|
|
1.IntroductionMolecular imaging refers to remote-sensing the characteristics of the biological process and interactions between molecules at the molecular level.1 It has great potential for the early detection and more effective treatment of diseases, because aberrations at the cellular and molecular levels occur much earlier than morphological changes. Molecular imaging methods have developed for many different imaging modalities, including optical imaging,2 ultrasonic imaging,3 magnetic resonance imaging,4 and nuclear-medicine-based imaging.5 Here, we provide the photoacoustic (PA) imaging technique using molecularly targeted single-walled carbon nanotubes (SWNTs) to detect highly proliferative cancerous cells. PA imaging is a new imaging modality under preclinical development that has been applied to several biomedical applications for obtaining structural and functional information, including diagnosing breast cancer,6, 7 imaging of gene overexpression,8 monitoring of vascular damage during photodynamic therapy treatment of tumors,9 noninvasive monitoring of traumatic brain injury and post-traumatic rehabilitation,10 and imaging of cerebrovascular activities in small animals and hemoglobin oxygen saturation variations.11 PA imaging using near-infrared (NIR) illumination in the range of has attracted the most attention, because light can penetrate several centimeters into tissue at these wavelengths. Ideally, the tissue medium should have low optical absorption for deep penetration, while the objects of interest (such as tumors in cancer detection) should have high absorption for the best image contrast. Exogenous staining can provide high contrast when the natural optical contrast is not sufficient. Several PA imaging studies utilized various NIR absorbing agents, such as indocyanine green,12 and several types of gold nanoparticles, such as nanoshells13and nanorods.14 SWNTs are an important class of artificial nanomaterials with remarkable mechanical, thermal, electronic, and optical properties. These properties suggest diverse future biomedical uses in areas such as targeted chemotherapeutics, in vitro cell markers, diagnostic imaging contrast agents, biochemical sensors, and photoablative therapy agents. The high optical absorbance of SWNTs in the NIR regime causes heating under laser irradiation, which is useful for destroying cancer cells that are selectively internalized with nanotubes. Various groups have found that well-water-solubilized nanotubes with high hydrophilicity are nontoxic, even at high concentrations.15 We present here PA molecular imaging with antibody-functionalized SWNTs for tumor early detection in vivo. To lay the groundwork for this goal, optical properties of SWNTs for molecular imaging of cancer have been studied. Images were collected in tissue-simulating phantoms to determine appropriate detectable concentrations of SWNTs. Preliminary in vitro and in vivo results showed that a high contrast and a high efficient targeting of integrin positive U87 human glioblastoma tumors in mice could be achieved. The nontoxicity of functionalized SWNTs has also been demonstrated in our experiment. 2.Materials and Methods2.1.MaterialsIn our experiments, SWNTs were purchased from the Chinese Academy of Sciences (Chengdu, China). Nude mice were purchased from Sun Yat-Sen University (Guangzhou, China). Phospholipid- (PL-PEG) was purchased from Avanti Polar Lipids, Inc. (Shanghai, China). Integrin antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA), and Protein A was purchased from Bei Jing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). 2.2.Administration of SWNTsSWNTs were sonicated in an aqueous solution of PL-PEG at a ratio of SWNTs: PL-PEG: water for , followed by centrifugation at for . Then, the supernatant was collected and underwent an additional centrifugation at for to obtain well-suspended PL-PEG-functionalized SWNTs in the supernatant. Excess phospholipids were thoroughly removed by repeated filtration through filters (Millipore) and rinsed thoroughly with water, then resuspended SWNTs in either water or phosphate-buffered saline (PBS) by sonication for . The PL-PEG-functionalized SWNTs (denoted as SWNT-PEG-COOH) were finally resuspended in PBS. 2.3.Anti-integrin Conjugation to SWNTsSWNT-PEG-COOH solution was reacted with -hydroxysulphonosuccinimide (NHS) in the presence of 1-ethyl-3-[3-(dimethylamino)-propyl] carbodiimide at 1:1:1 molar ratio, then Protein A was mixed with the SWNT-PEG-COOH solution at pH 7.4 and incubated for . The SWNT-PEG-Protein A was filtrated through filters (Millipore) to remove excess Protein A. Then, integrin antibody was added into a solution of SWNT-PEG-Protein A. The mixture was allowed to react for at room temperature and protected from light. After reaction, the solution was dialyzed against PBS using a membrane (molecular weight ) to remove dissociative antiintegrin . The dialysis was carried out for with frequent replacement of the buffer to obtain the final sample SWNT-PEG-Anti-integrin solution (see Fig. 1 ). Fig. 1Water-soluble SWNT functionalized with PEG and integrin antibodies. Schematic drawings of noncovalently functionalized SWNT- with Protein A and integrin antibody. The hydrophobic carbon chains of the phospholipids strongly bind to the sidewalls of the SWNTs, and the PEG chains render water solubility to the SWNTs. The integrin antibody on the SWNTs are used to specifically target the integrin -positive tumor.  2.4.Cell Lines and the Animal ModelU87MG human glioblastoma cancer cell lines (from American Type Culture Collection) were cultured under standard conditions. The U87MG tumor models were generated by subcutaneous injection of cells in PBS into the back of the nude balb/c mice. PEGylated SWNTs were intramuscular-injected into the tumor-free nude mice tail for the in vivo PA molecular imaging experiment. Targeted SWNTs with anti-integrin molecules were intravenously injected into the tumor-bearing nude mice tail veins for tumor-targeting experiment 2.5.PA Molecular Imaging System for In Vivo SWNTs Selective TargetingThe PA molecular imaging system for in vivo SWNTs selective targeting is shown in Fig. 2 . The photoacoustic signal is captured by a linear ultrasound transducer array (L7L38A, SIUI). The 128 elements piezoelectric linear transducer has a central frequency and a 70% nominal bandwidth. The physical dimensions of the elements are given by an elemental elevation aperture of and a lateral pitch of . Each element of the transducer array has a thin cylinder ultrasonic lens that produces a geometric focus approximately in front of the transducer array to select the 2-D image plane and suppress the out-of-plane signals. Fig. 2Schematic of the experimental setup for the photoacoustic molecular imaging system. For optical absorption distribution reconstruction, the photoacoustic signal is captured by a linear ultrasound transducer array (L7L38A, SIUI). The 128 elements piezoelectric linear transducer has a central frequency and a 70% nominal bandwidth. An OPO-provided laser beam is expanded and homogenized by the concave lens and the ground glass to irradiate on the backs of the mice. The linear ultrasound transducer array, driven by a computer-controlled step motor to scan around the mice, captures the PA signal after targeted SWNTs injection. 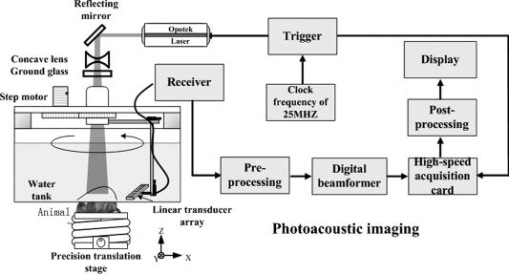 A frequency-doubled Nd:yttrium aluminum garnet laser pumping an optical parametric oscillator (Vibrant 532 I, Opotek, Carlsbad, CA) was employed to provide laser light with a pulse duration of and a pulse repetition rate of . The laser beam was expanded and homogenized to provide an incident energy density of and a beam diameter of to irradiate the sample for generating photoacoustic signals. A clock signal, provided by a control circuit, was divided into for triggering the pulse laser and controlling the multielement linear transducer array to acquire photoacoustic signals. The signals from the transducers, after preprocessing, including ultralow-noise time-gain compensate amplifiers, anti-aliasing bandpass filters, analog-to-digital converters, and several beamforming strategies (various f-number, aperture apodization, element size, etc.), were acquired with the NI PCI-6541 High-Speed Digital Wfm ( , selectable; voltage, 32 channels, /channel). The collected data were stored in a computer for later processing. Next, the multielement linear transducer array was driven by a step motor to circularly scan around the sample, detecting the photoacoustic signals in the imaging plane at each position. The linear transducer array and the samples were both immersed in a tank of water for better coupling. The induced photoacoustic waves were captured every , and a total of 20 positions of photoacoustic waves are recorded for a full view of the circularly scanning angle. After the series data were transferred to the computer, further postprocessing was done using the MATLAB program (version 7.0, Mathworks). Projections were calculated with the improved limited-field filtered back projection algorithm.16 At last, the photoacoustic images were shown on the display. 3.Results3.1.Optical Properties of SWNTs for PA Molecular ImagingSWNTs were dispersed in the aqueous phase by noncovalently adsorbing PL-PEGs. Figure 3 presents visible spectrometry (VIS)/NIR absorption spectra of the initial blood and functionalized SWNT samples in water. Absorption spectra of functionalized SWNTs were compared with pure blood absorption at the same concentration. The two absorption spectra are significantly different and clearly show that the SWNT can enhance absorption in the NIR region, in which the biological tissue is almost transparent with very low absorbance. Longer wavelengths are more desirable, as the depth of penetration through the tissues is increased; the photoacoustic spectra suggest that is the preferable wavelength, because the photoacoustic signal of the SWNTs is higher than blood absorption at that wavelength. The high absorbance of SWNTs in the NIR originates from electronic transitions between the first or second van Hove singularities of the nanotubes.17 High optical absorbance of SWNTs in the NIR window transparent to biological systems is exploited in the current work at a single wavelength by using a pulse laser for radiation. Fig. 3VIS/NIR absorption spectra of the initial blood and functionalized SWNT samples in water. Absorption spectra of functionalized SWNTs were compared with pure blood absorption at the same concentration. The two absorption spectra are significantly different and clearly show that the SWNT can enhance absorption in the NIR region, where the optical transmission through biological tissues is optimal. 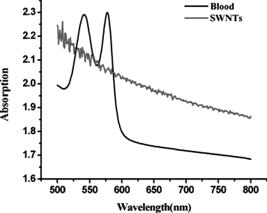 In contrast to NIR-absorbing dyes, the absorption properties of SWNTs are dependent upon a rigid structure rather than on molecular orbital electronic transitions. SWNTs are also not susceptible to photobleaching, a problem commonly associated with other absorbing dyes. These features make SWNTs suitable as a contrast agent for photoacoustic molecular imaging in the NIR region. 3.2.SWNTs for Photoacoustic Molecular ImagingThis experiment was performed to determine the concentration of SWNTs as a NIR contrast agent for effective enhancement in photoacoustic molecular imaging. The optical absorption spectrum of PL-PEG functionalized SWNT was tested at different concentrations [see Fig. 4a ]. Linear curve fitting for peak absorption at a wavelength of was shown in Fig. 4b. For the PA signal peak intensity of different concentrations of SWNT solution, linear curve fitting for absorption was evaluated to estimate the concentration of SWNTs [see Fig. 4c]. Different concentrations of SWNT solution mixed with blood were also investigated in vitro to estimate the appropriate concentration for in vivo application in Fig. 4d. Fig. 4SWNTs as IR contrast agent with varying concentrations for photoacoustic molecular imaging. (a) Absorption spectra of -functionalized SWNT suspensions in PBS with varying concentrations. (b) Linear fitting of peak absorbance of spectrum versus concentration of SWNTs at . (c) PA signal peak intensity of different concentrations of SWNT solution; linear curve fitting for absorption of was evaluated to estimate the concentration of SWNTs. (d) Different concentrations of SWNT solution suspended in blood in vitro. The series of experiments indicated that a SWNT solution concentration of can achieve effective enhancement. 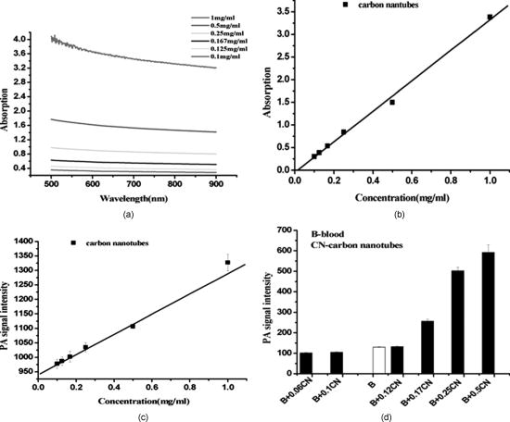 These data suggest that a SWNT solution concentration of 0. can achieve effective enhancement using a pulse laser. We thus expect, based on the concentrations of SWNTs expected to be bound in vivo, that it will be possible to detect the uptake of the proposed in vivo tumor probe. 3.3.In Vitro PA MeasurementsSWNTs as IR contrast agent for photoacoustic molecular imaging were demonstrated in an in vitro experiment. Two identical -diameter tubes, one with SWNTs of and one with pure blood, were placed side by side in the imaging tank at a depth of in a mimic phantom of Intralipid-20% dilution. The image in Fig. 5a was obtained by photoacoustic scanning by the linear transducer array and represents a clearer map of tube A containing than of tube B containing blood only. With the exogenous contrast agent, the optical absorption of the blood was increased, and the contrast between the vessels and the background brain tissues was enhanced. Reconstructed profiles of these two tubes shown in Fig. 5b also show higher signal-to-noise ratios using a SWNT-enhanced sample than using blood only at the excitation wavelength of . Fig. 5SWNTs as IR contrast agent for photoacoustic imaging in vitro. (a) Photoacoustic images of A gel phantom with two tubes, tube A containing and tube B containing blood only. (b) The line normal to object axis profile of the reconstructed image shown in (a) with , which shows a better imaging contrast with enhanced SWNTs than blood only at a wavelength of . 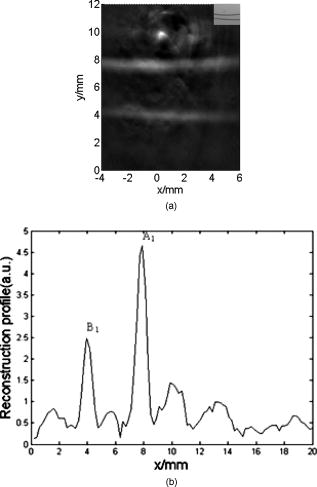 3.4.Cellular Toxicity Tests of SWNTsCell cytotoxicity assay was performed with a colorimetric tetrazolium salt-based assay, Cell Counting Kit-8 (CCK8). To determine the cytotoxicity of SWNTs, tumor cells ( per well) were cultured in a 96-well microplate for and then co-incubated with functionalized SWNTs of different concentrations for , rinsed with PBS, and incubated for another . OD450, with an absorbance value of , was read with a 96-well plate reader (Infinite M200, Tecan, Switzerland) to determine the viability of the cells. The viability of cells was calculated as: cell viability (where ODTre was the absorbance value at of treated cells and ODCon was the absorbance value at of control cells). Cell-toxicity tests (see Fig. 6 ) show that SWNTs display satisfactory biosafety at the dosages expected for in vivo applications. Fig. 6Cell toxicity of SWNT measurement. The viability of cells was calculated as: cell viability (where ODTre was the absorbance value at of treated cells and ODCon was the absorbance value at of control cells). SWNT-treated cells show little decreased viability in the colorimetric tetrazolium salt-based assay at when compared to control cells (ODCon). With the CCK8 assay, at all concentrations, SWNT-treated cells also show no significantly decreased viability when compared to control cells. 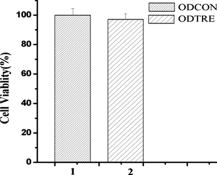 3.5.In Vivo PA Imaging of SWNTsSWNTs as IR contrast agent for photoacoustic molecular imaging were demonstrated in an in vivo experiment. Female nude mice ( body weight) were employed in this work. General anesthesia was administered on the mice by an intramuscular injection of ketamine hydrochloride , xylazine hydrochloride , acepromazine maleate , and atropine . During the experiment, the mouse was placed on a water-circulating heating pad, and additional heating was provided by an overhead surgical lamp. During the data acquisition, the rat was provided pure oxygen for breathing, and the arterial blood oxygenation level and heart rate were monitored by a pulse oximeter ( , Nonin) with the fiberoptic probe wrapped around a paw of the animal. The SWNT was successively intramuscular-injected into the tail of the mouse at a dose of SWNTs dispersed in PBS with a concentration of . Image acquisition of the mouse tail began approximately following administration. Two of the photoacoustic angiographs of the rat tail are presented in Fig. 7a and 7c . Compared to the tail image based on the intrinsic optical contrast [Fig. 7a], the image with SWNTs contrast agent [Fig. 7c] shows greater clarity. It also shows a better signal-to-noise ratio when comparing Fig. 7b with 7d. Fig. 7In vivo PA imaging of SWNTs as contrast agent in mouse tail. (a) Control PA image without SWNTs- injection. (b) Reconstruction profile of mouse tail shown in (a). (c) PA image at after SWNTs-PEG injection. (d) Reconstruction profile of mouse tail shown in Fig. 7d. 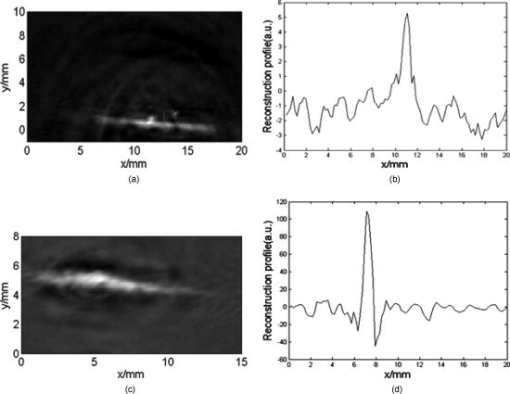 3.6.In Vivo PA Imaging of SWNTs Targeted TumorIn vivo PA imaging of SWNTs targeted tumor has been performed in this experiment. The targeted SWNTs with anti-integrin molecules and SWNTs-PEG (control group) were injected into the tail veins of two mice. In each mouse, the targeting process was imaged after injection. Photoacoustic scanning was performed after tumor-cell inculcation for two weeks. The PA images of a U87 tumor for injections with targeted SWNTs and SWNTs-PEG (control group) in Fig. 8 demonstrate the specific targeting ability of the targeted SWNTs probe. The contrast is higher for the targeted SWNTs data postinjection ( after injection) within the tumor region [Fig. 8b] than for the SWNTs-PEG injection [Fig. 8a]. The contrast between the experimental group (targeted SWNTs injection) and the control group (SWNTs-PEG injection) indicated specific targeting of SWNTs targeted with anti- to U87 cells. Fig. 8In vivo PA imaging of U87 tumor before and after injection of targeted SWNTs. The PA signals were generated by pulsed laser at a wavelength of . (a) Photograph of U87 tumor on the back of nude mice. Dotted line shows the tumor area. (b) PA image at after SWNTs-PEG injection. (c) PA image at after SWNT-PEG2000-Protein A-anti-integrin injection. 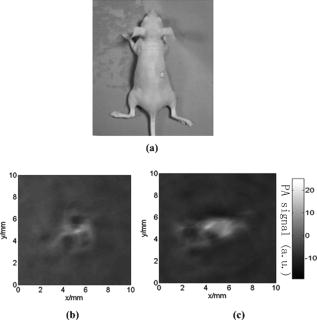 4.DiscussionAntibody-functionalized SWNTs have been designed for photoacoustic molecular imaging to specifically target early tumors. In the present study, preliminary in vitro and in vivo experiments were performed to lay the groundwork for this goal. The salient feature of this work is the use of functionalized SWNTs as both antibody shuttle and photoacoustic contrast agents. This can significantly suggests that photoacoustic molecular imaging with antibody-functionalized SWNTs has the potential to be an effective early tumor-diagnosis method. Biocompatibility has been an important issue for in vivo applications of SWNTs. Results from analyses of SWNTs in vivo systemic toxicity tests (see Fig. 6) show that SWNTs are biocompatible and hemocompatible for in vivo tests. SWNTs exhibit satisfactory biosafety at the dosages expected for in vivo applications. The results of an in vivo toxicity test for SWNTs administered to nude mice via tail-vein injection were also satisfactory, with all mice surviving the observation period. In addition, the temporal biodistribution of SWNTs in mice was analyzed to determine their metabolic clearance in some literatures.18, 19 Intravenous administration of these functionalized SWNTs indicated that SWNTs are not retained in any of the reticuloendothelial system organs (liver or spleen). SWNTs are rapidly cleared from systemic blood circulation through the renal excretion route. The observed rapid blood clearance and half-life of f-SWNT has major implications for all potential clinical uses of carbon nanotubes.19 So, the PA signal begun to obtain after SWNT injection. The U87 glioblastoma tumor, which highly expresses integrin , was clearly imaged by photoacoustic molecular imaging as shown in Fig. 8b. Clinical translation of SWNT-PEG2000-ProteinA-anti- is critical for the maximum benefit of Abegrin-based anticancer agents, as imaging can provide a straightforward and convenient way to monitor the biological changes at the molecular level in vivo. Future developments of anti- -directed therapy and translation of these encouraging experimental data to clinical studies would be greatly facilitated by noninvasive techniques that allow serial studies of -positive tumors. This work demonstrates that photoacoustic molecular imaging with antibody-functionalized SWNTs can be applied in early tumor detection. PA molecular imaging with targeted SWNTs has been demonstrated using SWNTs on a U87 tumor model in vivo. The results reveal that information about the oncogene surface molecules of cancer cells can be obtained with PA techniques, which will help improve our understanding of cancer cells and develop effective diagnosis tools as well as indications for effective treatments. Safe and effective SWNT-based cancer diagnoses have great potential in the pharmaceutical industry and could also make significant contributions in the biomedical field. AcknowledgmentsThis research is supported by the National Natural Science Foundation of China (Grants No. 30870676, No. 30627003, No. 30800261) and the Natural Science Foundation of Guangdong Province (Grant No. 7117865). We also acknowledge the technical assistance by Dr. Baoyan Wu and helpful conversations with Dr. Lei Huang. ReferencesK. Shah, A. Jacobs, X. O. Breakefield, and R. Weissleder,
“Molecular imaging of gene therapy for cancer,”
Gene Ther., 11 1175
–1187
(2004). https://doi.org/10.1038/sj.gt.3302278 0969-7128 Google Scholar
X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung, and S. Nie,
“In vivo cancer targeting and imaging with semiconductor quantum dots,”
Nat. Biotechnol., 22 969
–976
(2004). https://doi.org/10.1038/nbt994 1087-0156 Google Scholar
D. B. Ellegala, H. Leong-Poi, J. E. Carpenter, A. L. Klibanov, S. Kaul, M. E. Shaffrey, J. Sklenar, and J. R. Lindner,
“Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to ,”
Circulation, 108 336
–341
(2003). 0009-7322 Google Scholar
L. Josephson, M. F. Kircher, U. Mahmood, Y. Tang, and R. Weissleder,
“Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes,”
Bioconjugate Chem., 13 554
–560
(2002). https://doi.org/10.1021/bc015555d 1043-1802 Google Scholar
S. R. Cherry,
“In vivo molecular and genomic imaging: new challenges for imaging physics,”
Phys. Med. Biol., 49 R13
–R48
(2004). https://doi.org/10.1088/0031-9155/49/3/R01 0031-9155 Google Scholar
A. A. Oraevsky, V. A. Andreev, A. A. Karabutov, R. D. Fleming, Z. Gatalica, H. Singh, and R. O. Esenaliev,
“Laser optoacoustic imaging of breast: detection of cancer angiogenesis,”
Proc. SPIE, 3597 352
–363
(1999). https://doi.org/10.1117/12.356829 0277-786X Google Scholar
R. A. Kruger, K. D. Miller, H. E. Reynolds, W. L. Kiser, D. R. Reinecke, and G. A. Kruger,
“Breast cancer in vivo contrast enhancement with thermoacoustic CT at -feasibility study,”
Radiology, 216 279
–283
(2000). 0033-8419 Google Scholar
L. Li, R. Zemp, G. Lungu, G. Stoica, and L. V. Wang,
“Photoacoustic imaging of lacZ gene expression in vivo,”
J. Biomed. Opt., 12
(02), 020504-1
–3
(2007). https://doi.org/10.1117/1.2717531 1083-3668 Google Scholar
L. Zh. Xiang, D. Xing, H. M. Gu, D. W. Yang, S. H. Yang, L. M. Zeng, and W. R. Chen,
“Real-time optoacoustic monitoring of vascular damage during photodynamic therapy treatment of tumor,”
J. Biomed. Opt., 12 014001-1
–8
(2007). https://doi.org/10.1117/1.2437752 1083-3668 Google Scholar
S. H. Yang, D. Xing, Y. Q. Lao, D. W. Yang, L. M. Zeng, L. Zh. Xiang, and W. R. Chen,
“Noninvasive monitoring of traumatic brain injury and post-traumatic rehabilitation with laser-induced photoacoustic imaging,”
Appl. Phys. Lett., 90 243902-1
–3
(2007). https://doi.org/10.1063/1.2749185 0003-6951 Google Scholar
S. Yang, D. Xing, Q. Zhou, L. Zh. Xiang, and L. Q. Lao,
“Functional imaging of cerebrovascular activities in small animals using high-resolution photoacoustic tomography,”
Med. Phys., 34 3294
–3301
(2007). https://doi.org/10.1118/1.2757088 0094-2405 Google Scholar
K. M. Stantz, B. Liu, M. Cao, D. Reinecke, K. Miller, and R. Kruger,
“Photoacoustic spectroscopic imaging of intra-tumor heterogeneity and molecular identification,”
Proc. SPIE, 6086 608605
(2006). https://doi.org/10.1117/12.645106 0277-786X Google Scholar
Y. W. Wang, X. Y. Xie, X. D. Wang, G. Ku, K. L. Gill, D. P. O’Neal, G. Stoica, and L. H. V. Wang,
“Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain,”
Nano Lett., 4
(9), 1689
–1692
(2004). https://doi.org/10.1021/nl049126a 1530-6984 Google Scholar
P. Ch. Li, W. W. Chen, C. K. Liao, C. D. Chen, K. C. Pao, C. R. C. Wang, Y. N. Wu, and D. B. Shieh,
“Multiple targeting in photoacoustic imaging using bioconjugated gold nanorods,”
Proc. SPIE, 6086 60860M
(2006). https://doi.org/10.1117/12.645248 0277-786X Google Scholar
C. K. Paul, J. G. Christopher, K. L. Tonya, K. S. Howard, E. S. Richard, A. C. Steven, and R. B. Weisman,
“Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence,”
Proc. Natl. Acad. Sci. U.S.A., 103
(50), 18882
–18886
(2006). https://doi.org/10.1073/pnas.0609265103 0027-8424 Google Scholar
D. W. Yang, D. Xing, S. H. Yang, and L. Zh. Xiang,
“Fast full-view photoacoustic imaging by combined scanning with a linear transducer array,”
Opt. Express, 15
(23), 15566
–15575
(2007). https://doi.org/10.1364/OE.15.015566 1094-4087 Google Scholar
M. B. Sergei, S. S. Michael, K. Carter, H. H. Robert, E. S. Richard, and R. B. Weisman,
“Structure-assigned optical spectra of single-walled carbon nanotubes,”
Science, 298 2361
–2366
(2002). https://doi.org/10.1126/science.1078727 0036-8075 Google Scholar
Zh. Liu, W. B. Cai, L. N. He, N. Nakayama, K. Chen, X. M. Sun, X. Y. Chen, and H. J. Dai,
“In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice,”
Nat. Nanotechnol., 2
(1), 47
–52
(2007). https://doi.org/10.1038/nnano.2006.170 1748-3387 Google Scholar
R. Singh, D. Pantarotto, L. Lacerda, G. Pastorin, C. Klumpp, M. Prato, A. Bianco, and K. Kostarelos,
“Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers,”
Proc. Natl. Acad. Sci. U.S.A., 103
(9), 3357
–3362
(2006). 0027-8424 Google Scholar
|


