|
|
1.IntroductionA number of small clinical studies have shown that fluorescence spectroscopy can diagnose oral neoplasia with sensitivity and specificity in the range 80 to 100%.1, 2, 3, 4, 5 Clinical data indicate that neoplastic progression in the oral cavity is associated with a number of characteristic spectral changes, including a drop in fluorescence intensity and a shift in peak fluorescence emission to longer wavelengths. These trends are thought to be caused by a series of physiological changes that accompany neoplastic progression in oral mucosa, such as increased light scattering and metabolic activity of cells throughout the epithelium, angiogenesis, and the remodeling of the stromal matrix. While the pilot studies reported in Refs. 1, 2, 3, 4, 5 show promise, two factors are believed to limit the performance of spectroscopic algorithms for detection of oral neoplasia. First, the fluorescence properties of keratinized and nonkeratinized anatomic sites in the oral mucosa differ substantially.6 Diagnostic algorithms must therefore account for variations in the degree of epithelial keratinization; different algorithms may be required to identify precancerous lesions arising in different anatomic sites such as the keratinized gingiva and the nonkeratinized buccal mucosa. Second, the spectra of lesions with benign inflammatory changes can mimic those of oral premalignant lesions, reducing diagnostic specificity.7 A recent study used high-resolution autofluorescence confocal microscopy of fresh oral biopsies to investigate the biological basis for optical spectroscopy as a diagnostic aid for oral neoplasia and to help devise strategies to address these limitations.6 Precancerous tissues showed a loss of stromal autofluorescence and increased epithelial cell fluorescence compared to normal tissue; in contrast, lesions with chronic inflammation showed epithelial fluorescence similar to normal tissue, but exhibited a similar loss of stromal autofluorescence as lesions diagnosed with dysplasia.6 Thus, optical spectroscopy devices that separately record signals from the epithelium and stroma may improve the ability to distinguish benign inflammation from dysplasia. Improving the accuracy of fluorescence spectroscopy requires a detailed understanding of how changes in the morphology and biochemistry of a specific oral tissue site affect measured autofluorescence spectra. This understanding must include variations that occur with anatomic location, benign inflammation, and neoplasia. Monte Carlo modeling of light propagation in tissue can be an important tool to investigate the relationship between the optical properties of tissue and the resulting fluorescence emission spectrum. Monte-Carlo-based models have been used to study light propagation in multilayered tissue to investigate the effect of changes in optical properties on predicted spectra in several organ sites.8, 9, 10, 11 We designed a Monte Carlo model to predict fluorescence spectra of normal and neoplastic oral mucosa; this model incorporated a three-dimensional distribution of fluorescent sources determined by high-resolution fluorescence microscopy.12 We used this model to predict depth-resolved fluorescence spectra from normal and dysplastic oral mucosa.12 The model was validated by simulating average normal and dysplastic fluorescence spectra and comparing the predictions to average clinical data. Spectra of neoplastic tissue predicted using Monte Carlo analysis had a lower intensity and a longer peak emission wavelength than those predicted for normal oral mucosa; these predictions are consistent with changes observed in the average spectra measured from normal and neoplastic oral mucosa. However, sensitivity analysis of Monte Carlo predictions suggests that results of the depth-sensitive Monte Carlo model are sensitive to changes in the optical properties of the epithelium and the superficial stroma.12 Small changes in the total epithelial thickness, epithelial keratinization, and fluorescence intensity of the superficial stroma can lead to significant changes in the shape and intensity of predicted spectra. Thus, while Monte Carlo predictions of spectra of normal and neoplastic oral tissue agree with average clinical data, it has been difficult to assess whether Monte Carlo models can accurately predict the spectrum of a particular tissue site, given information about the three-dimensional distribution of chromophores within that particular tissue. The goal of this study was to evaluate the ability of a Monte Carlo model with site-specific input properties to predict individual depth-resolved spectra from normal, inflammatory, and neoplastic oral mucosa. Fluorescence spectra were measured in vivo from clinically normal and abnormal sites in the oral mucosa. Measured sites were biopsied, and high resolution fluorescence confocal microscopy was used to record the autofluorescence patterns as a function of depth at each individual site. These fluorescence distributions were used as input to the Monte Carlo model to predict tissue fluorescence spectra at each individual site. Predicted spectra were compared to measured spectra on a site-by-site basis. The results of the Monte Carlo model were used to elucidate possible biological reasons for variations in clinical depth-resolved spectra measured from normal, inflammatory, and neoplastic oral tissue. We show that developing an accurate modeling approach to predict site-specific fluorescence spectra could improve our understanding of how morphological and biochemical changes associated with specific benign, precancerous, and cancerous conditions influence the shape and intensity of clinically measured spectra. While validation of this model requires input obtained from biopsy, once validated, the model can be used to aid in designing optical instrumentation and diagnostic algorithms to noninvasively distinguish among spectra measured from benign, dysplastic, and cancerous sites. 2.Methods2.1.OverviewThe study was reviewed and approved by the Institutional Review Boards at the University of Texas at Austin, the University of Texas M.D. Anderson Cancer Center, and Rice University. Fluorescence spectra were measured in vivo from clinically normal and abnormal oral sites using a fiber optic probe that preferentially collects fluorescent light generated in the epithelium and superficial stroma. Biopsies were obtained from measured sites, and high resolution fluorescence confocal microscopy was used to record autofluorescence patterns as a function of depth at each site. Using these site-specific fluorescence distributions as input to a Monte Carlo model, we predicted the tissue fluorescence spectrum for each site. Predicted fluorescence spectra for six oral sites with different pathological diagnoses and/or anatomical types were compared to the clinical spectra measured at each site. 2.2.Depth-Sensitive Monte Carlo Modeling of Oral MucosaA fixed weight Monte Carlo program was used to simulate the collection of fluorescence spectra using a fiber optic probe designed to preferentially collect fluorescence generated in the epithelium and superficial stroma. The program was designed to account for generation and propagation of fluorescent light in multilayered oral tissue. Details regarding the Monte Carlo code and the depth-sensitive fiber optic probe geometry have been described previously.12 Briefly, excitation photons were initially propagated through the tissue using the scattering coefficient and absorption coefficient at the excitation wavelength according to the layer they are traveling through. A photon has a probability of being absorbed at a scattering site. If a photon is absorbed by a fluorophore, an emission photon may be generated and is propagated according to the and for the emission wavelength in the layer in which it is traveling. The photons are propagated until they reach the surface or are reabsorbed. The number of photons that reach the surface and are detected by a detector are calculated. A single simulation output predicts the fluorescence emission at excitation, and consists of the detected fluorescence intensity (total number of detected fluorescence photons) at emission wavelengths of 400, 420, 440, 450, 460, 480, 500, 520, 540, and . Each simulation was performed with excitation photons. For each detected photon, the depth at which fluorescence emission occurred was recorded. The fraction of detected photons originating in the epithelium and the fraction originating in the stroma were calculated. 2.3.Clinical Measurements, Biopsy Collection, and High Resolution Tissue ImagingFluorescence emission spectra at excitation were measured from clinically normal and abnormal sites using a depth-sensitive fiber optic probe. Detailed descriptions of the clinical spectroscopic device, the depth-sensitive fiber optic probe, and procedures for patient recruitment, data acquisition, calibration, and processing have been reported in Ref. 5. The optical probe contains four depth channels, each consisting of a set of illumination and collection fibers arranged in a geometric configuration designed to preferentially collect light from a specific depth region in the tissue. The shallow channel, used to collect the clinical data presented here, uses a ball lens at the tip of the probe to redirect the angle of the illumination light such that its intersection with the collection region is localized to the tissue surface. This enables preferential collection of signal from the epithelium and superficial stroma. Data from six sites in three patients were used to validate the Monte Carlo model. To explore variations in spectroscopic data with the presence of benign inflammation and neoplasia, data were selected from a patient with measurements made from four sites in the tongue with histologic diagnoses of normal, inflammation, dysplasia, and cancer. To explore variations with tissue anatomic sites, measurements were selected from two additional patients with measurements of normal palate and normal buccal mucosa. After spectroscopic measurement, each site was biopsied and the tissue sample was divided into two portions. One portion of each sample was sent for histologic examination. Transverse -thick tissue slices were immediately prepared from the second portion of each sample and imaged with a confocal fluorescence microscope while the tissue was still viable. Confocal images were obtained at ultraviolet (UV 350 and ) excitation and at excitation to determine input parameters to the Monte Carlo model for that site. Detailed procedures for tissue preparation, confocal imaging, and quantitative analysis of autofluorescence patterns are described in Ref. 6. 2.4.Tissue Geometry and Model Input ParametersFor normal, inflammatory, and dysplastic sites, tissue was modeled as a multilayered turbid medium with five sublayers: superficial epithelium, intermediate epithelium, basal epithelium, superficial stroma, and deep stroma.6, 12 Each sublayer was assumed to contain a single dominant fluorophore, characteristic of that sublayer and diagnosis. At each site, the UV confocal fluorescence images were analyzed to determine the thickness and average fluorescence intensity of each sublayer. These values were used as site-specific input to the Monte Carlo model. Previous work has shown that the superficial epithelium includes cells that retain keratin, and the main fluorophore in this layer was assumed to be keratin.6 The primary fluorophore in the intermediate epithelium was assumed to be mitochondrial flavin adenine dinucleotide (FAD).6 The dominant fluorophore in the basal epithelium was assumed to be mitochondrial reduced nicotinamide adenine dinucleotide (NADH).6 For the normal and dysplastic sites, the primary fluorophores in both the superficial and deep stroma were assumed to be collagen crosslinks.13 In oral lesions with severe stromal inflammation, confocal microscopy studies demonstrate stromal fluorescence that appears to originate from inflammatory cells with strong cytoplasmic fluorescence at UV excitation. Thus for the inflammatory site considered in this study, the dominant stromal fluorophore was assumed to be mitochondrial NADH. The site diagnosed as invasive cancer was modeled using a different tissue geometry, because the normal structure is lost with invasion. This site was modeled as a turbid medium with cellular and fibrous layers. The geometry was derived from visual examination of the confocal images obtained from the measured site. Five sublayers were defined: cellular region 1, with dominant keratin fluorescence and optical properties similar to noncancerous superficial epithelium; cellular region 2, with weak cytoplasmic fluorescence from FAD; cellular region 3, with bright cytoplasmic fluorescence from NADH; matrix region 1, with dominant NADH fluorescence associated with a significant cellular component consisting of cancer, inflammatory, and atypical cells; and matrix region 2, a fibrous region with collagen as the primary fluorophore and optical properties similar to those of noncancerous deep stroma. The scattering coefficients of the epithelial and stromal layers were obtained from previous studies of oral, cervical, and bronchial tissue. The values of the scattering coefficients at and absorption coefficients at are summarized in Table 1 . The scattering coefficient of the intermediate and basal epithelium was derived from reflectance confocal images of normal, dysplastic, and cancerous nonkeratinized oral epithelium.14 The scattering coefficient of the superficial epithelium in all oral sites considered in this study was derived from reflectance confocal measurements of cervical keratinized epithelium.15 In normal oral tissue, the scattering coefficients for the superficial and deep stromal layers were assumed to be the same and were based on values from Ref. 16. To accurately model the drop in stromal scattering associated with oral dysplasia, the scattering coefficient in the superficial stroma in the dysplastic case was decreased by 20%.17 Scattering coefficients obtained from the literature were extrapolated to lower wavelengths using the inverse proportionality relationship of cellular scattering to wavelength.18 Table 1Scattering and absorption coefficients for each oral tissue site and sublayer. Values were used as input to the Monte Carlo model.
The absorption coefficient for the epithelium was assumed to be constant for all sites considered in this study and was based on values derived in Ref. 16. The stromal absorption coefficient used in this model has contributions from three different absorbers: collagen, oxyhemoglobin, and deoxyhemoglobin. The collagen absorption coefficient was based on measurements from bloodless skin samples.19 The oxy- and deoxyhemoglobin absorption coefficients were calculated as described by Jacques.20 An oxygen saturation level of 80% and a hemoglobin concentration of were used. For the normal tongue site and the dysplastic tongue site, the volume fraction of blood in the stroma was determined from quantitative analysis of images from hematoxylin and eosin (HE) stained slides from that specific site. HE images of these sites were analyzed to estimate the fraction of the total stromal area occupied by blood. The estimated value included areas occupied by the lumen of blood vessels. The volume fraction of blood in the superficial stromal layer of the normal tongue site was estimated to be in the range 0.05 to 0.1%, while the value for deep stroma was in the range 0.2 to 0.3%. The superficial stromal layer underlying the epithelium in the dysplastic tongue site was estimated to have a volume fraction of blood in the range 0.10 to 0.20%, while the value for deep stroma was similar to that of the normal case. The values obtained for the normal tongue site were used for the other normal sites as well. For the cancer site and the inflammatory site, the volume fraction of blood in the stroma was estimated based on information from the literature. For the cancer site, the volume fraction of blood was estimated as four times higher than that of normal tissue, based on previous research indicating that the microvessel density of oral cancers is about three to four times higher than in normal oral tissue.21 For the inflammatory site, the volume fraction of blood was estimated to be similar to the blood volume fraction of normal tissue. 3.Results3.1.Site-Specific Spectral Changes Caused by Neoplastic Progression in Oral MucosaFigure 1 shows confocal fluorescence images and measured clinical spectra obtained at UV excitation from four different sites on the tongue of a single patient. One of the four sites was clinically and histologically normal. The other three sites were oral lesions that were diagnosed histologically as inflammation, dysplasia, and cancer, respectively. The normal oral tissue is characterized by strong epithelial and stromal autofluorescence [Fig. 1a]. In contrast, the oral lesion diagnosed with severe inflammation displays a large drop in both epithelial and stromal fluorescence [Fig. 1b]. In addition, while stromal fluorescence in the normal tissue originates from collagen fibers, fluorescence from the inflammatory stroma originates predominantly from a large influx of inflammatory cells. The oral lesion diagnosed with dysplasia is characterized by increased epithelial thickness, brightly fluorescent cells throughout the epithelium, and a drop in fluorescence of the superficial stroma [Fig. 1c]. Confocal images of a malignant lesion diagnosed with moderately differentiated cancer [Figure 1d] illustrate the loss of layered architecture typical for cancers. In this example, the superficial of the tissue are occupied by cancer cells; underneath is a region of matrix infiltrated by cancer and inflammatory cells. Fig. 1(a) to (d) Fluorescence confocal images and (e) measured fluorescence spectra at UV excitation, showing neoplastic progression in the tongue. (a) Normal tongue; (b) normal tongue with severe stromal inflammation; (c) focal mild dysplasia; and (d) moderately differentiated cancer. In (a), (b), and (c), the white lines represent the basement membrane and the yellow lines represent the tissue surface. Scale bars represent . (e) Fluorescence spectra at excitation, measured from the same oral sites shown in the confocal images. Images (b) and (c) are reproduced from Ref. 6 with permission. 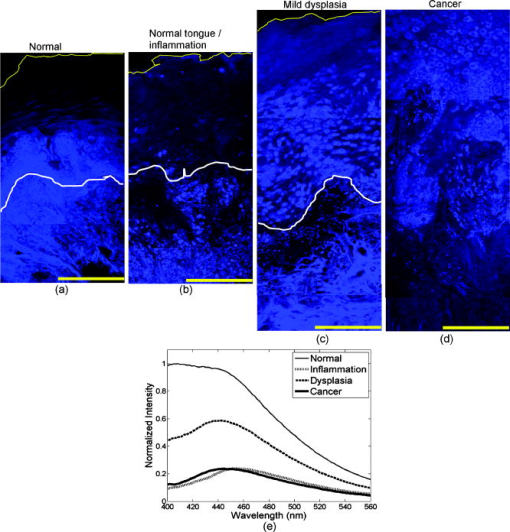 Clinically measured emission spectra at excitation from the four oral sites are displayed in Fig. 1e. The data illustrate the characteristic drop in overall intensity associated with neoplastic progression in oral mucosa. Spectra from the dysplastic and cancerous sites also have lower relative emission intensity in the wavelength region, giving them a different spectral shape compared to the normal case. The spectrum from the inflammatory site displays both a drop in fluorescence intensity and a shift to a longer peak emission wavelength compared to the normal spectrum. Table 2 summarizes the site-specific tissue properties that were derived from the confocal fluorescence images of each site. At a given site, each of the five tissue sublayers was assigned a thickness, a dominant fluorophore, and a relative fluorescence intensity based on quantitative analysis of the confocal fluorescence images. Depth-resolved spectra for all tissue sites were simulated with the Monte-Carlo-based model at excitation using the input values shown in Tables 1, 2. Figure 2 compares the predicted spectra to the clinically measured spectra of the four tongue sites. The simulated spectra accurately predict the drop in fluorescence intensity and changes in spectral shape displayed in this example of neoplastic progression in oral mucosa [Figs. 2a, 2b, 2c, 2d]. Fig. 2Comparison of Monte Carlo model predictions with clinical fluorescence spectra measured from four oral tongue sites in the same patient. Clinical data and predictions at excitation from (a) normal tongue, (b) normal tongue with severe inflammation, (c) focal mild dysplasia, and (d) moderately differentiated cancer. Bar graphs showing the predicted fraction of detected photons originating from the epithelium and the stroma for the (e) normal, (f) inflammatory, and (g) dysplastic simulations. (h) Bar graph showing the predicted fraction of detected photons originating from the cellular region (upper , labeled as epithelium on the bar graph) and the deeper fibrous regions (labeled as stroma on the bar graph) for the cancer simulation. 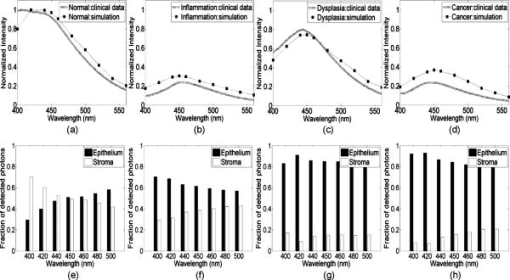 The fraction of photons originating from the epithelium and the fraction originating from the stroma were calculated for each simulation [Figs. 2e, 2f, 2g, 2h]. (In the normal case [Fig. 2e], the detected fluorescence includes both a significant epithelial and stromal component. For example, at , the fraction of photons originating from the epithelium is approximately equal to the fraction of photons originating from the stroma, and the stromal contribution dominates the total fluorescence in the region. With the presence of inflammation [Fig. 2f], the fraction of photons originating from the stroma substantially drops, while the fraction of epithelial photons increases. It should be noted that the decreased fraction of stromal photons is due to a large reduction in fluorescence originating from the stroma and not due to an increase in fluorescence originating from the epithelium. Thus the overall intensity of the predicted spectrum from the inflammation site is very low compared to the simulations from normal tissue. In the dysplastic tissue sample [Fig. 2g], the fraction of photons originating from the epithelium is very high for all emission wavelengths. Here, the high fraction of epithelial photons is due to a very large reduction of stromal fluorescence and a significant increase of epithelial fluorescence compared to the normal case. As a result, the overall fluorescence intensity of the simulated dysplastic spectrum is lower than the normal case but not as low as in the inflammation case. Thus, while both the dysplastic and inflammatory lesions show a drop in stromal fluorescence, the overall fluorescence intensity and fraction of epithelial photons of the dysplastic tissue are greater than that of the inflammatory tissue due to the increase in fluorescence originating from the dysplastic epithelium. In the cancerous tissue case [Fig. 2h], the fraction of fluorescence from the deep fibrous region is extremely low, and the overall fluorescence intensity is low as well. This is due to sharply reduced fluorescence from the deeper matrix region and slightly reduced fluorescence from the upper cellular regions of the cancer lesion relative to the analogous regions in normal tissue. 3.2.Site-Specific Spectral Changes Caused by Variations in KeratinizationFigure 3 shows high resolution confocal images of normal oral mucosa taken from two different anatomic sites that display different epithelial fluorescence patterns. The normal buccal site [Fig. 3a] is characterized by a weakly fluorescent superficial layer, while the normal palate site [Fig. 3b] displays a brightly fluorescent superficial epithelium. Clinical fluorescence spectra at excitation measured from both sites are displayed in Fig. 3c. Fig. 3(a) and (b) Fluorescence confocal images and (c) measured fluorescence spectra at UV excitation, showing changes in autofluorescence patterns due to anatomic site variation. (a) Normal buccal mucosa; and (b) normal palate. In (a) and (b), the white lines represent the basement membrane and the yellow lines represent the tissue surface. Scale bars represent . (c) Fluorescence spectra at excitation, measured from the same oral sites shown in the confocal images. 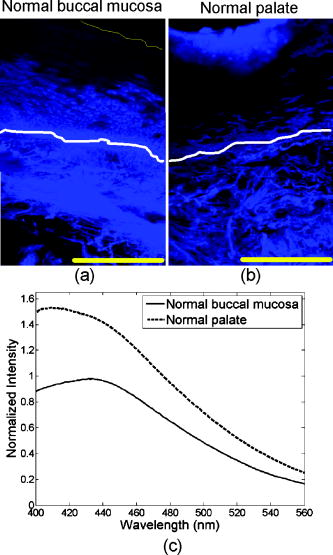 Figure 4 compares the Monte Carlo predictions to the clinically measured spectra of the normal buccal mucosa and normal palate sites. The fluorescence spectrum of the palate site has greater intensity and is blue shifted relative to the spectrum measured from the buccal site. These trends are accurately predicted by the model [Figs. 4a and 4b]. Predictions for the buccal mucosa site are within 10% of the measured spectra. However, the predicted intensity of the palate site is lower than that measured in vivo, possibly due to experimental error in estimating the thickness of the superficial epithelial layer. Previous model sensitivity analysis performed with the depth-sensitive Monte Carlo model has shown that even variations of in the thickness of the superficial layer can result in a noticeable effect on the total intensity of the prediction.12 The estimated thickness of the strongly fluorescent keratin layer from the confocal images has a large standard deviation, which could explain the difference in intensity between the simulated and clinical spectra. Fig. 4Comparison of Monte Carlo model predictions with clinical fluorescence spectra measured at different anatomic sites in the oral cavity. Clinical data and predictions at excitation from (a) normal buccal mucosa and (b) normal palate. Bar graphs showing the predicted fraction of detected photons originating from the epithelium and stroma for (c) normal buccal mucosa and (d) normal palate. 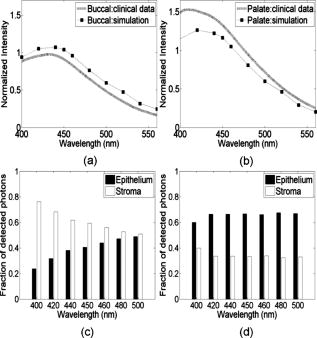 Table 2Thickness, dominant fluorophore, and relative fluorescence intensity for each oral tissue site and sublayer. Values were used as input to the Monte Carlo model.
Figure 4 also summarizes the fraction of photons originating from the epithelium and the stroma derived from depth analysis of the simulated spectra. The buccal mucosa has a large fraction of photons originating from the stroma [Fig. 4c], compared to the palate site [Fig. 4d]. Here, the larger fraction of epithelial photons can be attributed mainly to increased fluorescence from the epithelial layer of the palate site. Additional analysis (data not shown) indicates that the increased epithelial fluorescence originates from the superficial, keratinized region of the epithelium in the palate site. We suggest that the presence of a brightly fluorescent keratin layer in the palate site increases the fluorescence originating from the superficial epithelium compared to the buccal site, which accounts for the higher intensity of the palate spectrum compared to the buccal spectrum. The increased contribution from keratin also affects the spectral shape of the fluorescence from the palate site. The measured spectrum of the palate site shows similarities to the emission spectrum of keratin, which displays a peak at .12 4.DiscussionOur results demonstrate that a Monte Carlo modeling approach with site-specific tissue parameters measured using confocal fluorescence microscopy can predict variations in clinically measured fluorescence spectra of oral tissue. Further, the model shows how specific morphological and biochemical differences among anatomic sites and disease states can affect the fluorescence signal. The output of Monte-Carlo-based models is dependent on the input parameters and geometry used to represent the tissue of interest. Multilayered Monte Carlo models tend to have a large number of input parameters and may produce similar outputs with different sets of parameters. Some of these predictions, while agreeing well with clinical data, could be physiologically unrealistic. The use of experimentally derived site-specific input parameters can improve the biological validity of the Monte-Carlo-based predictions. The approach described in this study enables the prediction of spectra using optical characteristics specific for the tissue site of interest, rather than using general tissue properties for a given anatomic site and disease status. This brings the model closer to representing the actual tissue that produced the measured spectra. In addition, it allows for a more accurate analysis of the relationship between alterations in optical properties of a specific oral lesion and the fluorescence spectra measured from that lesion. This approach is useful primarily for exploring the origins of autofluorescence in normal and abnormal tissue; it depends, of course, on the availability of a tissue sample from the measured site. The site-specific Monte Carlo model accurately predicted the general trends in fluorescence intensity and spectral shape observed in the clinical data from the six sites analyzed in this study (Figs. 2 and 4). Agreement between the predicted and measured spectra was closest for the nonkeratinized oral sites with layered morphology: the normal and dysplastic tongue sites and the normal buccal mucosa. The prediction was slightly less accurate for the keratinized site (normal palate) and for the lesions diagnosed with inflammation and cancer. Determination of accurate tissue geometry from confocal images is more difficult for cases with large local differences in morphology, such as the cancer lesion, or in cases with variations in the thickness of a strongly fluorescent superficial epithelium, such as the palate site. Thus, spectra from keratinized or cancerous sites may be more challenging to predict accurately using this model than spectra from nonkeratinized sites with layered morphology. In this study, variations were observed in the spectra measured from normal tissue at different anatomic sites. Analysis of the site-specific predictions shows that the origins of the detected autofluorescence are different in the nonkeratinized and keratinized sites. For the nonkeratinized normal sites (buccal mucosa and tongue), the measured signal was attributed to a combination of stromal fluorescence and epithelial fluorescence. For the keratinized normal site (palate), the blue shift and increase in overall fluorescence intensity were attributed to a significant increase in the contribution of photons originating from the superficial keratinized layer to the total detected signal. These observations are consistent with reports in the literature that superficial epithelium in oral mucosa can vary significantly in the degree of keratinization depending on the anatomic site, and that these variations significantly affect autofluorescence spectra. Muller reported differences in fluorescence spectra measured in vivo from keratinized and nonkeratinized oral mucosa,2 related to a reduction in the depth of penetration of excitation light due to scattering from the keratin layer. De Veld reported that the different degree of keratinization displayed by oral lesions is a factor that complicates accurate differentiation of benign from malignant oral lesions using autofluorescence spectroscopy.7 The site-specific predictions of the normal, dysplastic, and cancerous fluorescence spectra displayed a decrease in fluorescence intensity and a shift to longer peak emission wavelength with neoplastic progression. Analysis of the origin of predicted fluorescence indicated that the lower fluorescence intensity seen in the dysplastic and cancerous spectra was due predominantly to a decreased fluorescence originating from the stroma or deeper fibrous regions, whereas the spectral red shift was attributed to an increase in the fraction of photons originating from the epithelium or superficial cellular layers. These results were similar to those obtained in a previous study, in which the depth-dependent Monte Carlo model was used to predict average normal and dysplastic oral fluorescence spectra.12 In addition, results from a mathematical model analysis for extracting the intrinsic fluorescence spectra from in-vivo fluorescence spectra from the oral cavity and the cervix2, 22 confirm that malignant progression is characterized by an increase in the epithelial contribution and a decrease in the stromal contribution to the total detected fluorescence. The Monte Carlo simulations of spectra from the inflammatory site and the cancer site are of particular interest, since inflammation can be a confounding factor in spectroscopic diagnosis of neoplasia. As shown in Fig. 2, the intensity and spectral shape of the measured fluorescence spectra are quite similar for the inflammatory and cancer sites. However, the origin of the signal is different in the two cases. The fluorescence signals from both the inflammatory and cancer sites are characterized by low stromal fluorescence and moderately low epithelial fluorescence relative to normal tissue. The cancer site, however, displays a more pronounced drop in stromal fluorescence compared to the inflammation site. In addition, the contribution from the cellular regions is higher in the cancer site than the epithelial contribution in the inflammatory site. Thus, while the total predicted fluorescence from the cancer site is dominated by epithelial photons, the spectrum from the inflammatory lesion has a significant stromal component. While these results are specific for a particular example of neoplastic progression in the oral tongue, they suggest that depth-sensitive spectroscopy, with further improvements in depth resolution and instrumentation to fully isolate the signal originating from the epithelium and/or stroma, may provide a potentially useful method to distinguish inflammation from neoplasia. In addition to using the model to suggest an optimal depth to improve differentiation between inflammation and cancer, similar analysis can be done to improve differentiation between the varying degrees of dysplasia and cancer. Another important application for this model is determining whether different anatomical sites can be grouped together for diagnosis, or whether inherent differences such as keratinization cause the spectra to differ enough so that the classification is compromised. The model can address this by studying the spectral effect caused by a keratin layer of varying thickness compared to the changes cause by neoplastic progression. If there is a depth at which the spectral signature of the neoplastic progression is independent of the keratin layer, then a device that measures at that depth would not be sensitive to the anatomical site keratinization. The results of this study indicate that a Monte Carlo model with site-specific input parameters extracted from confocal images can accurately predict depth-resolved fluorescence spectra from individual oral sites. The model provides information regarding the origin of detected photons within different tissue sublayers. This information may be useful in designing strategies to improve the ability of optical spectroscopy to accurately distinguish among normal, benign, and malignant oral sites. AcknowledgmentsThe authors thank Adel K. El-Naggar for providing pathology diagnoses, and Bimal Patel and Erica M. Smith for performing clinical measurements. The authors gratefully acknowledge support from National Institutes of Health grant R01 CA095604. ReferencesD. L. Heintzelman, U. Utzinger, H. Fuchs, A. Zuluaga, K. Gossage, A. M. Gillenwater, R. Jacob, B. Kemp, and R. R. Richards-Kortum,
“Optimal excitation wavelengths for in vivo detection of oral neoplasia using fluorescence spectroscopy,”
Photochem. Photobiol., 72
(1), 103
–113
(2000). https://doi.org/10.1562/0031-8655(2000)072<0103:OEWFIV>2.0.CO;2 0031-8655 Google Scholar
M. G. Muller, T. A. Valdez, I. Georgakoudi, V. Backman, C. Fuentes, S. Kabani, N. Laver, Z. Wang, C. W. Boone, R. R. Dasari, S. M. Shapshay, and M. S. Feld,
“Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma,”
Cancer, 97 1681
–1692
(2003). 0008-543X Google Scholar
D. C. G. de Veld, M. Skurichina, M. J. H. Witjes, R. P. W. Duin, H. J. C. M. Sterenborg, and J. L. N. Roodenburg,
“Autofluorescence and diffuse reflectance spectroscopy for oral oncology,”
Lasers Surg. Med., 36 356
–364
(2005). https://doi.org/10.1002/lsm.20122 0196-8092 Google Scholar
C. Y. Wang, T. Tsai, H. M. Chen, C. T. Chen, and C. P. Chiang,
“PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis,”
Lasers Surg. Med., 32 318
–326
(2003). https://doi.org/10.1002/lsm.10153 0196-8092 Google Scholar
R. A. Schwarz, W. Gao, D. Daye, M. D. Williams, R. Richards-Kortum, and A. M. Gillenwater,
“Autofluorescence and diffuse reflectance spectroscopy of oral epithelial tissue using a depth-sensitive fiber-optic probe,”
Appl. Opt., 47
(6), 825
–834
(2008). https://doi.org/10.1364/AO.47.000825 0003-6935 Google Scholar
I. Pavlova, M. Williams, A. El-Naggar, R. Richards-Kortum, and A. Gillenwater,
“Understanding the biological basis of autofluorescence imaging for oral cancer detection: High-resolution fluorescence microscopy in viable tissue,”
Clin. Cancer Res., 14 2396
–2404
(2008). 1078-0432 Google Scholar
D. C. G. de Veld, M. Skurichina, M. J. H. Witjes, R. P. W. Duin, H. J. C. M. Sterenborg, and J. L. N. Roodenburg,
“Clinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopy,”
J. Biomed. Opt., 9
(5), 940
–950
(2004). https://doi.org/10.1117/1.1782611 1083-3668 Google Scholar
S. K. Chang, N. Marin, M. Follen, and R. Richards-Kortum,
“Model-based analysis of clinical fluorescence spectroscopy for in vivo detection of cervical intraepithelial dysplasia,”
J. Biomed. Opt., 11
(2), 024008
(2006). https://doi.org/10.1117/1.2187979 1083-3668 Google Scholar
D. Arifler, C. MacAulay, M. Follen, and R. Richards-Kortum,
“Spatially resolved reflectance spectroscopy for diagnosis of cervical precancer: Monte Carlo modeling and comparison to clinical measurements,”
J. Biomed. Opt., 11
(6), 064027
(2006). https://doi.org/10.1117/1.2398932 1083-3668 Google Scholar
J. Qu, C. MacAulay, S. Lam, and B. Palcic,
“Laser-induced fluorescence spectroscopy at endoscopy: tissue optics, Monte Carlo modeling, and in vivo measurements,”
Opt. Eng., 34
(11), 3334
–3343
(1995). https://doi.org/10.1117/12.212917 0091-3286 Google Scholar
D. Hidovic-Rowe and E. Claridge,
“Modelling and validation of spectral reflectance for the colon,”
Phys. Med. Biol., 50 1071
–1093
(2005). https://doi.org/10.1088/0031-9155/50/6/003 0031-9155 Google Scholar
I. Pavlova, C. R. Weber, R. A. Schwarz, M. Williams, A. El-Naggar, A. Gillenwater, and R. Richards-Kortum,
“Monte Carlo model to describe depth selective fluorescence spectra of epithelial tissue: Applications for diagnosis of oral precancer,”
J. Biomed. Opt., 13 064012
(2008). https://doi.org/10.1117/1.3006066 1083-3668 Google Scholar
K. Sokolov, J. Galvan, A. Myakov, A. Lacy, R. Lotan, and R. Richards-Kortum,
“Realistic three-dimensional epithelial tissue phantoms for biomedical optics,”
J. Biomed. Opt., 7
(1), 148
–156
(2002). https://doi.org/10.1117/1.1427052 1083-3668 Google Scholar
A. L. Clark, A. Gillenwater, R. Alizadeh-Naderi, A. K. El-Naggar, and R. Richards-Kortum,
“Detection and diagnosis of oral neoplasia with an optical coherence microscope,”
J. Biomed. Opt., 9
(6), 1271
–1280
(2004). https://doi.org/10.1117/1.1805558 1083-3668 Google Scholar
T. Collier, M. Follen, A. Malpica, and R. Richards-Kortum,
“Sources of scattering in cervical tissue: determination of the scattering coefficient by confocal microscopy,”
Appl. Opt., 44
(11), 2072
–2081
(2005). https://doi.org/10.1364/AO.44.002072 0003-6935 Google Scholar
J. Qu, C. MacAulay, S. Lam, and B. Palcic,
“Optical properties of normal and carcinomatous bronchial tissue,”
Appl. Opt., 33
(31), 7397
–7405
(1994). 0003-6935 Google Scholar
D. Arifler, I. Pavlova, A. Gillenwater, and R. Richards-Kortum,
“Light scattering from collagen fiber networks: micro-optical properties of normal and neoplastic stroma,”
Biophys. J., 92 3260
–3274
(2007). https://doi.org/10.1529/biophysj.106.089839 0006-3495 Google Scholar
J. R. Mourant, J. P. Freyer, A. H. Hielscher, A. A. Eick, D. Shen, and T. M. Johnson,
“Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics,”
Appl. Opt., 37
(16), 3586
–3593
(1998). https://doi.org/10.1364/AO.37.003586 0003-6935 Google Scholar
I. S. Saidi,
“Transcutaneous optical measurement of hyperbilirubinemia in neonates,”
Rice University,
(1992). Google Scholar
S. L. Jacques,
“Skin optics,”
Oregon Medical Laser Center News,
(1998) http://omlc.ogi.edu/news/jan98/skinoptics.html Google Scholar
M. Macluskey, L. M. Chandrachud, S. Pazouki, M. Green, D. M. Chisholm, G. R. Ogden, S. L. Schor, and A. M. Schor,
“Apoptosis, proliferation, and angiogenesis in oral tissues. Possible relevance to tumour progression,”
J. Pathol., 191 368
–375
(2000). https://doi.org/10.1002/1096-9896(2000)9999:9999<::AID-PATH652>3.0.CO;2-Y 0022-3417 Google Scholar
I. Georgakoudi, B. C. Jacobson, M. G. Muller, E. E. Sheets, K. Badizadegan, D. L. Carr-Locke, C. P. Crum, C. W. Boone, R. R. Dasari, J. Van Dam, and M. S. Feld,
“NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes,”
Cancer Res., 62 682
–687
(2002). 0008-5472 Google Scholar
|

