|
|
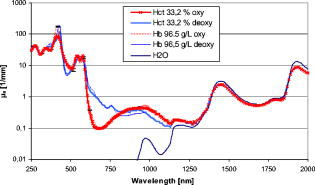
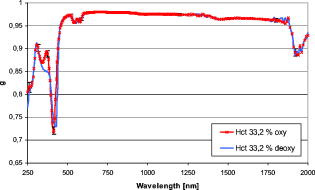
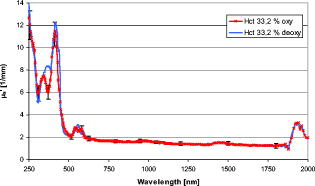
1.IntroductionThe development of spectroscopic methods for blood analysis requires knowledge of the light-scattering and absorption properties of human red blood cells (RBCs). It is also important for many diagnostic and therapeutic applications in laser medicine, hematology, and medical routine diagnosis. Hemoglobin oxygenation, which has a significant influence on the optical behavior of red blood cells, is an especially important diagnostic parameter in the fields of heart surgery, intensive care, and neonatology. Possible applications are measurement of the blood oxygen saturation in cardiopulmonary systems or measurement of the brain’s oxygen supply. According to the radiation transport theory, the optical properties of red blood cells can be described by the intrinsic optical parameters: absorption coefficient , scattering coefficient , and anisotropy factor , together with the appropriate phase function. Various approaches have been taken to determine the optical properties of blood cells.1, 2, 3, 4, 5, 6, 7, 8 In most cases, the investigated wavelength range did not extend to 1100 or . Due to the high optical density of blood, especially at physiological concentrations, it has not been possible, in most cases, to determine the parameters and separately. Only the effective scattering coefficient could be determined for single wavelengths or for small spectral ranges where the absorption of hemoglobin is low. Using the double integrating sphere technique combined with an inverse Monte Carlo simulation (iMCS),2, 4, 9 all three optical parameters for a flowing red blood cell suspension could be determined independently in the spectral range of , including the spectral areas of high hemoglobin absorption and the two distinct absorption peaks of water. It is known that the optical behavior of blood depends on various physiological parameters,10, 11, 12 one of the most important being the oxygen saturation of the hemoglobin. It is well known that a change in the oxygen saturation causes characteristic changes in the absorption behavior of hemoglobin, which determines the absorption of red blood cells. It is also known that changes in can influence the scattering properties, especially the anisotropy factor .4, 13 With regard to the physiological importance of the oxygen saturation and the increasing application of optical methods in medicine, it is a matter of some interest as to whether the oxygen saturation can influence the scattering properties. The aim of the present study is to determine the optical parameters , , and of a flowing red blood cell suspension at physiological hematocrit (Hct) in the wavelength range above and to investigate the influence of oxygen saturation on the optical properties in the wavelength range . To do this, the optical parameters , , and were determined for human red blood cells (RBCs) for both the fully oxygenated (SAT 100%) and deoxygenated states (SAT 0%). In order to facilitate comparison with data from the literature, was also calculated. In all experiments, native RBCs were suspended in saline solution and kept flowing with a wall shear rate of to avoid cell aggregation.4 2.Material and Methods2.1.Blood Cell PreparationIn order to measure native RBCs in saline solution, three samples of fresh human erythrocytes from a healthy blood donor were centrifuged three times and washed with isotonic phosphate buffer ( , pH 7.4) to remove the blood plasma and free hemoglobin. This procedure does not influence the biological function of the RBCs but inhibits the formation of aggregates. The hematocrit was determined using a red blood cell counter (Micros 60 OT 18, ABX Diagnostics, Montpellier, France). To investigate the influence of the oxygen saturation, the blood sample was adjusted to a Hct of 32.2% and the sample was divided in half. One part was equilibrated with a gas mixture of 96% and 4% to keep the sample in the fully oxygenated state; the other part was equilibrated with a gas mixture of 96% and 4% , after addition of 0.3% sodium dithionite to ensure complete deoxygenation.9 The oxygen saturation was determined with a blood gas analyzer (OPTI Care, AVL Medizintechnik GmbH, Bad Homburg). A miniaturized blood circulation setup was used with a roller pump (Sorin Group, Germany) and a blood cell reservoir, which was constantly aerated with the gas mixture. The temperature was kept constant at . The red blood cell suspension was gently stirred to avoid uncontrolled sedimentation or cell aggregation within the reservoir. The cell suspension was kept flowing by a customized turbulence-free cuvette with laminar flow and a sample thickness of . A cuvette thickness of approximately ensures multiple scattering and significant light transmission as well as avoiding obstruction phenomena. The flow was adjusted for each sample to keep constant wall shear rates of at the inner cuvette surfaces, avoiding aggregation or sedimentation at flow stop and shear-rate-induced deformation of the cells at high shear rates above (Refs. 11, 14). 2.2.Spectral MeasurementsThe diffuse reflectance , the total transmission , and the diffuse transmission ( within an aperture angle of ) of all blood cell samples were measured using an integrating sphere spectrometer (Lambda 900, Perkin Elmer, Rodgau-Jügesheim, Germany) in the spectral range of at intervals in the range and at intervals above . The optical arrangement of a sample and a reference beam compensates for light intensity shifts and changes in the inner sphere reflectivity by positioning the glass cuvette in front of or behind the sphere. For the measurement of , the reflectance port of the sphere is closed with a diffuse reflecting Spectralon standard. is measured after the standard is removed so that nonscattered and scattered transmitted light leaves the sphere within an angle of . is measured relative to a certified reflectance standard, and the Fresnel reflectance of the cuvette glass leaves the sphere. The experimental setup allowed measurement of the macroscopic radiation distribution with an error of less than 0.1% and has already been described by Friebel 4 A special inverse Monte Carlo simulation (iMCS) program was used, including forward Monte Carlo simulations to calculate the parameters , , and iteratively on the basis of a given phase function and the measured values for reflection and transmission. It considers all kinds of radiation losses by simulating the exact geometry of the illuminating light beam and of the sample, the cuvette, and the integrating sphere, including all diaphragms and apertures. As starting values, the parameters , , and were estimated from the Kubelka-Munk theory, and the approximation to the measured values was carried out by calculating a gradient matrix.4 Hct-dependent effective phase functions for RBCs flowing with a shear rate of were evaluated previously using the double integrating sphere technique.4 The iMCS was carried out using the Reynolds-McCormick phase15 function with for Hct 33.2%. An error threshold of 0.1%, i.e., the difference between measured and simulated macroscopic radiation distribution, was used for the calculation of the intrinsic optical parameters of the red blood cells. A total of three independent measurement series were carried out for all three samples, and independently simulated, using for each simulation. Due to the strong interindividual differences of RBCs, standard data can be obtained only by taking the mean values of many different samples. In order to exclude the optical influence due to this biological variability of the RBCs, all measured changes in the optical properties induced by reducing the oxygen saturation in the wavelength range were calculated as relative values related to those determined at 100% oxygen saturation. The absolute values were obtained by multiplication with the averaged parameters of a standard RBC suspension with the same Hct 33.2% (Ref. 12). As standard data are available only in the spectral range , the optical parameters determined in this experiment for the wavelength range were linearly adapted to the standardized data in the range to reach a smooth junction in the region of . 3.ResultsIt is known that the optical parameters of blood samples from different donors with identical hematocrit can show considerable variability.12 Therefore, in prior studies, Hct-dependent standard blood parameters were defined as averaged values of a number of individual blood samples measured in the spectral range under standard conditions (wall shear rate , oxygen saturation 98 to 100%, osmolarity , pH 7.4, ).12 The resulting standard deviations of these standardized data, caused by the biological variability, averaged over all wavelengths are 4.9% for , 8.4% for , 12.8% for , and 16.0% for (Ref. 12) The standard deviations of three independent measurements made in this paper are for 2 to 4%, for and 3 to 8%, and for 8-12%, as shown in the diagrams. 3.1.AbsorptionFigure 1 shows the absorption spectrum of red blood cells of Hct 33.2% with an oxygen saturation of 100% and 0% in the wavelength range . For comparison with data from the literature, the absorption spectra of pure water and of hemoglobin solutions extrapolated to a hemoglobin concentration of (Ref. 16), are also depicted in the wavelength range .17 At complete oxygenation, the absorption spectrum shows the characteristic peaks at and the double peak at with values of . The extrapolated hemoglobin curve is between 250 and , within the error tolerance identical to the RBC absorption spectrum, with the exception of the maximum of at . This difference results from the phenomenon of absorption flattening and can be explained by the Sieve effect.18 Above , the curve increasingly approximates that of water absorption. Above , the RBC absorption follows exactly the shape of the water curve but with values 20 to 30% below the water absorption values. This can be explained by the absence of significant hemoglobin absorption in this spectral area leading to a decrease due to the reduced water content in the cells as a result of the replacement by the hemoglobin molecules. The curve of the deoxygenated sample shows characteristic changes in the absorption behavior with a wavelength shift of the maximum peak of and a unique peak at . This result also corresponds to the absorption curve of deoxygenated hemoglobin from the literature, taking into account that the Sieve effect induces a lower absorption of the red blood cell suspension compared to the hemoglobin solution with at . In the spectral range above , is independent of the hemoglobin oxygen saturation. 3.2.ScatteringFigure 2 shows the spectrum of the scattering coefficient of red blood cells of Hct 33.2% with an oxygen saturation of 100% and 0% in the wavelength range . In the spectral range up to , the spectrum shows the characteristic decreases correlated with the absorption peaks at and as described earlier.4 According to the phenomenon of the absorption-induced decrease in the curve of completely deoxygenated RBCs shows the corresponding changes. The wavelength of the local minimum shifts from , and the double peak at is replaced by the single peak at . After reaching the maximum in the region of , with values between 83 and , decreases continuously with increasing wavelength to values of about at , independent of the oxygen saturation. This decrease is with and near to the theoretically expected value of according to Mie theory when the sphere size is large compared to the wavelength.19 3.3.AnisotropyFigure 3 shows the values of the anisotropy factor of RBCs suspended in saline solution for two different oxygen saturation states, dependent on wavelength. The anisotropy factor follows the absorption spectrum inversely with the distinct minimum of 0.72 at at an oxygen saturation of 100%, as described earlier.4 In the completely deoxygenated state, the minimum shifts to . The local absorption minimum in the oxygenated state at results in a relative maximum, whereas this maximum disappears as expected in the deoxygenated state. The double minimum at in the oxygenated state turns into a single minimum at with complete deoxygenation. Above , where is at the maximum level of 0.977, the spectrum of the oxygenated RBCs and the deoxygenated RBCs are equal. The value of decreases continuously to about 0.96 at , with the exception of a slight minimum at and a distinct local minimum at with a value of 0.87. In contrast to the scattering parameter , appears to be influenced not only by the hemoglobin absorption in the wavelength range below , i.e., absorption within the scattering cells, but also by the absorption maxima of water in the region of and , i.e., absorption within the medium and a reduced absorption within the scatterer. 3.4.Effective Scattering CoefficientFigure 4 gives the calculated values of the effective scattering coefficient for both oxygen saturation states in the wavelength range . If compared to the anisotropy factor, shows inverse spectra for both 100% and 0% oxygen saturation, which reflects the absorption spectrum. The maximum for the deoxygenated state is at in the observed spectral range. The other distinct maximum is at . At , the characteristic relative single maximum is about . At complete oxygen saturation, the respective relative maxima are at , at , and the double peak at with values in the region of . Above , is in the range of and decreases continuously and independently of oxygen saturation to values of about at . At , shows the water-absorption-induced maximum with values of . Corresponding to the minimum value of at , a local maximum is visible but not significant. 4.Discussions4.1.Oxygenation-Induced EffectsIt could be shown that differences in the oxygen saturation induce changes not only in absorption but also in the optical scattering parameters , and . This is due to the characteristic change in the absorption behavior of hemoglobin. As the hemoglobin absorption is invariant against the oxygen saturation between 1300 and but exhibits strong saturation dependence in the range , the induced changes of the scattering parameter occur mainly between 250 and , whereas the saturation-induced changes of and occur only between 250 and . The strongest oxygen saturation dependence of , in relation to the oxygenated state, is at wavelengths and , with a decrease of 4.5%, and in the range , with a maximal decrease of 12%. Due to changes in saturation, the maximum change in is 15% at and 5% at . In the range , decreases by about 2%. Above , the induced changes in are not significant. Apart from absorption, shows the strongest oxygen dependence at the same wavelength regions as . The maximum change in of about 35% is at , up to 25% in the range and 15% at At increases by 10%, which means that significant changes have to be considered when the scattering behaviour of RBCs is investigated and there are changes in the oxygen saturation. The results partly contradict earlier results published by our group9 because the scattering has now been shown to be influenced by the absorption properties. This has been shown using a commercially available spectrometer, which offers a much higher degree of accuracy and considerably shorter measurement times, leading to a reduction in the influence of hemolysis-induced optical effects. These results are partly in agreement with the data presented by Faber,10 who calculated the optical parameters in the wavelength range via Mie theory from the complex refractive indices of oxygenated and deoxygenated hemoglobin solutions taken from porcine blood. In that paper, shows similar characteristic differences between deoxygenated RBCs and fully oxygenated RBCs. The difference in the absolute values are of minor importance since Faber’s values are linearly calculated from the scattering cross sections for a hematocrit of 50%, assuming independent scattering of the cells, which is not obvious at such high cell concentrations. The minimum value at shifts to , and the maximum value in the area of shifts to a more pronounced peak at . The double minimum at changes into a single minimum at , and in the range , the curve of the deoxygenated RBCs runs below the curve of oxygenated RBCs. It is only in the spectral range that the data do not fit with the data presented here. This is possibly due to differences in the calculated complex refractive index of hemoglobin by Faber, which is quite different from other measured index values in this spectral range.16 The anisotropy factor of oxygenated RBCs shows also a minimum near , which shifts to longer wavelengths when deoxygenated. In the same way, the double minimum at vanishes. The constant higher level of the deoxygenation curve compared to the oxygenation curve above together with the behavior of in the wavelength range could not be observed in the experiments presented here. Also, the absolute values of Faber are higher than the ones presented here, which can also be explained by assuming independent scattering within the calculation. Even though Mie theory fails to predict the absolute values of the scattering parameters and at physiological hematocrit values,4 special phenomena can be recognized in the spectra that could be calculated according to Mie theory. For example, the relative minima of at 540 and induced by the relative absorption maxima at the same wavelengths can also be seen in Mie calculations. With this in mind, the influence of the absorption on is also explainable in principle by the Mie theory.4 However, the phenomenon of the significant decrease of the anisotropy factor when is increased is more difficult. This behavior shows Mie theory only if were to be 100 times greater. Since the decrease of tended to become smaller when the RBCs were changed to a more spherical shape, it can be assumed that the nonspherical shape is responsible for this discrepancy.4 Independent of this, it seems to be feasible that an increase in the complex refractive index results in an increase in the reflection and a decrease in transmittance, resulting in increased backscattering of the photons, which means a smaller . There are further indications in the literature that absorption peaks in light scattering media may lead to a reduction of and and an increase in (Refs. 8, 10, 13). It is of considerable interest that the changes in water absorption at 1450 and induce changes in in the same way as the strong changes in hemoglobin absorption do. In both cases, absorption is increased within the scatterer. In the case of water absorption, there is an additional and stronger absorption increase within the surrounding medium. shows no comparable change at the spectral regions of high water absorption, whereas and are influenced by the kind of absorption peaks described earlier. Classical Mie theory does not describe the scattering behavior of spheres in an absorbing medium. However, by using a modified Mie theory, it could be shown that under special conditions, similar to the blood situation for spheres and cylinders absorption within the surrounding medium, a decrease in and , could be induced.20, 21 These results at least partly correspond to the data presented in this study and the results of other previous studies.4, 11, 18 5.ConclusionsThis work presents the optical parameters , , and of oxygenated and deoxygenated human RBCs with hematocrit 33.2% in the wavelength range . It shows that the oxygen saturation of hemoglobin has an influence not only on the absorption coefficient , but also on the scattering parameters , and of human RBCs, which are induced by changes in the absorption behavior of hemoglobin. In addition, as the investigated wavelength range includes the spectral region above , where the absorption of hemoglobin tends to be negligible and the absorption of water has increasing values, it is possible to compare the physically interesting optical effect of absorption within the blood cell and the surrounding medium. It could be shown that in both cases, an increase in absorption can lead to a decrease in and an increase in . Therefore, it follows that the oxygen saturation has to be taken into account in order to estimate the scattering properties of blood. Moreover, not only the hemoglobin absorption within the blood cell but also the water absorption within the cell and the medium can influence the scattering properties of RBCs. AcknowledgmentsThis work was supported by the Federal Ministry of Education and Research (Grant No. 13N7522). The authors also want to thank the Sorin Group Deutschland GmbH, Munich, for placing the equipment at our disposal. The blood bags were kindly provided by the Department of Transfusion Medicine, Charité-Universitätsmedizin Berlin, Germany. ReferencesL. G. Lindberg and P. A. Öberg,
“Optical properties of blood in motion,”
Opt. Eng. (Bellingham), 32
(2), 253
–257
(1993). https://doi.org/10.1117/12.60688 0091-3286 Google Scholar
M. Meinke, G. Müller, J. Helfmann, and M. Friebel,
“Optical properties of platelets and blood plasma and their influence on the optical behaviour of whole blood in the VIS-NIR wavelength range,”
J. Biomed. Opt., 12 014024
(2007). https://doi.org/10.1117/1.2435177 1083-3668 Google Scholar
A. N. Yaroslavsky, I. V. Yaroslavsky, T. Goldbach, and H. J. Schwarzmaier,
“The optical properties of blood in the near infrared spectral range,”
314
–324
(1996). Google Scholar
M. Friebel, A. Roggan, G. Müller, and M. Meinke,
“Determination of optical properties of human blood in the spectral range using Monte Carlo simulations with hematocrit-dependent effective scattering phase functions,”
J. Biomed. Opt., 11
(3), 031021
(2006). https://doi.org/10.1117/1.2203659 1083-3668 Google Scholar
M. Hammer, D. Schweitzer, B. Michel, E. Thamm, and A. Kolb,
“Single scattering by red blood cells,”
Appl. Opt., 37
(31), 7410
–7418
(1998). https://doi.org/10.1364/AO.37.007410 0003-6935 Google Scholar
A. Nilsson, P. Alsholm, A. Karlson, and S. Andersson-Engels,
“T-matrix computation of light scattering by red blood cells,”
Appl. Opt., 37
(13), 2735
–2748
(1998). https://doi.org/10.1364/AO.37.002735 0003-6935 Google Scholar
J. M. Steinke and A. P. Sheperd,
“Comparision of Mie theory and light scattering of red blood cells,”
Appl. Opt., 27
(19), 4027
–4033
(1988). https://doi.org/10.1364/AO.27.004027 0003-6935 Google Scholar
D. J. Faber, F. J. van der Meer, M. C. G. Aalders, D. M. de Bruin, and T. G. van Leeuwen,
“Hematocrit-dependence of the scattering coefficient of blood determined by optical coherence tomography,”
58610W1
–9
(2005). Google Scholar
A. Roggan, M. Friebel, K. Dörschel, A. Hahn, and G. Müller,
“Optical properties of circulating human blood in the wavelength range ,”
J. Biomed. Opt., 4
(1), 36
–46
(1999). https://doi.org/10.1117/1.429919 1083-3668 Google Scholar
D. J. Faber, M. C. G. Aalders, E. G. Mik, B. A. Hooper, M. J. C. van Gemert and T. G. van Leeuwen,
“Oxygen saturation-dependent absorption and scattering of blood,”
Phys. Rev. Lett., 93 028102
(2004). https://doi.org/10.1103/PhysRevLett.93.028102 0031-9007 Google Scholar
M. Friebel, G. Müller, J. Helfmann, and M. Meinke,
“Influence of shear rate on the optical properties of human blood in the spectral range ,”
J. Biomed. Opt., 12 054005
(2007). https://doi.org/10.1117/1.2799154 1083-3668 Google Scholar
M. Meinke, G. Müller, J. Helfmann, and M. Friebel,
“Empirical model functions to calculate hematocrit dependent optical properties of human blood,”
Appl. Opt., 46 1742
–1753
(2007). https://doi.org/10.1364/AO.46.001742 0003-6935 Google Scholar
A. N. Bashkatov, E. A. Genina, V. I. Kochubey, A. A. Gavrilova, S. V. Kapralov, V. A. Grishaev, V. V. Tuchin,
“Optical properties of human stomach mucosa in the spectral range from : prognosis for gastroenterology,”
Med. Laser Appl., 22 95
–104
(2007). https://doi.org/10.1016/j.mla.2007.07.003 1615-1615 Google Scholar
A. Priezzhev, O. M. Ryaboshapka, N. N. Firsov, and I. V. Sirko,
“Aggregation and disaggregation of erythrocytes in whole blood: study by backscattering technique,”
J. Biomed. Opt., 4
(1), 76
–84
(1999). https://doi.org/10.1117/1.429923 1083-3668 Google Scholar
L. Reynolds and N. McCormick,
“Approximate two-parameter phase function for light scattering,”
J. Opt. Soc. Am., 70 1206
–1212
(1980). https://doi.org/10.1364/JOSA.70.001206 0030-3941 Google Scholar
M. Meinke and M. Friebel,
“Determination of the complex refractive index of highly concentrated hemoglobin solutions using transmittance and reflectance measurements,”
J. Biomed. Opt., 10 064019
(2005). https://doi.org/10.1117/1.2138027 1083-3668 Google Scholar
UV-VIS Atlas of Organic Compounds, 2nd ed.Wiley-VHC, Weinheim, Germany
(1992). Google Scholar
R. N. Pittman, Ann. Biomed. Eng., 14 119
–137
(1986). https://doi.org/10.1007/BF02584263 0090-6964 Google Scholar
M. Kerker, Scattering of Light and Other Electromagnetic Radiation,
(1969) Google Scholar
Q. Fu and W. Sun,
“Mie theory for light scattering by a spherical particle in an absorbing medium,”
Appl. Opt., 40 1354
–1361
(2001). https://doi.org/10.1364/AO.40.001354 0003-6935 Google Scholar
W. Sun, N. G. Loeb, and B. Lin,
“Light scattering by an infinite circular cylinder immersed in an absorbing medium,”
Appl. Opt., 44 2338
–2342
(2005). https://doi.org/10.1364/AO.44.002338 0003-6935 Google Scholar
|