|
|
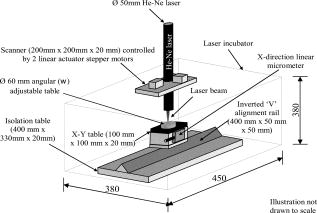
1.IntroductionTissue-engineered constructs are designed, and used, to replace diseased or damaged tissues, particularly the load-bearing soft connective tissues, such as cartilage,1 tendon,2 ligament,3 blood vessels,4 heart valves,5 and skin,6, 7, 8 including tissue-engineered skin products approved by the US Food and Drug Administration. Tissue-engineered structures replicate some aspects of tissue function, but the central aim is to allow the bioresorption of the construct and its replacement by natural body tissue. Tissue-engineered skin substitutes generally consist of cells and extracellular matrix materials either alone or in combination. Epidermal keratinocytes facilitate the development of an epidermal layer on the surface of the skin substitute, and fibroblasts facilitate the development of a dermal layer. The extracellular matrix provides a three-dimensional (3-D) framework for the fibroblasts and provides biological support both for the fibroblasts and the overlying keratinocytes. Following the pioneering work of Burke,9 collagen has been widely used as a matrix material in the form of sponges, gels, or lattices.10 Naturally occurring glycosaminoglycans (GAG), such as chondroitin-6-sulphate , and cross-linking agents may be added to matrix materials to improve their mechanical and biological properties.11, 12, 13 Fibroblast-populated collagen lattices (FPCL) have been extensively studied and used in tissue-engineered skin replacements, and fibroblasts cultured in a lattice mimic the in vivo situation in the dermis, displaying similar cell shape, mitotic activity, and collagen synthesis compared to human dermis.14 The main drawback of FPCL is that the interaction between fibroblasts and the matrix produces contraction of the lattice, which can lead to a reduction up to 20% of the initial area.15 The contraction of the skin substitute reduces the area available for skin replacement and risks exposing the wound to tissue inflammation, maceration, or desiccation and bacterial infection.16 FPCLs have been used as models of healing wounds, in which contraction may also occur. Mester 17 reported that low-level laser irradiation induced healing of chronic nonhealing soft tissue ulcers, and this formed the genesis of clinical low-level laser treatment (LLLT). Other studies18 produced equivocal results because of the variability of clinical wounds and patient status, and other poorly controlled variables, such as patient mobility. In vitro studies, however, show that LLLT can modulate a variety of biological processes within cells.19 We investigated the use of LLLT in vitro to determine whether it represented a means of modifying the contraction of FPCLs. In this context, we used FPCL following the compositions recommended by Yannas and coworkers,20 which led to the collagen-chondroitin sulphate dermal regeneration template known as Integra. Contraction of the lattices was measured at a density of fibroblasts previously observed to accomplish a reduction in area of greater than 50% in in a model of wound healing.12 2.Materials and Methods2.1.Preparation of Collagen LatticesThe test specimens were collagen lattices containing 0.3% (w/v) collagen supplemented with 20% w/v GAG. Lattices were formed by mixing type I collagen solution acid extracted from rat tail tendon,21 a mixture of ’s Modified Eagle’s Medium (DMEM) and NaOH (2:1), and the GAG, , prepared at in -free DMEM, at a ratio of 7:1:2. The pH was adjusted to 8 to 8.5 by dropwise addition of NaOH. Five ml aliquots of the collagen lattice solution were pipetted into a polycarbonate (Stockline Plastics Ltd., Glasgow, UK) cell chamber measuring with an outer diameter of . In order to have a hydrophobic cell chamber to inhibit cell attachment, the cell chamber was siliconized for in a fume cupboard using Sigmacote. Before cell seeding, the collagen lattices were washed twice by incubating them in complete DMEM containing 10% v/v fetal calf serum, penicillin/ streptomycin, and nonessential amino acids. 3T3 mouse fibroblasts were seeded onto the set collagen lattice at a density of in of complete DMEM and incubated for at (Heraeus Electronics, Hanau, Germany) in an atmosphere of 5% in air to allow cell attachment. After this time period, the FPCLs were detached from the walls of the cell chamber and floated freely in the culture medium. Laser treatment was carried out after this attachment period. Thereafter, cells were cultured for up to with a daily change of of the culture medium. Collagen lattices were also cultured in the absence of cells and laser treated by following the same protocol as described earlier, but omitting the cells. 2.2.Laser IrradiationLattices were irradiated using a continuous wave, linearly polarized He-Ne laser (3225H-PC, Hughes Aircraft Co.) operating at with a beam profile at TEM00 mode delivering a laser beam with a spot size of . The output power was measured using Molectron MAX 5200 and Metrologic power meters calibrated at . The body of the laser was attached to a scanner, which was controlled by two linear actuator stepper motors. A host personal computer with a Pentium II processor running on LabVIEW 6.0 (Laboratory Virtual Instrument Engineering Workbench, National Instruments Corp.) and a development environment based on the graphical programming language G were used to control the scanning speed of both motors, (cm/s), which determined the laser fluence or energy density, , the relation being: where (cm) is the total distance travelled by the laser beam, (W) is the output power, and is the area of treatment.The laser, delivering an output power of , was positioned above the surface of the specimen. A holder specially fabricated to accommodate the cell chamber was attached to the top of the angular table together with a linkage connecting the linear micrometer with the table, and an alignment rail enabled precise positioning of the fibroblast-populated collagen lattices (FPCL) for LLLT treatment (Fig. 1 ). The entire computer-assisted experimental setup was housed in a controlled environment of . 2.3.Analysis of Cell Viability, Morphology, Mitochondrial Membrane Depolarization, and Collagen Fiber Content of the MatrixTo examine the viability of the fibroblasts in the FPCL, ethidium bromide (EB) and 5-carboxyfluorescein diacetate (CFDA) staining was used.22 CFDA stains the viable 3T3 fibroblasts green, and EB stains the nuclei of cells with damaged membranes red. The lattices were washed twice with phosphate buffered saline (PBS), and then of 0.1% (w/v) EB in PBS was added. The lattices were kept in the dark at room temperature for before washing them again three times with PBS. Three ml of CFDA in PBS was then added, and the lattices were kept in the dark at . After , the lattices were again washed three times with PBS and viewed under the confocal laser scanning microscope (Leica CLSM). For the detection of cytoskeletal damage, fluorescein isothiocyanate (FITC)-labeled phalloidin was used to stain the actin, as described by Wulf 23 The lattices were fixed by immersing them in PBS containing 4% formaldehyde for at room temperature. Fixed samples were then washed three times with PBS and incubated with 1:500 dilution of FITC-phalloidin for in the dark at room temperature, washed again three times with PBS, and examined by CLSM. To visualize the collagen fibers in the collagen-GAG lattices, both the LLLT-treated and control lattices were immersed for at room temperature in 0.1% (w/v) acriflavine, a mixture of 3,6-diamino-10-methylacridinium (acriflavine) and 3,6-diaminoacridine (proflavine). Samples were washed three times with PBS to remove excess stain before viewing on the CLSM. Examination by CLSM for CFDA/EB, FITC-phalloidin, and acriflavine stained samples used excitation with a dichroic beamsplitter in place. Data from the two channels were collected, using a barrier filter of in channel 1 and a bandpass filter of in channel 2. The objective lens was a immersion lens (NA 0.75). To determine the mitochondrial membrane potential, accumulation of the fluorescent potentiometric dye tetramethylrhodamine ethyl ester (TMRE) was measured in the control and LLLT-treated 3T3 cells. For this method, the cells were cultured on polystyrene Petri dishes and not in collagen lattices. Cells were seeded at in complete DMEM and treated with the laser after allowing for attachment as with the FPCL samples. As a positive control, cultured cells were also incubated with carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP) for at to disrupt the mitochrondrial membrane potential. Cultures of control, LLLT-treated, and FCCP-treated cells were incubated with TMRE in complete DMEM for , after which they were washed three times with PBS and imaged by -sectioning. Each section was , and a total of 18 sections were imaged. The images were collected using Lasersharp 2000 software (Bio-Rad Laboratories) running OS/2. Images were recorded using an MRC 1024 CLSM (Bio-Rad) coupled to a Nikon E600FN upright microscope using a water dipping lens . TMRE was excited at using a Krypton/Argon laser. A LP filter (Chrom, Inc.) was used to collect emission of light greater than . Time-dependent TMRE fluorescent changes were measured in frame scan mode. Projections of the image sections were created using a “maximum” alogarithm in the Bio-Rad Lasersharp 2000 software. Subsequently, the intensity of the cells was quantified in Scion Image. 2.4.Measurement of ContractionThe area of the lattices in all the experiments was measured daily using a light box over which the cell chambers were placed on a standard millimeter grid. The area of the specimen was then calculated using digital planimetry and imaging software (Scion Image, www.scioncorp.com). Using Scion Image, the file containing the digitized image of the collagen lattices was opened, the perimeter of the cell chamber outlined, and the original area calculated. The areas of the contracted lattices were measured in a similar way by first plotting the outline of the contracted lattices using a freehand selection tool in Scion Image and then instructing the program to measure the outlined area. The contracted area of the lattices was expressed as a percentage of the original area. 3.Results3.1.Lattice ContractionBoth the unseeded collagen lattices and the fibroblast populated lattices contracted, but the magnitude and time scale of the contraction were markedly different. Seeded lattices showed progressive contraction over the for which it was monitored (Figs. 2 and 3 ). The rate of contraction was greatest initially, during the first day of monitoring, and then it decreased nonlinearly. The maximum contraction occurred on day 7, the last day of monitoring, and this value was used to characterize the contraction of the FPCL. Collagen lattices in the absence of cells reached a maximum contraction on the second day and remained constant thereafter (Figs. 2 and 3). Fig. 2Contraction of control and LLLT-treated unseeded collagen lattices and FPCL at . Results are expressed as percentages of the original area of the collagen lattices and are the mean deviation (SD) of five repeated experiments. Diamonds indicate control (FPCL); triangles indicate control (collagen lattices); squares indicate LLLT at (FPCL); and circles indicate LLLT at (collagen lattices). 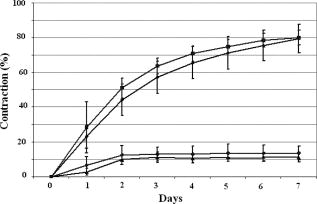 Fig. 3Contraction of control and LLLT-treated unseeded collagen lattices and FPCL at . Results are expressed as percentages of the original area of the collagen lattices and are the , . , comparing values from the LLLT-treated lattices with and without cells with those from untreated seeded and unseeded collagen lattices, respectively, by two-tailed Student’s -test. Diamonds indicate control (FPCL); triangles indicate control (collagen lattices); squares indicate LLLT at (FPCL); and circles indicate LLLT at (collagen lattices). 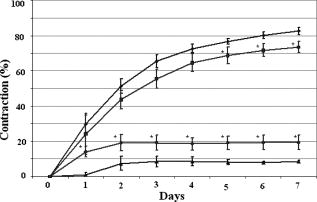 The values of the parameters used to characterize the contraction of the lattices in control and LLLT-treated samples are shown in Table 1 . Comparison of the values for control and laser-treated specimens using two-tailed Student’s -test showed that irradiation at fluences of 1, 2, and had no statistically significant effect. The effect of is shown on Fig. 3. Irradiation at produced significantly greater contraction of the unseeded lattices and reduced contraction of FPCL compared with values from the control unseeded collagen lattices and FPCL, respectively . The increase in contraction of the unseeded lattices was significant for all days after day 1, but the decrease in contraction of FPCL was significant for only the last of the monitoring period (Fig. 3). Table 1Contraction of FPCL and unseeded collagen lattices. Results are expressed as a percentage of the original area of the collagen lattice and represent the values after 7days in culture. Values are means of five experiments, with the standard deviation in parentheses. * Denotes that the differences between laser-treated and control values are statistically significant, by two-tailed Student’s t -test.
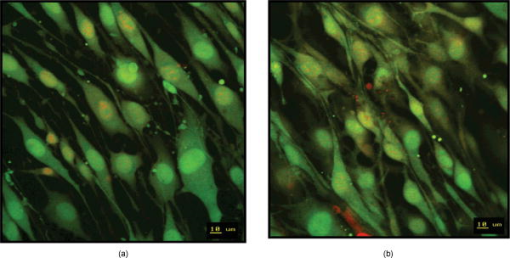
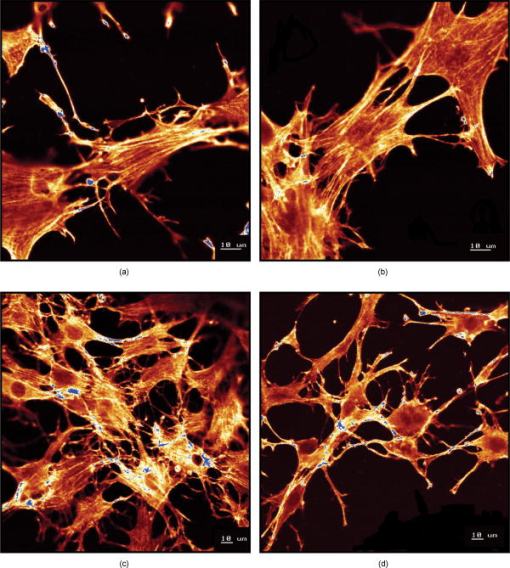
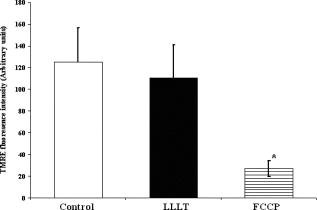
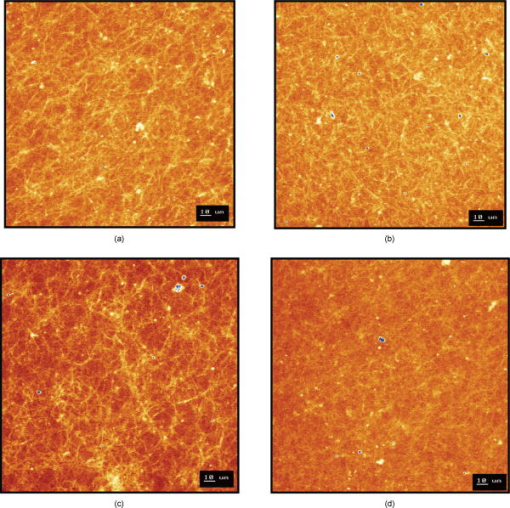
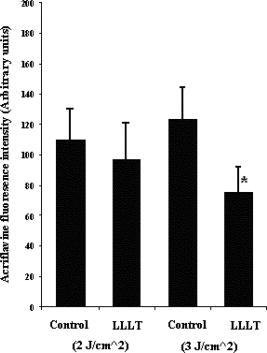
Using CLSM, no significant visual difference was observed in the morphology and viability of the cells using CFDA and EB between the control and LLLT-treated FPCL [Figs. 4a and 4b ]. Elongated fibroblastic morphology was maintained for up to after treatment, with typical alignment of cells in one direction. There was no discernable alteration in the number of nonviable cells after LLLT treatment. 3.2.Actin FilamentsFigure 5 shows the staining with FITC-labeled phalloidin after treatment with 2 and . Intracellular actin filaments are less pronounced in cells treated with than in either the controls or the cells treated with . There are few prominent fibers crossing the interior of the cells, and actin staining appears to be mainly around the cell perimeter. Figure 6 shows that LLLT treatment has no significant effect on the accumulation of TMRE within cells, indicating that it does not alter the mitochondrial membrane potential. In contrast, FCCP treatment markedly decreased TMRE accumulation. 3.3.Collagen FibersThe effect of LLLT treatment on the collagen fibers was investigated in cell-free matrices in order to distinguish the effect of laser irradiation from cell-induced changes. CLSM images of acriflavine stained collagen fibers at a depth of into the collagen-GAG matrices are shown in fig. 7 . The images show that there are fewer collagen fibers in the matrices treated with than in either controls or in samples treated with . This was observed in three separate experiments, and Fig. 8 illustrates the mean fluorescent intensities measured in all experiments. After treatment with , there was a significant decrease in fluorescent intensities measured at a depth of in the matrices. 4.DiscussionThe greatest rate of contraction of the FPCL was measured within the first day following cell attachment, and this is thought to be due to the forces generated during cell elongation.24 Freyman and coworkers also reported that the contractile force developed by fibroblasts bending or buckling collagen fibrils in the matrix during elongation reached a maximum in the first few hours of culture.24, 25 Laser irradiation modifies the extent of the contraction of unseeded and suspension fibroblast seeded collagen lattices, but only at an energy density of . The occurrence of significant photobiostimulation at , which was not observed at other fluencies, could be explained by Karu,26, 27 who reported that the fluence versus response curve was bell-shaped with a threshold, maximum, and phase of decline. Recent studies of the enhancement of porcine skin graft adherence using a light-activated process,28 the effect of pulsed radiation on HeLa cell attachment to extracellular matrix,29 and the effect of a He-Ne laser on the cloning efficiency of Chinese hamster ovary cells (CHO) and human skin fibroblasts (HSF)30 further endorse a nonlinear fluence-response relationship. The reduction in contraction in FPCL produced by laser irradiation at is statistically significant but is too small to be of great practical significance. Laser irradiation does, however, represent a simple applicable method of modifying contraction; it may be possible to further reduce contraction of FPCL by optimizing the procedure. Unfortunately, the mechanisms of the laser effect and contraction are not well understood. Nevertheless, both mechanisms must involve the fibroblast cell, the collagen substrate, or both. Investigations of the influence of low-level irradiation of 3T3 mouse fibroblasts cultured on collagen-GAG substrates previously showed that postattachment cells showed no difference in proliferation rates, evaluated by 5-carboxyflourescein diacetate, nor in cell viability, assessed using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay.8 CLSM studies using CFDA and EB showed that LLLT treatment did not alter the fibroblasts, viability, morphology, or ability to align in culture. There was some indication of an alteration in the distribution of actin with the cells after treatment with , and this may be related to their ability to contract in FPCL. The contractile activity of chondrocytes seeded in collagen-GAG matrices has been shown previously to parallel expression of smooth muscle actin,31, 32 and treatment that depleted actin fibers reduced the ability of the cells to contract. Accumulation of actin around the perimeter of a cell has been associated with early apoptosis.33 However, there was no evidence of an alteration in mitochondrial membrane potential after treatment with . Loss of the mitochondrial membrane potential is associated with the early stages of apoptosis and coincides with the opening of the mitochondrial permeability transition pores, allowing passage of ions and small molecules, resulting ultimately in release of cytochrome c into the cytosol.34 There is no evidence from the data shown that the redistribution of actin inside cells treated with is accompanied by apopotic events. Acriflavine staining showed a decrease in the number of collagen fibers in the cell-free collagen-GAG matrices that had been treated with . When fibroblasts contact with fibrils of collagen in the matrix, they attach to them, and compact them by drawing the fibrils toward themselves, thereby causing contraction of the matrix. With fewer fibrils to attach to, the cell-mediated contraction of the irradiated matrices is decreased. There may, however, be an unwanted side effect. The stiffness and strength of collagen lattices are correlated with fibril density,35 and fewer fibers may compromise the mechanical properties of FPCL. The contraction of the unseeded collagen substrate was significantly increased by laser irradiation at . The mechanism responsible for this increase in the absence of cells is unclear. There may be physical cross-linking or laser-induced photobiostimulatory effects on the collagen fibers causing an increase in the volume of water expressed from the lattice, resulting in increased contraction. It appears that the altered contraction of the FPCL caused by LLLT may be due to effects of the laser on both the collagen fibers of the matrix and the actin fibers inside the cell. Further study of the mechanical affects of laser irradiation of cell-free matrix may provide an explanation of the effect of irradiation and a route to the reduction of the contraction of FPCL. 5.ConclusionsLaser irradiation at an energy density of altered the contraction of FPCL over a subsequent period of . The contraction of FPCL was significantly less than control unirradiated specimens on the last three days of monitoring. Energy density was a significant factor—1, 2, and produced no alteration in the magnitude of contraction. The effect of irradiation was accompanied by altered distribution of actin fibers intracellularly and collagen fibers in the matrix. This resulted in altered interaction between the two proteins, which contributed to the decrease in contraction. AcknowledgmentsG.H. received an Overseas Research Scholarship (ORS), a University of Strathclyde Studentship, the Kenedi Bursary from the Bioengineering Unit, and support from the Agency for Science, Technology, and Research, Singapore. ReferencesL. Galios, A. M. Freyria, L. Grossin, P. Hubert, D. Mainard, D. Herbage, J. F. Stolz, P. Netter, E. Dellacherie, and E. Payan,
“Cartilage repair: surgical techniques and tissue engineering using polysaccharide- and collagen-based biomaterials,”
Biorheology, 41 433
–443
(2004). 0006-355X Google Scholar
J. Garvin, J. Qi, M. Maoloney, and A. J. Banes,
“Novel system for engineering bioartificial tendons and application of mechanical load,”
Tissue Eng., 9 967
–979
(2003). https://doi.org/10.1089/107632703322495619 1076-3279 Google Scholar
E. Gentleman, A. N. Lay, D. A. Dickerson, E. A. Nauman, G. A. Livesay, and K. C. Dee,
“Mechanical characterization of collagen fibers and scaffolds for tissue engineering,”
Biomaterials, 24 3805
–3813
(2003). https://doi.org/10.1016/S0142-9612(03)00206-0 0142-9612 Google Scholar
M. Rothenburger, W. Volker, J. P. Vischer, E. Berendes, B. Glasmacher, H. H. Scheld, and M. Deiwick,
“Tissue engineering of heart valves: formation of a three-dimensional tissue using porcine heart valve cells,”
ASAIO J., 48 586
–591
(2002). https://doi.org/10.1097/00002480-200211000-00003 1058-2916 Google Scholar
L. T. Jensen and N. B. Host,
“Collagen: scaffold for repair or execution,”
Cardiovasc. Res., 33 535
–539
(1997). https://doi.org/10.1016/S0008-6363(96)00247-7 0008-6363 Google Scholar
B. Pomahac, T. Svensjo, and F. Yao,
“Tissue engineered skin,”
Crit. Rev. Oral Biol. Med., 9 333
–344
(1998). https://doi.org/10.1177/10454411980090030601 1045-4411 Google Scholar
W. H. Eaglstein and V. Falanga,
“Tissue engineering for skin: an update,”
Adv. Chem. Ser., 39 1007
–1019
(1998). 0065-2393 Google Scholar
G. Ho, M. H. Grant, J. C. Barbenel, and C. J. Henderson,
“Low-level laser therapy on tissue-engineered skin substitutes: effect on the proliferation rate of 3T3 mouse fibroblast cells,”
Proc. SPIE, 5610 1469
–1475
(2004). 0277-786X Google Scholar
J. F. Burke, I. V. Yannas, W. C. Quinby Jr., C. C. Bondoc, and W. K. Jung,
“Successful use of physiologically acceptable artificial skin in the treatment of extensive burn injury,”
Ann. Surg., 194 413
–428
(1981). https://doi.org/10.1097/00000658-198110000-00005 0003-4932 Google Scholar
R. S. Kirsner, V. Falanga, and W. H. Eaglstein,
“The development of bioengineered skin,”
TIBTECH, 16 246
–249
(1998). Google Scholar
Y. S. Pek, M. Spector, I. V. Yannas, and L. J. Gibson,
“Degradation of a collagen-chondroitin-6-sulfate matrix by collagenase and chondroitinase,”
Biomaterials, 25 473
–482
(2004). https://doi.org/10.1016/S0142-9612(03)00541-6 0142-9612 Google Scholar
C. S. Osborne, W. H. Reid, and M. H. Grant,
“Investigation into the biological stability of collagen/chondroitin-6-sulphate gels and their contraction by fibroblasts and keratinocytes: the effect of crosslinking agents and diamines,”
Biomaterials, 20 283
–290
(1999). https://doi.org/10.1016/S0142-9612(98)00179-3 0142-9612 Google Scholar
C. S. Osborne, J. C. Barbenel, D. Smith, M. Savakis, and M. H. Grant,
“Investigation into the tensile properties of collagen/chondroitin-6-sulphate gels: the effect of crosslinking agents and diamines,”
Med. Biol. Eng. Comput., 36 129
–134
(1998). https://doi.org/10.1007/BF02522870 0140-0118 Google Scholar
F. Berthod and F. A. Auger, Skin Substitute Production by Tissue Engineering, 211
–237 Springler-Verlag, Heidelberg, Germany
(1997). Google Scholar
F. Berthod, D. Hayek, O. Damour, and C. Collombel,
“Collagen synthesis by fibroblasts—cultured within a collagen sponge,”
Biomaterials, 14 749
–754
(1993). https://doi.org/10.1016/0142-9612(93)90039-5 0142-9612 Google Scholar
E. Bell, B. Ivarsson, and C. Merrill,
“Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro,”
Proc. Natl. Acad. Sci. U.S.A., 6 1272
–1278
(1979). 0027-8424 Google Scholar
E. Mester, T. Spiry, B. Szende, and J. Tota,
“Effect of laser rays on wound healing,”
Am. J. Surg., 122 523
–535
(1971). https://doi.org/10.1016/0002-9610(71)90482-X 0002-9610 Google Scholar
C. Lucas, L. J. Criens-Poublon, C. T. Cockrell, and R. J. de Haan,
“Wound healing in cell studies and animal model experiments by low level laser therapy; were clinical studies justified? A systematic review,”
Lasers Med. Sci., 17 110
–134
(2002). https://doi.org/10.1007/s101030200018 0268-8921 Google Scholar
T. Karu,
“Effects of visible radiation on cultured cells,”
Photochem. Photobiol., 52 1089
–1098
(1990). https://doi.org/10.1111/j.1751-1097.1990.tb08450.x 0031-8655 Google Scholar
I. V. Yannas, J. F. Burke, P. L. Gordon, C. Huang, and R. H. Rubenstein,
“Design of an artificial skin. II. Control of chemical composition,”
J. Biomed. Mater. Res., 14 107
–131
(1980). https://doi.org/10.1002/jbm.820140203 0021-9304 Google Scholar
T. Elsdale and J. Bard,
“Collagen substrata for studies on cell behavior,”
J. Cell Biol., 54 626
–637
(1972). https://doi.org/10.1083/jcb.54.3.626 0021-9525 Google Scholar
G. C. McKay, C. Henderson, E. Goldie, G. Connel, C. Westmoreland, and M. H. Grant,
“Cryopreservation of rat hepatocyte monolayers: cell viability and cytochrome P450 content in post-thaw cultures,”
Toxicol. In Vitro, 16 71
–79
(2002). https://doi.org/10.1016/S0887-2333(01)00096-0 0887-2333 Google Scholar
E. A. Wulf, F. A. Deboben, H. Bautz, and T. H. Wieland,
“Fluorescent phallotoxin, a tool for the visualization of cellular actin,”
Proc. Natl. Acad. Sci. U.S.A., 76 4498
–4502
(1979). https://doi.org/10.1073/pnas.76.9.4498 0027-8424 Google Scholar
T. M. Freyman, I. V. Yannas, Y. S. Pek, R. Yokoo, and L. J. Gibson,
“Micromechanics of fibroblast contraction of a collagen-GAG matrix,”
Exp. Cell Res., 269 140
–153
(2001). https://doi.org/10.1006/excr.2001.5302 0014-4827 Google Scholar
T. M. Freyman, I. V. Yannas, R. Yokoo, and L. J. Gibson,
“Fibroblast contraction of a collagen-GAG matrix,”
Biomaterials, 22 2883
–2891
(2001). https://doi.org/10.1016/S0142-9612(01)00034-5 0142-9612 Google Scholar
T. I. Karu, O. Tiphlova, R. O. Esenaliev, and V. Letokhov,
“Two different mechanisms of low-intensity laser photobiological effects on Escherichia coli,”
J. Photochem. Photobiol., B, 24 155
–161
(1994). https://doi.org/10.1016/1011-1344(94)07016-4 1011-1344 Google Scholar
T. I. Karu, L. V. Pyatibrat, G. S. Kalendo, and R. O. Esenaliev,
“Effects of monochromatic low-intensity light and laser irradiation on adhesion of HeLa cells in vitro,”
Lasers Surg. Med., 18 171
–177
(1996). https://doi.org/10.1002/(SICI)1096-9101(1996)18:2<171::AID-LSM7>3.0.CO;2-P 0196-8092 Google Scholar
B. P. Chan, I. E. Kochevar, and R. W. Redmond,
“Enhancement of porcine skin graft adherence using a light-activated process,”
J. Surg. Res., 108 77
–84
(2002). https://doi.org/10.1006/jsre.2002.6516 0022-4804 Google Scholar
T. I. Karu, G. S. Kalendo, and V. S. Letokhov,
“Control of RNA synthesis rate in tumor cells HeLa by action of low-intensity visible light of copper laser,”
Lett. Nuovo Cimento, 32 55
–59
(1981). https://doi.org/10.1007/BF02745112 0024-1318 Google Scholar
F. A. H. Al-Watban and B. L. Andres,
“The effect of He-Ne laser and Solcoseryl in vitro,”
Lasers Med. Sci., 16 267
–275
(2001). https://doi.org/10.1007/PL00011363 0268-8921 Google Scholar
J. M. Zaleskas, B. Kinner, T. M. Freyman, I. V. Yannas, L. J. Gibson, and M. Spector,
“Contractile forces generated by articular chondrocytes in collagen-glycosaminoglycan matrices,”
Biomaterials, 25 1299
–1308
(2004). https://doi.org/10.1016/j.biomaterials.2003.08.005 0142-9612 Google Scholar
C. R. Lee, A. J. Grodzinsky, and M. Spector,
“Modulation of the contractile and biosynthetic activity of chondrocytes seeded in collagen-glycosaminoglycan matrices,”
Tissue Eng., 9 27
–36
(2003). https://doi.org/10.1089/107632703762687500 1076-3279 Google Scholar
M. Gunaratnam and M. H. Grant,
“Damage to F-actin and cell death induced by chromium VI and nickel in primary monolayer cultures of rat hepatocytes,”
Toxicol. In Vitro, 18 245
–253
(2004). https://doi.org/10.1016/j.tiv.2003.08.014 0887-2333 Google Scholar
D. R. Green and J. C. Reed,
“Mitochondria and apoptosis,”
Science, 281 1309
–1312
(1998). https://doi.org/10.1126/science.281.5381.1309 0036-8075 Google Scholar
B. A. Roeder, K. Kokini, J. E. Sturgis, J. P. Robinson, and S. L. Voytik-Harbin,
“Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure,”
J. Biomech. Eng., 124 214
–222
(2002). https://doi.org/10.1115/1.1449904 0148-0731 Google Scholar
|
|||||||||||||||||||||||||||||