|
|
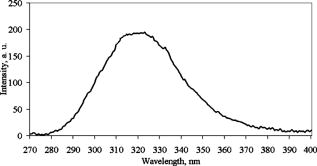
1.IntroductionFree radicals are molecular species with an unpaired electron on the external orbital and of high chemical reactivity. All radicals existing in a human organism can be divided into two kinds, natural and alien. The former are those which are inherently produced in the organism during chemical reactions: radical oxygen species (ROS) such as superoxide , singlet oxygen , and hydroxyl radical (OH), as well as nitric oxide (NO), etc. They play an important role as regulatory mediators in signalling processes such as regulation of vascular tone, monitoring of oxygen tension in the control of ventilation and erythropoietin production and signal transduction from membrane receptors in various physiological processes.1, 2 Under normal conditions, the amount of free radicals is balanced by enzymes and antioxidants. An excessive increase in ROS production is involved, for example, in the pathogenesis of cancer, diabetes mellitus, atherosclerosis, neurodegenerative diseases, rheumatoid arthritis, and ischemia/reperfusion injury.1 The alien free radicals appear as a consequence of the effect of ionizing radiation, UV light, xenobiotics, etc. on human tissue and are harmful. Studying of free radicals can be carried out by direct and indirect methods. The direct methods implement the effects of either electron paramagnetic resonance (EPR) or chemoluminescence. The indirect methods include investigations of the end products of the reactions with free radicals involved or application of inhibitors. The EPR (also called electron spin resonance) technique is based on the absorption of microwave radiation by an unpaired electron of a molecule located in a magnetic field. Direct detection of free short-living radicals by the EPR method is possible only at a quite low temperature ( , liquid nitrogen) due to their sufficient steady-state concentrations only under such conditions.3 In order to achieve suitable concentrations at room temperature, spin traps and spin markers are used (e.g., PCA, DPPH, Tempol, TEMPO, DMPO, 4-POBN). Spin traps are molecules which bind to short-lived free radicals and form detectable stable forms of radicals (spin adducts). Spin markers are stabilized radicals themselves contributing to the EPR signal; however, being in contact with short-lived free radicals, they loose or add an electron and become undetectable. The decrease in the EPR signal in this case quantifies the free radicals under investigation. The EPR methodology is a useful tool for the noninvasive in vivo measurements of skin barrier function, drug/skin interaction and cutaneous oxygen tension.3 Skin is a tissue protecting deeper-located organs from various hazards of the environment, such as chemical, biological, and physical hazards, in particular, from UV light. Exceeding doses of UV radiation can cause direct or indirect (via formation of free radicals) DNA damage, leading to carcinogenesis.4 Skin is a suitable object for EPR investigations due to its surface location and relatively small thickness. Using the microwave radiation of , which penetrates as deep as into skin, it is possible to monitor penetration of spin traps and spin markers inside skin5, 6 and in-depth appearance of generated radicals by means of EPR imaging.7 According to investigations, UV-induced radicals include ROS and lipid radicals8, 9, 10 as well as melanin radicals.11 Formation of free radicals under UV irradiation and affect of various substances on this process in lipid-model systems12, 13 of different complexity as in vitro counterparts of the intercellular lipid matrix of the stratum corneum was investigated by Trommer, in particular, using the EPR technique.12 As shown by experiments with human skin in vivo,14 UVA part of the UV spectrum, is mainly responsible for the generation of free radicals (80–90% of the total amount) because of higher penetration depth, in contrast to UVB light, which contributes to radical generation only in the epidermis (up to a depth of ). Titanium dioxide nanoparticles are extensively used today in cosmetics, paints, air and water waste purification.15 Among three existing crystal modifications of (anatase, rutile, and brookite), anatase is the most photoactive, if irradiated by UV light, according to some authors.16 However, its photoactivity strongly depends on particle size, dopants,17 and coating and correlates with the reflectance spectra.18 Coatings are introduced to suppress photocatalytic properties of the particles.19 It is reported that both anatase and rutile particles used in sunscreens oxidize DNA and RNA in vitro and in human cell culture20, 21, 22 under UV irradiation. However, it is important to know whether the amount of radicals generated by the particles on skin exceeds that produced by the skin itself. In this paper, we experimentally investigate the ability of titanium dioxide particles (anatase) of two average sizes (25 and in diam) to produce free radicals under UV irradiation when applied to two different surfaces, glass and porcine skin in vitro. 2.Materials and MethodsTwo types of commercially available samples were used in the experiments. A placebo (sunscreen o/w emulsion without any filters, Creme Sante soleil SPF 8 F148-006, RoC S.A., France) with coated titanium dioxide nanoparticles embedded was applied either onto glass slides or onto porcine skin in vitro. The surface density of the substance was , corresponding to the recommendations of COLIPA23 for sunscreen applications. The placebo was weighted on precise-balance BP 211D (Sartorius, Göttingen, Germany) with an accuracy of using a syringe. One type of spin marker, 3-carboxy-2,2,5,5-tetramethylpyrrolidine-1-oxyl (PCA, Sigma-Aldrich Chemie GmbH, Munich, Germany), was used to detect short-lived free radicals emerging under UV irradiation. PCA was dissolved from powder in a water–ethanol (1:1) solution at a concentration of . It was chosen from other available spin markers due to satisfactory stability for our applications: after -long UV irradiation, the EPR signal decrease was , and after -long irradiation it was only 6%.24 Additionally, in porcine skin there is a low amount of antioxidants, thus prolonging the existence on PCA. As a source of UV radiation , a device TH-1E (Cosmedico Medizintechnik GmbH, Schwenningen, Germany) with the spectral maximum located at about was used. The radiation intensity was , measured by the powermeter HBM-1 (Hydrosun Medizintechnik GmbH, Mullheim, Germany), which corresponds to the solar UV intensity . The EPR system LBM MT 03 (Magnettech GmbH, Berlin, Germany) operating in L-band was used for detecting the EPR signal. The generated short-lived radicals react with the nitrogen oxide PCA, which is thereby reduced to the corresponding hydroxylamine. PCA looses its free electron and the signal intensity in the EPR signal decreases. 2.1.Experiments with Nanoparticles Applied onto Glass SlidesGlass slides had the following dimensions: width , length , thickness . There were three types of prepared slide samples (five couples of samples of each type): (i) placebo with small particles, (ii) placebo with large particles, and (iii) placebo only. Slides were covered homogeneously with a mixture of the corresponding substances and PCA . In each couple, one sample was irradiated with the UV lamp ( particles for , particles and placebo only for ); the other was used for control (was not irradiated). The samples were put into the EPR system and measured before and after the irradiation. The signals were displayed in real time on the computer screen in the software environment supplied with the system. They were stored as files and then analyzed. 2.2.Experiments with Nanoparticles Applied onto Porcine SkinNative ears of domestic pigs were delivered from a nearby farm (Gut Hesterberg, Neurupin, Germany) on the day following the slaughter. Before the experiments, the ears were washed with cold running water and gently wiped with paper napkins. All hairs on surfaces of the ears were completely removed with medical scissors with curved edges to avoid injuring the skin. Then, tesa adhesive tape (Beiersdorf AG, Hamburg, Germany) was used for tape stripping according to Weigmann 25 Twenty tape strips were taken from the same area to remove the stratum corneum (horny layer, superficial layer of skin) in part. Corresponding to Ref. 26, such an amount of strips means that about 50% of stratum corneum was removed. A roller was used to press the tape to the skin. This procedure was a prerequisite to ease penetration of PCA into the skin.6 PCA served as a marker for revealing the production of free radicals. In the investigation of free radicals with the EPR technique, the deeper the PCA penetrated, the higher the amount of skin volume was involved. The surface of each porcine ear area, in size, was marked with a permanent marker. The areas were divided into couples of small areas of or . One area in the couple was used immediately after the preparation procedure was completed; the other area was used after the application of substances. The borders of all areas were covered by a color glue to build barriers between neighbouring areas. As in experiments with application of particles onto glass slides, three different substances were investigated: placebo with particles, placebo with particles, and placebo without particles. PCA was added to all substances ( per each small area). The substances were topically applied onto the skin with a surface density of . During the application of the corresponding (according to the area) amount of the substances, the skin was massaged with a massage device (Petra PC 60, petra-electric GmbH Co.KG) covered with a latex glove finger saturated in advance with the substance. From each small area, two punch biopsies were taken with a round-edged sharp cutter; one was later irradiated with the UV lamp (stratum corneum side), while the other was used for control. The diameter of the skin samples was ; the thickness was (all skin layers from the surface to the cartilage in the middle of the ear). The biopsies were removed from the ear using a scalpel and pincers and were fixed onto a glass slide with acryl glue. Afterward, certain samples were irradiated by UV light for . The irradiated samples were measured in the EPR system before, immediately after and after the irradiation. Those serving as controls were measured at 0, 3, 7, 10, and (zero time corresponds to the start-time point of sample irradiation). Four to six samples were used for each measurement to collect statistical data. 2.3.Transmission Electron Microscopy (TEM)TEM photos of particles were obtained using a transmission electron microscope Philips Morgagni 280 D (Eindhoven, the Netherlands). 2.4.Raman SpectroscopyThe Raman spectroscopy system (LMTB, Berlin, Germany)27 was based on a CW laser operating at the wavelength of (power ). Its radiation was focused into an optical fiber connected to an optical imaging system, where the light was filtered and focused onto samples. The Raman signal reflected from samples was collected by a lens system and transferred into another fiber bundle connected to a spectrograph. The spectrum was recorded by a charge-coupled device camera and transferred to a PC for processing. Because of flexibility of light delivering and collecting fibers, samples ( particles as powder and imbedded in placebo) in cuvettes or applied onto glass slides were just put in contact with them. One measurement took a few seconds. 2.5.Mie CalculationsFor Mie calculations, MieTab 7.23 software28 was used. As input parameters, the radiation wavelength, refractive indices of the particles and the surrounding medium, as well as particle sizes were required. 3.Results and DiscussionTEM photographs of the particles in diluted placebo are represented in Fig. 1 . Magnification for the images of particles was 89, while for particles it was equal to 22. It is seen that the particles of both sizes form multipartilce structures. This process affects optical properties of the solutions. Fig. 1TEM images of the 25- (a) and (b) particles in a diluted placebo. Bars on the photographs correspond to 0.2 (a) and (b). Magnification: 89 (a) and 22 (b). 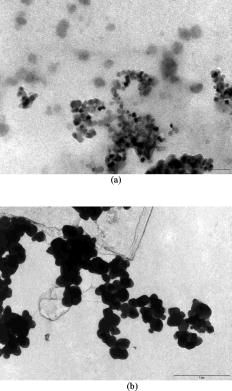 In order to reveal the crystal form of the used titanium dioxide particles, we measured Raman spectra of the powder of particles and of the placebo with embedded particles. The obtained spectra are presented in Fig. 2a . The signal produced by the placebo was much smaller than that of the powder due to a smaller concentration of particles (not shown). It is clearly seen that three peaks are present on the graph. This indicates, according to Ref. 29, that the crystal form of the particles under investigation is anatase. Fig. 2Signal of Raman scattering (a) from powder titanium dioxide nanoparticles with diameters of 400 (1) and (2); relative absorption efficiency factor referred to the particle diameter for 310 (1) and (2) UV radiation calculated according to the Mie theory; maxima of the curves correspond to the particle diameters of 74 and (b). 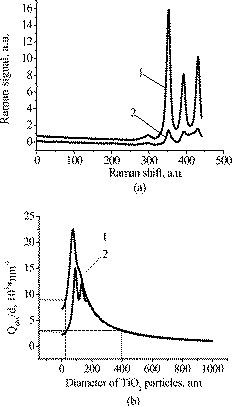 The Mie theory was used to describe the interaction between the particles and UV radiation. The spectrum of the used UV light source is depicted in Fig. 3 . For the calculations, two wavelengths were chosen: 310 and . The first was chosen because it corresponds both to the maximum of the source spectrum and to the maximum of the product of the solar spectral irradiance over wavelength with the erythemal action spectrum.30 The second wavelength was taken for comparison; the anatase particles do not absorb light with a wavelength longer than .31 Titanium dioxide is a birefringent crystal, with different refractive indices for light polarized perpendicular or parallel to the optic axis. In the “average index” approximation, the particles are supposed to be isotropic, with real and imaginary parts of the refractive index equal to and , where and ( and ) are the ordinary (extraordinary) real and imaginary parts of the refractive index, respectively.32 For the 310- and UV radiation, these constants taken from Ref. 31, result in: 0.83 (for ) and 0.25 (for ). The refractive index of the surrounding medium (placebo) was 1.4, and the diameters of the particles were considered to be with steps. The result of the calculation is shown in Fig. 2b. Efficiency factor is the ratio of the absorption and the geometrical cross sections of a particle. The value , where is a particle diameter, is proportional to the absorption coefficient of a particle suspension30, 33, 34, 35 and therefore takes into account the presence of other particles of the same type in the sample. Figure 4 illustrates typical signals obtained with the EPR system for the two investigated types of samples with particles: located on glass and on porcine skin. The signals are normalized to the highest amplitude of the signal from the sample before irradiation. It is seen that after -long exposure to UV light the signal decreases. This means that short-lived free radicals appear. Fig. 4EPR signals obtained from anatase particles on glass (a) and on porcine skin (b) before (1) and after (2) UV irradiation. The samples on glass were irradiated for and the samples on skin for . 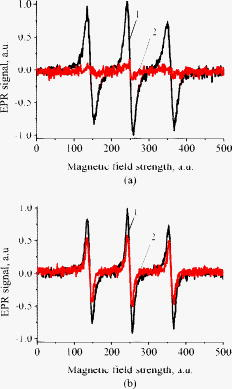 Figure 5 shows the mean values with standard deviations of the results obtained from five samples on glass with or without particles. Standard deviations vary between 0.02 and 0.24 for nonirradiated samples and between 0.10 and 0.26 for the irradiated samples. Dependencies of the EPR signal on time for the irradiated and nonirradiated samples are depicted. Statistically, there is no effect of UV radiation in the presence of large particles and placebo without particles. However, the effect is distinctly seen in the case of small particles. This phenomenon can be explained in frames of the Mie theory. Considering Fig. 2b representing the absorption efficiency curves for 310- and radiation, we can conclude that particles absorb UV light much more efficiently than the particles for or at the same level for at the same volume concentrations. As shown in Fig. 1, the particles form aggregates and agglomerates although ultrasonic stirring was used during the process of embedding particles into placebo. Formation of the above-mentioned structures causes an increase in the average size of particles, leading to the increased absorption efficacy of the particles and decreasing that of the particles. The larger absorption means more active production of free short-lived radicals. The curve corresponding to the nonirradiated large particles looks very similar to that of placebo and different from that of small particles. Fig. 5Temporal dependences of amplitudes of the EPR signals obtained from the samples on glass: 25 (a) and (b) particles and placebo without particles (c) UV-irradiated during 1 (a) or (b, c) (●) and not irradiated (◼). Zero time corresponds to the beginning of UV irradiation (for the irradiated samples). For averaging, five samples of each type were measured. 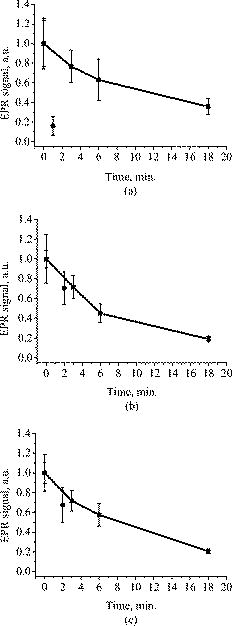 The other series of experiments was carried out with porcine ear skin. One group of skin samples was irradiated immediately after preparation and the other with -long delay. The results of the measurements of the first group with standard deviations are presented in Fig. 6 . Standard deviations vary between 0.04 and 0.25 for nonirradiated samples and between 0.08 and 0.30 for the irradiated samples. Taking into account the statistical errors, the effect of UV irradiation is clearly seen in all cases (the points corresponding to such samples are located lower than those of nonirradiated samples), and the magnitude of the effect is almost the same for the samples with the particles, with placebo only, and without placebo and particles. It means that the amounts of short-lived free radicals appearing under UV irradiation are comparable and do not depend on the presence of the particles on the skin surface. In other words, the contribution of skin to free-radical generation under UV irradiation exceeds that of the particles. Fig. 6Temporal dependences of amplitudes of the EPR signals obtained from the samples on porcine skin in vitro measured without -long delay: placebo with particles (a), placebo with (b) particles, placebo without particles (c), and skin without placebo and particles (d) UV-irradiated during (●) and not irradiated (◼). For averaging, four skin samples with placebo and each type of the particles, six skin samples with placebo only, and six skin samples without placebo and particles were measured. Zero time corresponds to the beginning of UV irradiation (for the irradiated samples). 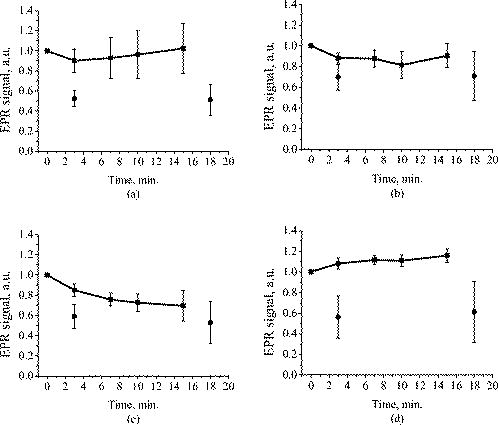 The effect of penetration time (0 versus ) of the substances before UV irradiation is depicted in Fig. 7 . There is no significant difference, but in the samples corresponding to large particles [Fig. 7b] as well as to the placebo [Fig. 7c] and to skin without placebo and particles [Fig. 7d], the lines connecting the average values relative to measurements without any delay are located above the lines corresponding to the delayed experiments. However, the situation is reverse for the samples with small particles [Fig. 7a]. Fig. 7Comparison of time-dependent amplitudes of the EPR signals after -long UV irradiation of the skin samples immediately after preparation (◼) and later (●): placebo with particles (a), placebo with particles (b), placebo without particles (c), and skin without placebo and particles (d). For averaging, four skin samples with placebo and each type of the particles, six skin samples with placebo only, and six skin samples without placebo and particles were measured. Zero time corresponds to the beginning of UV irradiation (for the irradiated samples). 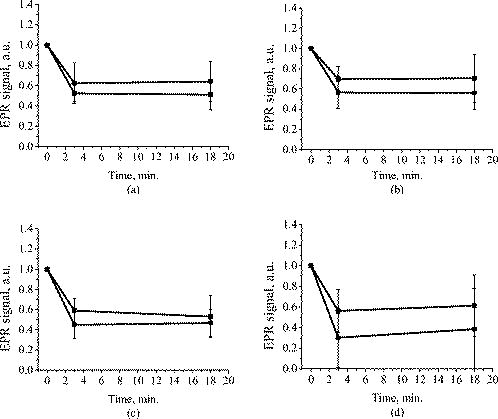 4.SummaryAs we proved experimentally by means of EPR spectroscopy, small ( in diam) nanoparticles of titanium dioxide (anatase form) are more photoactive than large ( in diam) particles. This effect is clearly seen if the particles embedded into the placebo are applied on glass and is in agreement with the Mie theory. However, if the particles are applied onto the porcine skin in vitro, no distinct difference is observed. This is caused by high skin contribution to the generation of radicals. In comparison to the skin’s ability to produce radicals, the nanoparticles do not play a significant role in the concentrations used . AcknowledgmentsThe authors thank Dr. Maxim Darvin for his assistance during the experiments and Dr. Elena Zagainova from Nizhny Nov gorod Medical Academy (Russia) for the TEM photos of the particles. A.P.P. thanks Infotech Oulu and the Tauno Tönning Foundation (both Finland) and DAAD (Germany) for support of this study. This work was partially supported by the RFBR Grant No. 07-02-01000. ReferencesW. Dröge,
“Free radicals in the physiological control of cell function,”
Physiol. Rev., 82 47
–95
(2002). 0031-9333 Google Scholar
D. Darr and I. Fridovich,
“Free radicals in cutaneous biology,”
J. Invest. Dermatol., 102 671
–675
(1994). 0022-202X Google Scholar
J. Fuchs, T. Herrling, and N. Groth,
“Detection of free radicals in skin: a review of the literature and new developments,”
Oxidants and Antioxidants in Cutaneous Biology, 29 1
–17 Karger, Basel
(2001). Google Scholar
H. N. Ananthaswamy and W. E. Pierceall,
“Molecular mechanisms of ultraviolet radiation carcinogenesis,”
Photochem. Photobiol., 52 1119
–1136
(1990). 0031-8655 Google Scholar
T. Herrling, J. Fuchs, and N. Groth,
“Kinetic measurements using EPR imaging with a modulated field gradient,”
J. Magn. Reson., 154 6
–14
(2002). https://doi.org/10.1006/jmre.2001.2459 1090-7807 Google Scholar
M. Meinke, S. Haag, N. Groth, F. Klein, R. Lauster, W. Sterry, and J. Lademann,
“Method for detection of free radicals in skin by EPR spectroscopy after UV irradiation,”
SÖFW-J., 134 2
–11
(2008). 0942–7694 Google Scholar
T. Herrling, K. Jung, and J. Fuchs,
“Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin,”
Spectrochim. Acta, Part A, 63 840
–845
(2005). 0584-8539 Google Scholar
J. Nishi, R. Ogura, M. Sugiyama, T. Hidaka, and M. Kohno,
“Involvement of active oxygen in lipid peroxide radical reaction of epidermal homogenate following ultraviolet light exposure,”
J. Invest. Dermatol., 97 115
–119
(1991). 0022-202X Google Scholar
R. Ogura, M. Sugiyama, J. Nishi, and N. Haramaki,
“Mechanism of lipid formation following exposure of epidermal homogenate to ultraviolet light,”
J. Invest. Dermatol., 97 1044
–1047
(1991). 0022-202X Google Scholar
T. Herrling, J. Fuchs, J. Rehberg, and N. Groth,
“UV-induced free radical in the skin detected by ESR spectroscopy and imaging using nitroxides,”
Free Radic Biol. Med., 35 59
–67
(2003). 0891-5849 Google Scholar
B. Collins, T. O. Poehler, and W. A. Bryden,
“EPR persistence measurements of UV-induced melanin free radicals in whole skin,”
Photochem. Photobiol., 62 557
–560
(1995). 0031-8655 Google Scholar
H. Trommer, R. Böttcher, A. Pöppl, J. Hoentsch, S. Wartewig, and R. H. H. Neubert,
“Role of ascorbic acid in stratum corneum lipid models exposed to UV irradiation,”
Pharmacol. Res., 19 982
–990
(2002). 1043-6618 Google Scholar
H. Trommer and R. H. H. Neubert,
“Screening for new antioxidative compounds for topical administration using skin lipid model systems,”
J. Pharm. Pharm. Sci., 8 494
–506
(2005). 1482-1826 Google Scholar
T. Herrling, L. Zastrow, J. Fuchs, and N. Groth,
“Electron spin resonance detection of UVA-induced free radicals,”
Skin Pharmacol. Appl. Skin Physiol., 15 381
–383
(2002). 1422-2868 Google Scholar
U. Diebold,
“The surface science of titanium dioxide,”
Surf. Sci. Rep., 48 53
–229
(2003). https://doi.org/10.1016/S0167-5729(02)00100-0 0167-5729 Google Scholar
L. Cao, A. Huang, F.-J. Spiess, and S. L. Suib,
“Gas-phase oxidation of 1-butene using nanoscale photocatalysts,”
J. Catal., 188 48
–57
(1999). https://doi.org/10.1006/jcat.1999.2596 0021-9517 Google Scholar
Z. Zhang, C.-C. Wang, R. Zakaria, and J. Y. Ying,
“Role of particle size in nanocrystalline -based photocatalysts,”
J. Phys. Chem. B, 102 10871
–10878
(1998). https://doi.org/10.1021/jp982948+ 1089-5647 Google Scholar
A. V. Vorontsov, A. A. Altynnikov, E. N. Savinov, and E. N. Kurkin,
“Correlation of photocatalytic activity and diffuse reflectance spectra,”
J. Photochem. Photobiol., A, 144 193
–196
(2001). 1010-6030 Google Scholar
J. Lademann, H.-J. Weigmann, H. Schaefer, G. Mueller, and W. Sterry,
“Investigation of the stability of coated titanium microparticles used in sunscreens,”
Skin Pharmacol. Appl. Skin Physiol., 13 258
–264
(2000). 1422-2868 Google Scholar
H. Hidaka, S. Horikoshi, N. Serpone, and J. Knowland,
“In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation,”
J. Photochem. Photobiol., A, 111 205
–213
(1997). https://doi.org/10.1016/S1010-6030(97)00229-3 1010-6030 Google Scholar
R. Dunford, A. Salinaro, L. Cai, N. Serpone, S. Horikoshi, H. Hidaka, and J. Knowland,
“Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients,”
FEBS Lett., 418 87
–90
(1997). https://doi.org/10.1016/S0014-5793(97)01356-2 0014-5793 Google Scholar
W. G. Warner, J.-J. Yin, and R. R. Wei,
“Oxidative damage to nucleic acids photosensitized by titanium dioxide,”
Free Radic Biol. Med., 23 851
–858
(1997). 0891-5849 Google Scholar
COLIPA, European Cosmetic, Toiletry and Perfumery Association, “COLIPA sun protection factor test method,”
(1994) Google Scholar
S. Haag, Nachweis von freien Radikalen in biologischen Proben mittels ESR-Spektroskopie,
(2007) Google Scholar
H.-J. Weigmann, J. Lademann, H. Meffert, H. Schaefer, and W. Sterry,
“Determination of the horny layer profile by tape stripping in combination with optical spectroscopy in the visible range as a prerequisite to quantify percutaneous absorption,”
Skin Pharmacol. Appl. Skin Physiol., 12 34
–45
(1999). 1422-2868 Google Scholar
A. P. Popov, J. Lademann, A. V. Priezzhev, and R. Myllylä,
“Reconstruction of stratum corneum profile of porcine ear skin after tape stripping using UV/VIS spectroscopy,”
Proc. SPIE, 6628 66281S-1
–6
(2007). https://doi.org/10.1117/12.729525 0277-786X Google Scholar
M. E. Darvin, I. Gersonde, M. Meinke, W. Sterry, and J. Lademann,
“Noninvasive in vivo determination of the carotenoids beta-carotene and lycopene concentrations in the human skin using the Raman spectroscopic method,”
J. Phys. D, 38 2696
–2700
(2005). https://doi.org/10.1088/0022-3727/38/15/023 0022-3727 Google Scholar
⟨MieTab is a Mie scattering program for Microsoft Windows,”
http://amiller.nmsu.edu/mietab.html Google Scholar
K. D. O. Jackson,
“A guide to identifying common inorganic fillers and activators using vibrational spectroscopy,”
Internet J. Vib. Spectros., 3
(1998) http://www.ijvs.com/volume2/edition3/section3.html#ackson Google Scholar
A. P. Popov, A. V. Priezzhev, J. Lademann, and R. Myllylä,
“ nanoparticles as effective UV-B radiation skin-protective compound in sunscreens,”
J. Phys. D, 38 2564
–2570
(2005). https://doi.org/10.1088/0022-3727/38/15/006 0022-3727 Google Scholar
G. E. Jellison Jr., L. A. Boatner, and J. D. Budai,
“Spectroscopic ellipsometry of thin film and bulk anatase ,”
J. Appl. Phys., 93 9537
–9541
(2003). https://doi.org/10.1063/1.1573737 0021-8979 Google Scholar
B. R. Palmer, P. Stamatakis, C. F. Bohren, and G. C. Salzman,
“A multiple scattering model for opacifying particles in polymer films,”
J. Coat. Technol., 61 41
–47
(1989). 0361-8773 Google Scholar
A. P. Popov, J. Lademann, A. V. Priezzhev, and R. Myllylä,
“Effect of size of nanoparticles embedded into stratum corneum on UVA and UVB sun-blocking properties of the skin,”
J. Biomed. Opt., 10 064037-1-9
(2005). https://doi.org/10.1117/1.2138017 1083-3668 Google Scholar
A. P. Popov, A. V. Priezzhev, J. Lademann, and R. Myllylä,
“The effect of nanometer particles of titanium oxide on the protective properties of skin in the UV region,”
J. Opt. Technol., 73 208
–211
(2006). 1070-9762 Google Scholar
A. P. Popov, A. V. Priezzhev, J. Lademann, and R. Myllylä,
“Effect of multiple light scattering by titanium dioxide nanoparticles implanted into a superficial skin layer on radiation transmission in different wavelength regions,”
Quantum Electron., 37 17
–21
(2007). 1063-7818 Google Scholar
|