|
|
|
Advances in high-resolution imaging capabilities and fluorescent probe development have enabled the study of physiological processes within single living cells. Intracellular monitoring of pH, calcium, chloride, and oxygen provide an opportunity to create real-time descriptions of the biomolecular cellular state.1, 2, 3 Calibration of fluorescent sensors sensitive to these molecules is necessary for accurate quantitative results. A recurring challenge is the inability to calibrate the fluorescent probe in a medium that truly mimics the intracellular environment. Excited-state fluorophore lifetime (and, hence, fluorescence intensity) is inherently dependent on microenvironment, which makes reproducing the intracellular molecular environment essential for accurate calibration. Toward this end, several types of solvents have been employed, including phosphate buffer saline (PBS), varied culture media, and even poisoned cells.2, 4, 5 These approaches are either simplistic or raise concern about cellular health and response. Coupled with the fact that different cell lines can vary significantly in their intracellular composition, any calibration of the fluorescent probe is unlikely to be universally applicable. The methods discussed here aim to provide a generic protocol for accurate intracellular oxygen sensing with any oxygen-sensitive fluorophore. The protocol developed employs a calibration correction derived from optical measurements on cellular lysate, which is representative of the intracellular environment and commonly used to recover enzymes and proteins in their active states. Oxygen is the terminal electron acceptor of oxidative phosphorylation in mitochondria and a key indicator of metabolic function in aerobic cells and organisms. Information obtained from accurate oxygen estimation has been applied to study mitochondrial function, signaling pathways, effects of various stimuli, membrane permeability, disease differentiation, and to screen for new drugs.6, 7, 8, 9 Various methods exist for estimating oxygen at different scales, whether in whole organs [magnetic resonance imaging (MRI) and nuclear magnetic resonance (NMR)], blood (absorption), tissues [electrochemical microelectrodes], or individual cells (phosphorescence, fluorescence, [electron paramagnetic resonance (EPR)].10, 11, 12, 13 Optical approaches, such as fluorescence microscopy, provide the most viable approach for intracellular oxygen sensing with high sensitivity and subcellular spatial resolution. Fluorescence lifetime-based methods additionally circumvent most complications associated with intensity-based studies, such as photobleaching, optical loss, and variable fluorophore concentration in cells.14 Figure 1 shows a representative intensity-overlay fluorescence lifetime imaging microscopy (FLIM) image of the fluorescent oxygen sensor ruthenium tris(2,2’-dipyridyl) dichloride hexahydrate (RTDP) in living cells. Such lifetime maps serve as the input to the Stern–Volmer equation for estimation of oxygen levels via collisional quenching. The oxygen sensitivity of RTDP was calibrated at in PBS and applied to extracellular and intracellular oxygen measurements using FLIM.2, 15 Here, for the first time, lysate-FLIM studies were performed to refine this calibration and to make it sample specific. All fluorescence-based oxygen measurements were then compared to EPR. Fig. 1Intensity-overlay FLIM image of the oxygen sensor RTDP in living cells ( diam). RTDP lifetime maps serve as the input to the Stern–Volmer equation for estimation of oxygen levels. 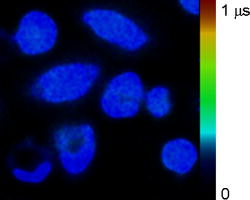 Cellular lysate of HET-1 (human normal squamous esophageal epithelial cells) and SEG-1 (human Barrett’s adenocarcinoma esophageal epithelial cells) were generated using the NP-40 buffer via the following protocol. Briefly, NP-40 was thawed on ice and of Halt Protease Inhibitor was added for inhibition of serine-, cysteine-, metallo-, and aspartic acid-proteases in addition to aminopeptidases. Because of DNA/RNA release, each of DNAse and RNAse was added to reduce viscosity. Cells grown in culture flasks were scraped off into PBS, centrifuged, and washed twice with ice-cold PBS. The NP-40 formulation was added in a ratio of buffer/ cells. The solution was placed on ice and vortexed for every . After , the solution was transferred to microcentrifuge tubes and run at 13,000 rpm for at . This allowed the heavier debris (e.g., cell membrane components) to sediment; the clear aliquot was removed and frozen at . For lysate-FLIM experiments, of lysate was combined with of RTDP in a heated dish and lifetime measurements were immediately taken, as described previously.2 EPR oximetry is a spectroscopic technique to detect materials with unpaired electrons, such as oxygen.11 Microcrystalline LiNc–BuO particles were suspended in of minimum essential medium (MEM). A probe sonicator was used to create particles with sizes by pulsing the sample 10 times for each, with cooling time (on ice) between successive pulses. The sample was placed on ice for to allow heavier, larger particles to settle. The supernatant liquid was then aliquoted out. For treatment with the EPR particles, both HET and SEG were grown to 70% confluence in dishes, then suspended in MEM. To this suspension, of the LiNc–BuO suspension was added and the cells were incubated for . The media were then replaced, the cells were resuspended, and of the suspension ( cells) was drawn into a glass tube. Previous reports have indicated successful internalization of LiNc–BuO into cells, as well as removal of remaining extracellular particles in the media by this approach.16 The tube was placed in a flat cell resonator that was carefully aligned in the microwave cavity of an EPR spectrometer (Bruker EMX). EPR spectra were acquired for both cell lines, as specified in the manufacturer’s guidelines for the spectrometer (www.bruker.com). A previously reported calibration was used to convert the spectral bandwidth to oxygen levels (0% line width, oxygen sensitivity for LiNc–Buo).16 Table 1 shows the analysis of results. Both HET-l and SEG-l were exposed to similar oxygen levels ( in solution), yet exhibited different RTDP lifetimes. Given that both cell lysates were generated in an identical fashion and that the experiments were not affected by factors such as pH, viscosity, temperature differences, or aggregation (shown previously in Ref. 2), the differences in lysate RTDP lifetime were likely indicative of cytosolic composition and/or of greater dynamic quenching in SEG versus HET. The differences in lysate RTDP lifetime were small compared to RTDP lifetime ( , or ) and were statistically significant . Table 1Lifetime differences Δτ=τHET−τSEG and oxygen estimates from FLIM experiments on living cells and cellular lysates. Revised values of Δτ (computed as a-b) were used to correct [O2]SEG levels. Corrected FLIM-derived oxygen estimates [O2]FLIM were in good agreement with EPR validation measurements [ [O2]EPR . Kq values were estimated via the Stern–Volmer equation with known lifetime and corrected oxygen values for both HET and SEG cell lines.
Live-cell FLIM revealed an average intracellular RTDP lifetime difference of between HET and SEG cell lines, which led to initial, uncorrected estimates of and , as shown in Table 1 and reported elsewhere.2 Given that extracellular oxygen was and no known mechanisms of active oxygen transport exists in cells, it is likely that was overestimated by the uncorrected calibration. Indeed, cell lysate studies indicated that the potential lifetime difference might have been overestimated by (Table 1). When corrected by this factor, we obtain a revised average intracellular RTDP lifetime difference and corrected value of . EPR oximetry provided a well-established means of validating these intracellular oxygen studies. Lysate studies on the experimental probe LiNc–BuO indicated little or no effect of the intracellular environment. As shown in Table 1 data in gray boxes), intracellular oxygen levels measured via EPR on living HET cells were very close to the values estimated via RTDP FLIM maps of living HET cells. Also (data in gray boxes), intracellular oxygen levels measured via EPR on living SEG cells were close to the values estimated via RTDP FLIM maps of living SEG cells that were corrected using correction values obtained from the cell lysate studies described above. We note that the trend of was maintained, despite some expected variations due to the differences between fluorescence imaging and EPR spectroscopic methods (e.g., the use of adherent vs. suspended cell samples). These results suggest a novel protocol for intracellular oxygen sensing in living cells by using a reference cell line with known oxygenation. In the study reported here, that purpose was served by the HET cells due to their stable oxygen levels across both fluorescence and EPR studies. However, any established cell line could be used in this role. For example, commercially available Chinese Hamster Ovary (CHO) cells have been used for EPR oxygenation studies with reproducible results .6 The protocol, extensible to any oxygen-sensitive fluorophore, would involve measuring for the fluorophore in solution. Both the reference and the experimental cell lines would then be incubated with the probe to estimate oxygen levels, which could be corrected via lysate-FLIM studies. Finally, corrected lifetime and oxygen values could be used to compute the Stern–Volmer quenching constant that is specific to the experimental cell line and valid for all future experiments without the need for any additional corrections. Linear subtraction of lifetimes obtained from the RTDP-cell and RTDP-lysate studies is a simplified assumption made due to the unknown nature of the biochemical component that quenches RTDP lifetime. Future work in this regard could involve use of more accurate and complicated mathematical models for correcting measured lifetime values. Compared to previous calibration efforts for intracellular oxygen sensing, the validated FLIM-based approach described here has the advantages of using a living reference biological sample, accounting for specific intracellular biochemical information, and enabling accurate, quantitative mapping of intracellular oxygen in vivo. AcknowledgmentsThe authors thank Dr. Periannan Kuppusamy (Ohio State University) for providing the LiNc–BuO particulates and Arjun Khullar (University of Michigan) for early efforts on the EPR project. This work was supported by a grant from the National Institutes of Health Grant No. NIH CA-114542 (to M.-A.M.). ReferencesC. Balut, M. vandeVen, S. Despa, I. Lambrichts, M. Ameloot, P. Steels, and I. Smets,
“Measurement of cytosolic and mitochondrial pH in living cells during reversible metabolic inhibition,”
Kidney Int., 73 226
–232
(2008). 0085-2538 Google Scholar
D. Sud, W. Zhong, D. G. Beer, and M. A. Mycek,
“Time-resolved optical imaging provides a molecular snapshot of altered metabolic function in living human cancer cell models,”
Opt. Express, 14 4412
–4426
(2006). https://doi.org/10.1364/OE.14.004412 1094-4087 Google Scholar
P. Rohrbach, O. Friedrich, J. Hentschel, H. Plattner, R. H. Fink, and M. Lanzer,
“Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes,”
J. Biol. Chem., 280 27960
–27969
(2005). 0021-9258 Google Scholar
C. J. Grauw and H. C. Gerritsen,
“Fluorescence lifetime imaging of oxygen in dental biofilm,”
Proc. SPIE, 4164 70
–78
(2003). 0277-786X Google Scholar
T. C. O’Riordan, A. V. Zhdanov, G. V. Ponomarev, and D. B. Papkovsky,
“Analysis of intracellular oxygen and metabolic responses of mammalian cells by time-resolved fluorometry,”
Anal. Chem., 79 9414
–9419
(2007). 0003-2700 Google Scholar
N. Khan, J. Shen, T. Y. Chang, C. C. Chang, P. C. Fung, O. Grinberg, E. Demidenko, and H. Swartz,
“Plasma membrane cholesterol, a possible barrier to intracellular oxygen in normal and mutant CHO cells defective in cholesterol metabolism,”
Biochemistry, 42 23
–29
(2003). 0006-2960 Google Scholar
C. Simpkins, S. Balderman, and E. Mensah,
“Mitochondrial oxygen consumption is synergistically inhibited by metallothionein and calcium,”
J. Surg. Res., 80 16
–21
(1998). 0022-4804 Google Scholar
H. M. Swartz,
“Using EPR to measure a critical but often unmeasured component of oxidative damage, Oxygen,”
Antioxidants Redox Signal., 6 677
–686
(2004) Google Scholar
H. M. Swartz, N. Khan, J. Buckey, R. Comi, L. Gould, O. Grinberg, A. Hartford, H. Hopf, H. G. Hou, E. Hug, A. Iwasaki, P. Lesniewski, I. Salikhov, and T. Walczak,
“Clinical applications of EPR: overview and perspectives,”
NMR Biomed., 17 335
–351
(2004). 0952-3480 Google Scholar
S. M. Evans, K. D. Judy, I. Dunphy, W. T. Jenkins, P. T. Nelson, R. Collins, E. P. Wileyto, K. Jenkins, S. M. Hahn, C. W. Stevens, A. R. Judkins, P. Phillips, B. Geoerger, and C. J. Koch,
“Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding,”
Cancer Res., 64 1886
–1892
(2004). https://doi.org/10.1158/0008-5472.CAN-03-2424 0008-5472 Google Scholar
B. Gallez, and H. M. Swartz,
“In vivo EPR: when, how and why?,”
NMR Biomed., 17 223
–225
(2004). 0952-3480 Google Scholar
H. M. Swartz, and J. Dunn,
“The difficulties in comparing in vivo oxygen measurements: Turning the problems into virtues,”
Oxygen Transport to Tissue XXVI, 566 295
–301 ,
(2005). Google Scholar
W. Zhong, P. Urayama, and M. A. Mycek,
“Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing,”
J. Phys. D, 36 1689
–1695
(2003). https://doi.org/10.1088/0022-3727/36/14/306 0022-3727 Google Scholar
P. K. Urayama, W. Zhong, J. A. Beamish, F. K. Minn, R. D. Sloboda, K. H. Dragnev, E. Dmitrovsky, and M. A. Mycek,
“A UV-visible fluorescence lifetime imaging microscope for laser-based biological sensing with picosecond resolution,”
Appl. Phys. B, 76 483
–496
(2003). 0946-2171 Google Scholar
D. Sud, G. Mehta, K. Mehta, J. Linderman, S. Takayama, and M. A. Mycek,
“Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture,”
J. Biomed. Opt., 11 050504
(2006). 1083-3668 Google Scholar
V. K. Kutala, N. L. Parinandi, R. P. Pandian, and P. Kuppusamy,
“Simultaneous measurement of oxygenation in intracellular and extracellular compartments of lung microvascular endothelial cells,”
Antioxid. Redox Signal, 6 597
–603
(2004). Google Scholar
|

