz,
K. J. Schuster, C. D. Clark III, L. E. Estlack, and
R. J. Thomas,
“An in vitro model reveals a sharp transition between laser damage mechanisms,”
Google Scholar
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

