|
|
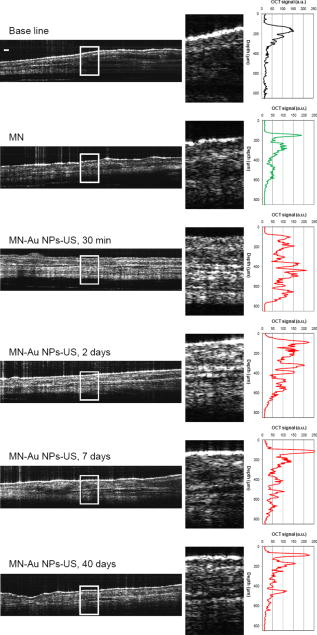
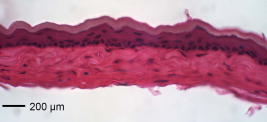
1.IntroductionThe sensitive and specific detection of premalignant and malignant lesions is a crucial and indispensable prerequisite for optimal treatment outcome and prognosis. Surgical biopsy, which is currently the primary diagnostic tool, often causes patient anxiety and discomfort, undesirable side effects, and sampling errors. For example, multiple prostate biopsies miss up to 30% of cancer present at the time of biopsy.1, 2, 3 An urgent need exists to develop cost-effective, minimally invasive imaging technologies to identify premalignancy and malignancy with high sensitivity and specificity.4 Optical imaging tools such as reflectance confocal microscopy, optical coherence tomography (OCT), and optical coherence microscopy (OCM) are promising diagnostic tools for detecting cancer at an early stage.5 Optical imaging is particularly useful for in vivo applications because it uses near-infrared wavelengths that avoid predominant absorption in tissue.6 OCT, which detects a reflected light source from the sample using refractive index mismatching, is particularly promising for early diagnosis of neoplasia because it provides resolution that is an order of magnitude higher than that of other minimally invasive diagnostic techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US).6, 7 Real-time OCT imaging with ultrafast image acquisition can minimize the errors generated by patient and operator motion artifacts.8, 9, 10, 11, 12 Because OCT uses broadband light, 2-D and 3-D cross-sectional images of subsurface tissue can be reconstructed at a high resolution for various biological and medical applications.13, 14 However, OCT-based early cancer detection is limited by the low contrast levels in biological tissues, particularly between normal and neoplastic tissues. Various approaches, including Doppler OCT,15, 16, 17, 18, 19 polarization-sensitive OCT,20, 21, 22, 23 chemico-physical enhancers (e.g., absorbing dyes),24, 25 and contrast agents26, 27, 28, 29 have been explored to overcome this fundamental limitation of OCT. Little progress has been reported using contrast agents to enhance OCT images in vivo.27, 30 The use of inorganic nanoparticles such as nanospheres,31, 32, 33, 34 nanocages,35, 36 nanoshells,37, 38 and nanorods39, 40 to overcome OCT limitations, in particular using surface plasmon resonance (SPR), has been investigated, but limited in vivo successes have been achieved compared with phantom studies and in vitro tests. Some of the limitations for the use of nanoparticles as OCT contrast agents include toxicity [e.g., in vivo toxicity of nanorods due to the indispensible use of cetyltrimethylammonium bromide (CTAB) during synthesis]41 and poor in vivo delivery and distribution.42 Gold nanoparticles (Au NPs) are promising in vivo OCT contrast agents because they are biocompatible, easy to synthesize, and functional with additional modalities. For example, Au NPs have been used in human rheumatoid arthritis treatment and microscopy at both low- and high-resolution levels as specific markers for a variety of macromolecules (e.g., polysaccharides, glycoproteins, proteins, lectins, and antibodies).43, 44 In addition, Au NPs are attractive as OCT contrast agents because the optical resonance properties of Au NPs can be controlled over a broad range by tailoring their sizes and shapes.4, 45, 46, 47, 48 A crossover from a low (absorption dominant) to a high (scattering dominant) albedo of spherical Au NPs at an diameter, which can be quantified by OCT, has been theoretically and experimentally proven.49 The efficient delivery of Au NPs is crucial to obtain a sufficient signal and a sensitivity-determining step. A topical administration of Au NPs is preferable because OCT can only image to an approximate depth of in human soft tissues.50 A topical delivery can avoid adverse systemic effects while delivering a large quantity of Au NPs to the epithelial layers where many types of oral, skin, and gastrointestinal cancers develop but a supporting vasculature is poorly developed or not available.51 The penetration of Au NPs to subsurface epithelial layers is greatly hampered by various biological barriers such as the stratum corneum (SC), the -thick outermost nonliving layer [Fig. 1a ].52 In this study, we hypothesized that this obstacle to Au NP delivery for OCT imaging could be overcome by generating micropassages through the SC using microneedles (MNs), followed by the enhanced distribution of Au NPs in underlying epithelial layers through increased movements by ultrasonic forces (i.e., multimodal delivery) [Figs. 1b and 1c].53, 54 Fig. 1(a) Stratum corneum and underlying epithelial layers as biological barrier to Au NP transport. (b) Penetration of Au NPs into subsurface epithelial layers through a passage generated by a microneedle. (c) Enhanced Au NP distribution in tissue by ultrasonic force. 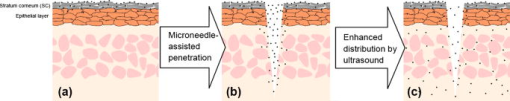 The purpose of this study was to improve the penetration and distribution of Au NPs using MNs and US and thereby enhance contrast in in vivo OCT images of oral dysplasia in a hamster model. Spherical Au NPs were prepared as previously reported55 and further conjugated with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies and polyethylene glycol (PEG), also as previously reported.56 Au NPs were applied topically with and without -long MNs and US, in vivo to the hamster cheek pouch model, and the changes in OCT signals with the same focal point were quantified by intensity profiles. 2.Materials and Methods2.1.Au NP SynthesisAu NPs ( in diameter) were prepared by the Frens method with modifications.55 A -diameter Au NP (highly scattering dominant) was used as the OCT contrast agent because OCT detects backscattering signals from a sample.49 All glassware was cleaned in aqua regia (three parts HCl, one part ), rinsed with deionized (DI) water, and then dried prior to use. Solutions of (0.01% w/w, solution A) and -citrate (1% w/w, solution B) were prepared, then of solution A was heated to its boiling point and of solution B was added with vigorous stirring. After , the boiling solution turned dark blue followed by violet after of stirring. The solution was refluxed for an additional and allowed to cool down to room temperature with continuous stirring. The final concentration of -diameter Au NPs was . Particle sizes were determined using a Carl Zeiss Ultra 55 scanning electron microscope (SEM), Phillips CM20 transmission electron microscope (TEM), and Melvern Zetasizer dynamic light scattering (DLS) particle analyzer. The Au NP solution was kept at . 2.2.Antibody Conjugation on Au NPsSolutions of Au NPs were washed three times with DI water using centrifugation at for . Monoclonal antibodies (Clone 29.1.1, Sigma, St. Louis, MO) binding to EGFR, which is over-expressed in oral cancer, were conjugated on the Au NP surfaces using protocols published elsewhere56 with modification. Briefly, of washed Au NP solution was diluted with of HEPES buffer (Fisher Scientific, Fair Lawn, NJ) and mixed with of an anti-EGFR antibody-containing solution in of HEPES. The pH of the solution was kept at and stirred for . The final concentration of the antibody-conjugated Au NP solution was , and the final volume was . Then of this solution was tested for a color change in the presence of of 10% NaCl, since it is well known that NaCl induces Au NP aggregation and results in a change to the solution’s original color.57 Au NPs that are completely coated with antibodies do not coagulate, so a color change to dark blue indicates the Au NP surfaces are not saturated with antibodies.56 Finally, of a 1% (w/v) PEG solution (Fluka, Switzerland) in DI water was added, and the mixed solution was incubated for at room temperature while stirred. The solution was centrifuged at for at room temperature, and the pellet was resuspended in of phosphate buffered saline (Fisher Scientific, Fair Lawn, NJ). 2.3.In Vivo Oral Cancer Model and Au NPs DeliveryFor in vivo imaging, the standard hamster cheek pouch model was used, whereby the cheek pouch on one side of each golden Syrian hamster (Mesocricetus auratus) was treated topically with 0.5% (v/v) 9, 10-dimethyl-1,2-benzanthracene (DMBA, Sigma, St. Louis, MO) three times per week for five months to induce cancer. The contra-lateral side was used as control, with only mineral oil applied topically to the cheek pouch surface. The anesthetized hamster’s cheek pouch was attached to a microscope stage using a custom-built ring-shaped clamp that was rigidly fastened to the stage surface. CR3 roller microneedles (MTS dermaroller with miniscule holes of diameter and depth; Clinical Resolution Laboratory, Inc., Beverly Hills, CA) were rolled on both the DMBA-untreated and DMBA-treated sides of the hamster cheek pouches three times at three different angles (i.e., , , and ). Two hundred of the anti-EGFR antibody-conjugated PEGylated Au NP solution was applied to the hamster’s cheek pouch for by dropping it directly into the -diameter aperture of the ring-shaped clamp. After the Au NP topical administration, of ultrasonic force was applied to the cheek pouch using the Dynatron 125 ultrasonicator (Dynatronics Corporation, Salt Lake City, UT) for . The typical ultrasonic intensity range for diagnostic applications is , and the frequency has been reported to be sufficient to facilitate NP dispersion into tissue by changing the diffusion coefficient (by factors of 2.6 to 15) of the tissue rather than partitioning (factors of 0.7 to 1.6).54, 58 2.4.Setup of Spectral-Domain Optical Coherence TomographySpectral-domain optical coherence tomography (SD-OCT) is a high-speed, high-resolution, minimally invasive, cross-sectional imaging technique based on a Michelson interferometer with four arms (Fig. 2 ). The source arm has a broadband light source with a center wavelength and a FWHM. The detector arm has a spectrometer with a spectral accuracy and a frame rate. An immobilized mirror is placed at the reference arm. A two-axis scanner with two galvanometers is located at the sample arm. Reflected light from the immobilized mirror and each scattering particle in a sample make a signal in the spectral domain that is detected by the spectrometer. When the path difference between the reference and sample arms is defined by and the refractive index of the sample by , the scattering event by each particle at a different depth is encoded in the frequency of the cosine function, and the signal is a sum of the cosine functions with different ’s, where the amplitude of each cosine is proportional to the scattering amplitude. An inverse Fourier transformation of the signal in the spectral domain gives a complex signal in the domain. The powers of peaks in the domain represent the scattering amplitudes and are converted to grayscale to make an array of images along the direction. While the two-axis scanner scans the plane, the array is acquired continuously to make a volume image. The current setup resolution is (in air) depthwise and laterally. Each pixel is in tissue with a refractive index of 1.33. All the SD-OCT images were taken with the same focal point. Fig. 2Schematic of a fiber-based SD-OCT in which the low-coherence light has a center wavelength and FWHM. A -wide spectrum was sampled by a InGaAs detector array at . Imaging depth and depth resolution were and in air, respectively. A two-axis scanner with two galvanometers was used. CM: collimator; DAQ: data acquisition system; DG: diffraction grating; FL: focusing lens; LCL: low-coherence light; LSC: line scan camera. 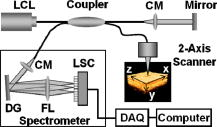 2.5.Histology of Excised TissuesThe excised hamster cheek pouch tissues were fixed in Karnovsky 2% paraformaldehyde and 3% glutaraldehyde in cacodylate buffer overnight and then postfixed in 1% osmium tetroxide for . The tissues were dehydrated in a graded series of ethanol solutions (30%, 50%, 70%, 90%, and 100%), then embedded in PolyBed® 812 (Polyscience, Inc., Warrington, PA) and polymerized at for . Half- semi-thin sections were prepared using an ultra microtome and then stained with Richardson’s stain59 for visualization under an upright microscope (BH-2, Olympus, Tokyo, Japan) equipped with an Olympus Microfire digital camera. To confirm the lack of inflammatory response, MNs, topical delivery of Au NPs, and US were applied to the cheek pouches of DMBA-untreated hamsters. The cheek pouches were stained with hematoxylin and eosin (H&E) after the multimodal delivery of Au NPs. 3.Results and DiscussionThe results shown in Fig. 3 clearly demonstrate that (1) Au NPs were able to penetrate deep into the tissue only when micropassages were provided by MNs (i.e., Au NP alone versus MN-Au NP and Au NP-US versus MN-Au NP-US for both DMBA-treated and DMBA-untreated tissues in Fig. 3); and (2) US enhanced Au NP distribution throughout the tissues (i.e., Au NP alone versus Au NP-US and MN-Au NP versus MN-Au NP-US for both DMBA-treated and DMBA-untreated tissues in Fig. 3). In the SD-OCT images, the tissue layers were clearly visible, and the difference between epithelial micromorphologies of the carcinogen-treated and untreated tissues was clearly visible [Figs. 3a and 3b]. Scion Image software (Scion Corp., Frederick, MA) was used to obtain mean intensities for each SD-OCT image with (length) by (width). The mean intensity of normal tissue was increased by multimodal delivery of Au NPs from 46.64 to 69.25 (149% increased), and the mean intensity of DMBA-treated tissue was increased from 34.59 to 61.08 (177%), as shown in Figs. 3a and 3b, respectively. The images shown in Figs. 3a and 3b were from the left and right cheek pouches of the same animal. The right cheek pouch was treated with DMBA for five months. In this standard model for oral carcinogenesis, neoplastic lesions will typically begin to develop after four to six weeks in the treated cheek pouch while the untreated cheek pouch is used as the control. However, this animal received the carcinogen for five months. While we had assumed that the untreated cheek pouch could still be used as a healthy control, our OCT images showed the unexpected development of dysplasia in the untreated cheek pouch (dotted box in Fig. 3), which was confirmed by subsequent histopathology. Fig. 3In vivo SD-OCT images of (a) normal (DMBA-untreated), and (b) DMBA-treated sides of hamster cheek pouches. treated; Au nanoparticles administered; applied. 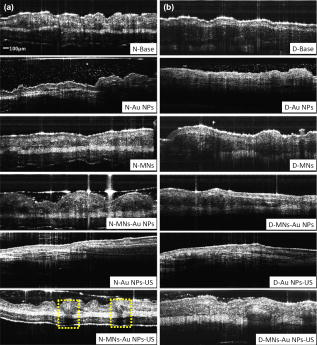 OCT intensity profiles along with imaging depth were assessed to identify distinctive optical properties of the abnormal tissue (Fig. 4 ). Five SD-OCT intensity profiles, averaged over laterally, were taken from the SD-OCT image of dysplasia tissue shown in Fig. 4a. Scion Image software was used for data processing. The actual SD-OCT image depth was calculated by converting the axial pixel number to actual scale by multiplying for each pixel in the tissue by a refractive index of 1.33. Several quantitative OCT markers of dysplasia were also identified: (1) considerably greater light scattering in the SC and upper epithelial layers [red square in Fig. 4b]; (2) reduced optical signals in subsurface epithelial layers [green square in Fig. 4b]; and (3) relatively low OCT signals in underlying connective tissue layers [blue square in Fig. 4b]. Fig. 4(a) Enlarged OCT image of dysplastic area. (b) Depth-resolved OCT signal profile in dysplastic and normal regions. Scale bar: . 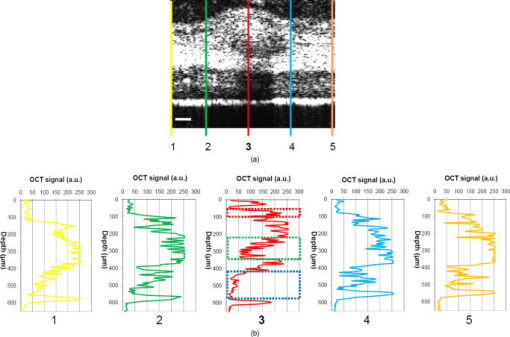 SD-OCT-based diagnoses (shown in Figs. 3 and 4) were verified by histological staining (Richardson’s stain)59 of the excised tissues. Figure 5 shows that Au NPs were dispersed along passages generated by the -long MNs. Au NPs were dispersed homogeneously into the connective tissue [Fig. 5a] but not into the hamster cheek pouch muscle bundles [Fig. 5b]. This may be due to the presence of a muscle outer layer that prevented penetration of the Au NPs. The greater presence of Au NPs in connective tissue rather than muscle bundles, as indicated by strong backscattering signals (Figs. 3 and 4), was confirmed by histology. Interestingly, Au NPs were able to penetrate deeper through the epithelial layers in the premalignant region [dotted area in Fig. 6a ] than in the normal region [Fig. 6b]. This finding explains the increased signals in the SC and upper epithelial layers in the early dysplasia region [red square in Fig. 4b]. Arrows in Fig. 6a indicate the disrupted epithelial layers of an early-stage dysplasia, thus demonstrating that mild dysplasia was differentiated from normal tissue by SD-OCT with enhanced Au NP distribution. Fig. 5Au NPs in a hamster cheek pouch (a) connective tissue and (b) muscle bundles after MN and US administration. Arrows indicate a MN passage in the tissue. 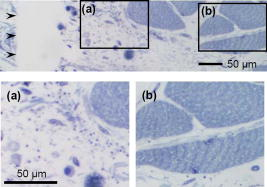 Fig. 6Distribution of Au NPs in SC and upper epithelial layers of (a) an early dysplasia, and (b) a normal region. 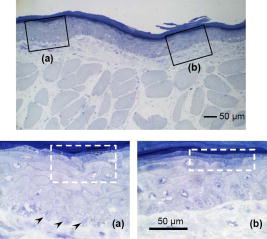 The duration of Au NP-induced OCT signal enhancement was also investigated (Fig. 7 ) by imaging the cheek pouches of DMBA-untreated hamsters over a period of after the initial administration using MNs and US. No noticeable changes were observed for two days. OCT signals in epithelial and connective tissue areas were found to be significantly diluted after a week, indicating that the Au NPs were cleared from the lower tissue. However, enhanced signals from the SC persisted over the entire . This may be due to poor (or no) active transport in the SC area. Thus, it was speculated that Au NPs were efficiently delivered by multimodal methods to both the epithelial and connective layers, where relatively active clearance of Au NPs is available. This could be confirmed by quantifying the concentration of Au NPs in the tissue. Importantly, no inflammation or other adverse effects were observed in the hamster cheek pouch over a period of , indicating the potential for an acceptable level of biocompatibility of the Au NPs (Fig. 8 ). PEGylated Au NPs with an antibody coating were used to prevent agglomeration of Au NPs while achieving EGFR-mediated specific binding to cancerous cells. Au NP agglomeration in vivo was effectively prevented, but no selective antibody-mediated binding of the Au NPs was observed in these studies (i.e., the low optical signals in the dysplasia shown in Figs. 3 and 4, and no selective accumulation of Au NPs in the dysplasial membrane shown in Fig. 6). This may be explained by the predominant enhanced permeation and retention (EPR) effects on relatively large particles in tissue.60 Due to their size (i.e., ), Au NPs may have been immobilized in the tissue with a low EGFR-encountering frequency. This unanswered question should be investigated in a subsequent study with a quantitative model and experimental data using nonspecific antibody-conjugated Au NPs. The antibody seemed to at least serve as a PEGylation anchor via its strong affinity to Au surfaces and efficient absorption of PEG on antibody layers.61 4.ConclusionIn conclusion, our multimodal delivery that employed MNs and US enabled the efficient transport of Au NPs by overcoming the SC and epithelial barriers and resulted in an approximately 150% increased OCT contrast level in the standard model for oral carcinogenesis. Early neoplasia was clearly identified by effectively delivered and distributed Au NPs, which also permitted meaningful quantitative signal analysis. The distribution of Au NPs in subepithelial layers through MN-assisted penetration and US-facilitated distribution was confirmed by histology. Diminishing OCT signals in sub-SC layers over time (up to ) imply that Au NPs were able to reach the area within the tissues where active and efficient clearance is available. This study demonstrates an effective approach to overcoming poor transport of OCT contrast-enhancing nanoparticles and may provide a new paradigm for enhancing in vivo OCT images for the early diagnosis of cancer. Further study on the molecular properties of Au NPs (e.g., Au NP size, shape, and surface functionalities) and on systemic tuning of multimodal delivery methods (e.g., MN geometry, level and duration of ultrasonic forces, and various combinations of the two modalities) are underway to further elucidate and optimize this new approach. AcknowledgmentsThis work was financially supported by the National Institutes of Health (EB-00293, NCI-91717, RR-01192), the Air Force Office of Scientific Research (FA9550-04-1-0101), the Beckman Laser Institute Endowment, seed grant funding from the University of California Cancer Center Support Grant (5P30CA062203-13), the University of California-Irvine (UCI) Council on Research Computing and Libraries (CORCL) Faculty Research and Travel Award, and a new faculty setup fund from UCI. The authors thank Dr. Matthew Brenner (UCI) for scientific discussion and Kenneth Lee (UCI) for technical assistance. The ultrasound system was generously loaned at no cost from Dynatronics (Salt Lake City, UT). ReferencesD. W. Keetch, W. J. Catalona, and D. S. Smith,
“Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values,”
J. Urol. (Baltimore), 151
(6), 1571
–1574
(1994). 0022-5347 Google Scholar
W. J. Ellis and M. K. Brawer,
“Repeat prostate needle biopsy: who needs it,”
J. Urol. (Baltimore), 153
(5), 1496
–1498
(1995). https://doi.org/10.1016/S0022-5347(01)67444-5 0022-5347 Google Scholar
C. G. Roehrborn, G. J. Pickens, and J. S. Sanders,
“Diagnostic yield of repeated transrectal ultrasound-guided biopsies stratified by specific histopathologic diagnoses and prostate specific antigen levels,”
Urology, 47
(3), 347
–352
(1996). https://doi.org/10.1016/S0090-4295(99)80451-8 0090-4295 Google Scholar
C. Loo, A. Lin, L. Hirsch, M. H. Lee, J. Barton, N. Halas, J. West, and R. Drezek,
“Nanoshell-enabled photonics-based imaging and therapy of cancer,”
Technol. Cancer Res. Treat., 3
(1), 33
–40
(2004). 1533-0346 Google Scholar
A. Gillenwater, V. Papadimitrakopoulou, and R. Richards-Kortum,
“Oral premalignancy: new methods of detection and treatment,”
Curr. Oncol. Rep., 8
(2), 146
–154
(2006). https://doi.org/10.1007/s11912-006-0050-4 Google Scholar
N. D. Gladkova, G. A. Petrova, N. K. Nikulin, S. G. Radenska-Lopovok, L. B. Snopova, Y. P. Chumakov, V. A. Nasonova, V. M. Gelikonov, G. V. Gelikonov, R. V. Kuranov, A. M. Sergeev, and F. I. Feldchtein,
“In vivo optical coherence tomography imaging of human skin: norm and pathology,”
Skin Res. Technol., 6
(1), 6
–16
(2000). https://doi.org/10.1034/j.1600-0846.2000.006001006.x 0909-752X Google Scholar
T. S. Troutman, J. K. Barton, and M. Romanowski,
“Optical coherence tomography with plasmon resonant nanorods of gold,”
Opt. Lett., 32
(11), 1438
–1440
(2007). https://doi.org/10.1364/OL.32.001438 0146-9592 Google Scholar
J. G. Fujimoto,
“Optical coherence tomography for ultrahigh resolution in vivo imaging,”
Nat. Biotechnol., 21
(11), 1361
–1367
(2003). https://doi.org/10.1038/nbt892 1087-0156 Google Scholar
C. A. Puliafito, M. R. Hee, J. S. Schuman, and J. G. Fujimoto, Optical Coherence Tomography of Ocular Disease,
(1995) Google Scholar
B. E. Bouma and G. J. Tearney, The Handbook of Optical Coherence Tomography,
(2002) Google Scholar
G. J. Tearney, M. E. Brezinski, B. E. Bouma, S. A. Boppart, C. Pitris, J. F. Southern, and J. G. Fujimoto,
“In vivo endoscopic optical biopsy with optical coherence tomography,”
Science, 276
(5321), 2037
–2039
(1997). https://doi.org/10.1126/science.276.5321.2037 0036-8075 Google Scholar
S. A. Boppart, B. E. Bouma, C. Pitris, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto,
“In vivo cellular optical coherence tomography imaging,”
Nat. Med., 4
(7), 861
–865
(1998). https://doi.org/10.1038/nm0798-861 1078-8956 Google Scholar
D. Huang, E. A. Swanson, C. P. Lin, J. S. Schuman, W. G. Stinson, W. Chang, M. R. Hee, T. Flotte, K. Gregory, C. A. Puliafito, and J. G. Fujimoto,
“Optical coherence tomography,”
Science, 254
(5035), 1178
–1181
(1991). https://doi.org/10.1126/science.1957169 0036-8075 Google Scholar
Z. Ding, H. Ren, Y. Zhao, J. S. Nelson, and Z. Chen,
“High-resolution optical coherence tomography over a large depth range with an axicon lens,”
Opt. Lett., 27
(4), 243
–245
(2002). https://doi.org/10.1364/OL.27.000243 0146-9592 Google Scholar
X. J. Wang, T. E. Milner, and J. S. Nelson,
“Characterization of fluid flow velocity by optical Doppler tomography,”
Opt. Lett., 20
(11), 1337
–1339
(1995). https://doi.org/10.1364/OL.20.001337 0146-9592 Google Scholar
Z. Chen, T. E. Milner, S. Srinivas, X. Wang, A. Malekafzali, M. J. van Gemert, and J. S. Nelson,
“Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography,”
Opt. Lett., 22
(14), 1119
–1121
(1997). https://doi.org/10.1364/OL.22.001119 0146-9592 Google Scholar
S. Yazdanfar, M. Kulkarni, and J. Izatt,
“High resolution imaging of in vivo cardiac dynamics using color Doppler optical coherence tomography,”
Opt. Express, 1
(13), 424
–431
(1997). 1094-4087 Google Scholar
J. Kehlet Barton, J. A. Izatt, M. D. Kulkarni, S. Yazdanfar, and A. J. Welch,
“Three-dimensional reconstruction of blood vessels from in vivo color Doppler optical coherence tomography images,”
Dermatology, 198
(4), 355
–361
(1999). https://doi.org/10.1159/000018148 Google Scholar
A. M. Rollins, S. Yazdanfar, J. K. Barton, and J. A. Izatt,
“Real-time in vivo color Doppler optical coherence tomography,”
J. Biomed. Opt., 7
(1), 123
–129
(2002). https://doi.org/10.1117/1.1428291 1083-3668 Google Scholar
J. F. de Boer, T. E. Milner, M. J. van Gemert, and J. S. Nelson,
“Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography,”
Opt. Lett., 22
(12), 934
–936
(1997). https://doi.org/10.1364/OL.22.000934 0146-9592 Google Scholar
M. J. Everett, K. Schoenenberger, B. W. Colston Jr, and L. B. Da Silva,
“Birefringence characterization of biological tissue by use of optical coherence tomography,”
Opt. Lett., 23
(3), 228
–230
(1998). https://doi.org/10.1364/OL.23.000228 0146-9592 Google Scholar
G. Yao and L. V. Wang,
“Two-dimensional depth-resolved Mueller matrix characterization of biological tissue by optical coherence tomography,”
Opt. Lett., 24
(8), 537
–539
(1999). https://doi.org/10.1364/OL.24.000537 0146-9592 Google Scholar
J. F. de Boer and T. E. Milner,
“Review of polarization sensitive optical coherence tomography and Stokes vector determination,”
J. Biomed. Opt., 7
(3), 359
–371
(2002). https://doi.org/10.1117/1.1483879 1083-3668 Google Scholar
C. Yang, L. E. McGuckin, J. D. Simon, M. A. Choma, B. E. Applegate, and J. A. Izatt,
“Spectral triangulation molecular contrast optical coherence tomography with indocyanine green as the contrast agent,”
Opt. Lett., 29
(17), 2016
–2018
(2004). https://doi.org/10.1364/OL.29.002016 0146-9592 Google Scholar
C. Xu, J. Ye, D. L. Marks, and S. A. Boppart,
“Near-infrared dyes as contrast-enhancing agents for spectroscopic optical coherence tomography,”
Opt. Lett., 29
(14), 1647
–1649
(2004). https://doi.org/10.1364/OL.29.001647 0146-9592 Google Scholar
C. Yang,
“Molecular contrast optical coherence tomography: a review,”
Photochem. Photobiol., 81
(2), 215
–237
(2005). https://doi.org/10.1562/2004-08-06-IR-266.1 0031-8655 Google Scholar
S. A. Boppart, A. L. Oldenburg, C. Xu, and D. L. Marks,
“Optical probes and techniques for molecular contrast enhancement in coherence imaging,”
J. Biomed. Opt., 10
(4), 041208
(2005). https://doi.org/10.1117/1.2008974 1083-3668 Google Scholar
J. K. Barton, J. B. Hoying, and C. J. Sullivan,
“Use of microbubbles as an optical coherence tomography contrast agent,”
Acad. Radiol., 9
(1), S52
–55
(2002). https://doi.org/10.1016/S1076-6332(03)80395-1 1076-6332 Google Scholar
T. M. Lee, A. L. Oldenburg, S. Sitafalwalla, D. L. Marks, W. Luo, F. J. Toublan, K. S. Suslick, and S. A. Boppart,
“Engineered microsphere contrast agents for optical coherence tomography,”
Opt. Lett., 28
(17), 1546
–1548
(2003). https://doi.org/10.1364/OL.28.001546 0146-9592 Google Scholar
E. V. Zagaynova, M. V. Shirmanova, M. Y. Kirillin, B. N. Khlebtsov, A. G. Orlova, I. V. Balalaeva, M. A. Sirotkina, M. L. Bugrova, P. D. Agrba, and V. A. Kamensky,
“Contrasting properties of gold nanoparticles for optical coherence tomography: phantom, in vivo studies and Monte Carlo simulation,”
Phys. Med. Biol., 53
(18), 4995
–5009
(2008). https://doi.org/10.1088/0031-9155/53/18/010 0031-9155 Google Scholar
R. G. Rayavarapu, W. Petersen, C. Ungureanu, J. N. Post, T. G. van Leeuwen, and S. Manohar,
“Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques,”
Int. J. Biomed. Imaging, 2007 29817
(2007). 1687-4188 Google Scholar
K. Sokolov, M. Follen, J. Aaron, I. Pavlova, A. Malpica, R. Lotan, and R. Richartz-Kortum,
“Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles,”
Cancer Res., 63
(9), 1999
–2004
(2003). 0008-5472 Google Scholar
K. Sokolov, J. Aaron, B. Hsu, D. Nida, A. Gillenwater, M. Follen, C. MacAulay, K. Adler-Storthz, B. Korgel, M. Descour, R. Pasqualini, W. Arap, W. Lam, and R. Richards-Kortum,
“Optical systems for in vivo molecular imaging of cancer,”
Technol. Cancer Res. Treat., 2
(6), 491
–504
(2003). 1533-0346 Google Scholar
I. H. El-Sayed, X. Huang, and M. A. El-Sayed,
“Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer,”
Nano Lett., 5
(5), 829
–834
(2005). https://doi.org/10.1021/nl050074e 1530-6984 Google Scholar
H. Cang, T. Sun, Z. Y. Li, J. Chen, B. J. Wiley, Y. Xia, and X. Li,
“Gold nanocages as contrast agents for spectroscopic optical coherence tomography,”
Opt. Lett., 30
(22), 3048
–3050
(2005). https://doi.org/10.1364/OL.30.003048 0146-9592 Google Scholar
J. Chen, F. Saeki, B. J. Wiley, H. Cang, M. J. Cobb, Z. Y. Li, L. Au, H. Zhang, M. B. Kimmey, X. Li, and Y. Xia,
“Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents,”
Nano Lett., 5
(3), 473
–477
(2005). https://doi.org/10.1021/nl047950t 1530-6984 Google Scholar
A. M. Gobin, M. H. Lee, N. J. Halas, W. D. James, R. A. Drezek, and J. L. West,
“Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy,”
Nano Lett., 7
(7), 1929
–1934
(2007). https://doi.org/10.1021/nl070610y 1530-6984 Google Scholar
A. Agrawal, S. Huang, A. Wei Haw Lin, M. H. Lee, J. K. Barton, R. A. Drezek, and T. J. Pfefer,
“Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells,”
J. Biomed. Opt., 11
(4), 041121
(2006). https://doi.org/10.1117/1.2339071 1083-3668 Google Scholar
A. L. Oldenburg, M. N. Hansen, D. A. Zweifel, A. Wie, and S. A. Boppart,
“Plasmon-resonant gold nanorods as low backscattering albedo contrast agents for optical coherence tomography,”
Opt. Express, 14
(15), 6724
–6738
(2006). https://doi.org/10.1364/OE.14.006724 1094-4087 Google Scholar
C. Wang, Z. Ma, T. Wang, and Z. Su,
“Synthesis, assembly, and biofunctionalization of silica-coated gold nanorods for colorimetric biosensing,”
Adv. Funct. Mater., 16
(13), 1673
–1678
(2006). https://doi.org/10.1002/adfm.200500898 1616-301X Google Scholar
H. Liao and J. H. Hafner,
“Gold nanorod bioconjugates,”
Chem. Mater., 17
(18), 4636
–4641
(2005). https://doi.org/10.1021/cm050935k 0897-4756 Google Scholar
V. P. Zharov, J. W. Kim, D. T. Curiel, and M. Everts,
“Self-assembling nanoclusters in living systems: application for integrated photothermal nanodiagnostics and nanotherapy,”
Nanomedicine, 1
(4), 326
–345
(2005). 1743-5889 Google Scholar
M. Horisberger,
“Colloidal gold: a cytochemical marker for light and fluorescent microscopy and for transmission and scanning electron microscopy,”
Scan Electron Microsc., Pt.2 9
–31
(1981). 0586-5581 Google Scholar
J. M. Gumpel,
“The role of radiocolloids in the treatment of arthritis,”
Rheumatol. Rehabil., 13
(1), 1
–9
(1974). 0300-3396 Google Scholar
Y. Wang, X. Xie, X. Wang, G. Ku, K. Gill, D. P. O’Neal, G. Stoica, and L. V. Wang,
“Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain,”
Nano Lett., 4
(9), 1689
–1692
(2004). https://doi.org/10.1021/nl049126a 1530-6984 Google Scholar
J. A. Copland, M. Eghtedari, V. L. Popov, N. Kotov, N. Mamedova, M. Motamedi, and A. A. Oraevsky,
“Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography,”
Mol. Imaging Biol., 6
(5), 341
–349
(2004). https://doi.org/10.1016/j.mibio.2004.06.002 1536-1632 Google Scholar
R. Wilson,
“The use of gold nanoparticles in diagnostics and detection,”
Chem. Soc. Rev., 37
(9), 2028
–2045
(2008). https://doi.org/10.1039/b712179m 0306-0012 Google Scholar
K. Chen, Y. Liu, G. Ameer, and V. Backman,
“Optimal design of structured nanospheres for ultrasharp light-scattering resonances as molecular imaging multilabels,”
J. Biomed. Opt., 10
(2), 024005
(2005). https://doi.org/10.1117/1.1899684 1083-3668 Google Scholar
A. L. Oldenburg, D. A. Zweifel, C. Xu, A. Wie, and S. A. Boppart,
“Characterization of plasmon-resonant gold nanorods as near-infrared optical contrast agents investigated using a double-integrating sphere system,”
Proc. SPIE, 5703 50
–60
(2005). https://doi.org/10.1117/12.589617 0277-786X Google Scholar
S. Santra, J. Xu, K. Wang, and W. Tan,
“Luminescent nanoparticle probes for bioimaging,”
J. Nanosci. Nanotechnol., 4
(6), 590
–599
(2004). https://doi.org/10.1166/jnn.2004.017 1533-4880 Google Scholar
P. Wilder-Smith, W. G. Jung, M. Brenner, K. Osann, H. Beydoun, D. Messadi, and Z. Chen,
“In vivo optical coherence tomography for the diagnosis of oral malignancy,”
Lasers Surg. Med., 35
(4), 269
–275
(2004). https://doi.org/10.1002/lsm.20098 0196-8092 Google Scholar
S. A. Coulman, D. Barrow, A. Anstey, C. Gateley, A. Morrissey, N. Wilke, C. Allender, K. Brain, and J. C. Birchall,
“Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedlesnancy,”
Curr. Drug Deliv., 3
(1), 65
–75
(2006). https://doi.org/10.2174/156720106775197510 Google Scholar
I. V. Larina, B. M. Evers, T. V. Ashitkov, C. Bartels, K. V. Larin, and R. O. Esenaliev,
“Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation,”
Technol. Cancer Res. Treat., 4
(2), 217
–226
(2005). 1533-0346 Google Scholar
S. E. Cross and M. S. Robers,
“Physical enhancement of transdermal drug application: Is delivery technology keeping up with pharmaceutical development?,”
Curr. Drug Deliv., 1
(1), 81
–92
(2004). https://doi.org/10.2174/1567201043480045 Google Scholar
G. Frens,
“Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions,”
Nature (London), Phys. Sci., 241 20
–22
(1973). 0300-8746 Google Scholar
A. C. Curry, M. Crow, and A. Wax,
“Molecular imaging of epidermal growth factor receptor in live cells with refractive index sensitivity using dark-field microspectroscopy and immunotargeted nanoparticles,”
J. Biomed. Opt., 13
(1), 014022
(2008). https://doi.org/10.1117/1.2837450 1083-3668 Google Scholar
K. Sato, K. Hosokawa, and M. Maeda,
“Rapid aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization,”
J. Am. Chem. Soc., 125
(27), 8102
–8103
(2003). https://doi.org/10.1021/ja034876s 0002-7863 Google Scholar
K. Ng and Y. Liu,
“Therapeutic ultrasound: its application in drug delivery,”
Med. Res. Rev., 22
(2), 204
–223
(2002). https://doi.org/10.1002/med.10004 0198-6325 Google Scholar
K. C. Richardson, L. Jarett, and E. H. Finke,
“Embedding in epoxy resins for ultrathin sectioning in electron microscopy,”
Stain Technol., 35 313
–325
(1960). 0038-9153 Google Scholar
V. P. Torchilin,
“Multifunctional nanocarriers,”
Adv. Drug Delivery Rev., 58
(14), 1532
–1555
(2006). https://doi.org/10.1016/j.addr.2006.09.009 0169-409X Google Scholar
L. R. Hirsch, A. M. Gobin, A. R. Lowery, F. Tam, R. A. Drezek, N. J. Halas, and J. L. West,
“Metal nanoshells,”
Ann. Biomed. Eng., 34
(1), 15
–22
(2006). https://doi.org/10.1007/s10439-005-9001-8 0090-6964 Google Scholar
|