|
|
1.IntroductionThe possibility of selective translucence of the superficial skin layers is very useful in developing functional imaging techniques, including optical coherence tomography (OCT) and reflectance spectroscopy. Recently, numerous works have been carried out in skin optical clearing, since it has a great potential in enhancing the capabilities of noninvasive light-based diagnostic and laser therapeutic techniques. Current challenges to skin optical clearing include epidermal penetration by optical clearing agents (OCA). The outermost layer of the skin, the stratum corneum (SC), presents a significant barrier to the most topically applied OCA and is hence responsible for the poor optical clearing effect. Slow diffusion of the index-matching agent through the skin barrier makes practical implementation of the approach difficult. To reduce barrier function of the skin during optical clearing, a number of different chemical and physical methods have been proposed. Chemical penetration enhancers, photothermal, sandpaper, and microneedle arrays have been shown to accelerate the skin permeability of OCA.1, 2, 3, 4, 5, 6 However, most of the current enhancing methods are invasive. Our recent studies have demonstrated that sonophoretic delivery (SP), as a noninvasive physical method, exhibited an enhancing the skin-clearing effect when applied topically with glycerol.7 The significant ultrasound-induced enhancement in OCT imaging depth and contrast of in vitro porcine skin and in vivo human skin was found.8, 9 A vast number of studies have been reported on the sonophoretic delivery of small molecules through the skin in transdermal drug delivery (TDD).10 It represents a useful noninvasive physical method to enhance epidermal permeability. In spite of the wide use of sonophoretic delivery in TDD, there are few studies to develop sonophoretic delivery for optical clearing of skin. In this study, we further examine the effect of ultrasound on a series of alcohols, i.e., polyethylene glycol (PEG), glycerol, propylene glycol, butanediol, and butanol using in vitro porcine skin. The aim of the study is to evaluate the effects of various alcohols mediated with ultrasound on optical skin clearing and to find more effective ultrasound-OCA combinations for skin clearing. 2.Materials and Methods2.1.MaterialsThe fresh porcine skin obtained from an accredited abattoir was cleaned with an alcohol wipe, and hair was removed before treatments. The tissue samples were sealed for preventing natural dehydration and stored at for no longer than before use. The samples were allowed to equilibrate at room temperature prior to the experiments. PEG200 (molecular weight ), PEG400 , glycerol, propylene glycol, butanediol, and butanol were tested at a concentration of 60% (v/v, aqueous solution), respectively as used in Ref. 11. Characteristics of the chemicals and solutions are listed in Table 1 . Table 1Characteristics of chemicals and osmolarity of 60% solutions in this study.
2.2.Ultrasound ApplicationA B-328 sonicator (Beauty & Health Instrument Co., Ltd., Guangzhou, China) operating at a frequency of equipped with a -diam probe was used for ultrasound application. Once the alcohol solution was applied, ultrasound treatment was performed on the samples for a period of . During sonication, the ultrasound probe was immersed in the agent solution that was topically applied onto the skin. Ultrasound at over the probe was applied in a pulsed mode at the frequency of to minimize thermal effects. After of ultrasound treatment, the sample was kept occluded with solution for a total of . At , both skin samples treated with alcohol or alcohol/SP are native. For the first of treatment, the samples with alcohol/SP were applied with the ultrasound, while the control samples were only applied with OCA for the whole .7, 8, 9 2.3.Spectroscopic MeasurementsTransmittance measurements were performed on a PE Lambda 900 UV/VIS/NIR spectrophotometer with an internal integrating sphere over a range from for visible and near-infrared measurement. The spectra of transmittance were acquired at the time intervals of 0 (before applying any alcohol onto the tissue), 15, 30, 45, and , respectively. The agent solution on the sample was removed right before acquiring the spectrum and added again right after the measurement.7 Enhancement of transmittance by the solutions at the time intervals of treatment were calculated according to where is the measured transmittance at the wavelength of . The subscripts control and treated refer to the samples before and after the application of agents at the different time intervals, respectively. The reason for choosing for the assessment of transmittance is that the chemical agents cause the greatest changes of optical properties of the tissue at the wavelength in the near-infrared region. All final results of transmittance are of triplicate experiments. A paired -test statistical analysis was used to determine differences in enhancement in transmission between control group (without ultrasound) and treated group (with ultrasound). For all tests, 0.05-level was chosen as a significance level.2.4.OCT MeasurementsA delay line OCT system with a central wavelength and bandwidth light source was used. The light source yields an axial resolution in free space.8 OCT images were taken of native and optically cleared skin with alcohol solution or alcohol/SP every until a total of . The solution was removed prior to OCT imaging. A cross-section image was obtained by taking 100 A-scans over the lateral length of and the axial depth of . Digital reregistration of the uneven skin surface in the OCT data based on an edge-detection algorithm was conducted to obtain a flat surface. Quantitative data were obtained by averaging the linearized signal intensity across the lateral imaging range as a function of depth. A best fit exponential curve covering epidermis and dermis in depth was applied to the averaged and normalized signal intensity data from which the corresponding light penetration depth was derived.5, 8 All data are the average of triplicate samples. 3.Results and DiscussionFigures 1a and 1b show the examples of the shift of light transmittance spectra of the fresh porcine skin specimen with the topical application of 60% PEG200 (control) and 60% PEG200 with a simultaneous application of ultrasound (PEG200/SP) over a range from , where the curves were obtained from the time intervals of 0, 15, 30, 45, and from bottom to top. Light transmittance increased against time for both treatments. The clearing capability of 60% PEG200 was much improved with the simultaneous application of ultrasound [Fig. 1b]. Other alcohol-ultrasound combinations exhibited similar effect with different levels (figures not shown). The dynamics of enhancement in transmittance at are summarized in Fig. 2 with (a) for the six alcohols alone and (b) for alcohol-ultrasound combinations at . For alcohols alone, glycerol exhibited the highest clearing effect and butanol had the lowest effect , consistent with previously reported results.11, 12 It is believed that smaller molecule with more hydroxyl groups in the chemical structure would penetrate the skin epidermis more easily, corresponding to the more enhanced transmittance. After application of alcohol-ultrasound combinations at , the total transmittance of the skin sample increased by 55.7%, 56.9%, 50.3%, 53.9%, 52.4%, and 31.2% for glycerol, PEG200, PEG400, propylene glycol, butanediol, and butanol, respectively [Fig. 2b]. The difference in clearing effect between alcohol alone and ultrasound-alcohol combination was significant for the six alcohols. Except for butanol, propylene glycol, PEG, and butanediol achieved almost the same enhanced effect as glycerol with ultrasound assistance. In addition, PEG200-ultrasound exhibited a 1.8-fold the enhanced effect of PEG200 alone, while glycerol-ultrasound did 1.5-fold of glycerol alone. Fig. 1Measured transmittance spectra over a range from for fresh skin treated with 60% PEG200 against time with intervals of 0, 15, 30, 45, and from bottom to top: (a) 60% PEG200, and (b) 60% PEG200 in implementation of ultrasound. 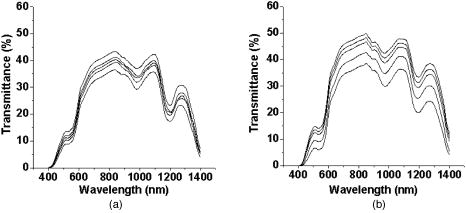 Fig. 2Enhancement in light transmittance at : (a) for alcohols alone, and (b) for alcohol-ultrasound combinations. 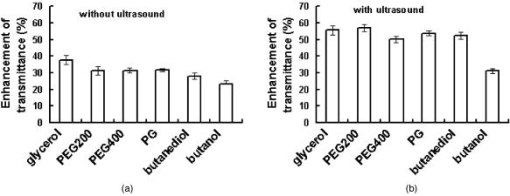 Increase of skin translucence can aid noninvasive aiming for diagnostic purposes. OCT was used as a noninvasive imaging modality. Figure 3 shows OCT images of the porcine skin treated with PEG200 alone and PEG200-ultrasound combination. It can be seen that light penetration is limited in native skin [see Figs. 3a and 3b, ], corresponding to a low imaging depth due to a shallow light penetration depth of approximately [see Figs. 4a and 4b, lower lines]. A small enhancement with 60% PEG200 was found from Fig. 3a and Fig. 4a, upper line. The light penetration was greatly enhanced after of PEG200/SP exposure [see Fig. 3b]. Sixty minutes of PEG200/SP exposure resulted in a significantly increased light penetration depth of [see Fig. 4b]. Fig. 3OCT images of in vitro porcine skin at 0, 15, 30, and after topical application of (a) 60% PEG200 and (b) 60% PEG200 in implementation of ultrasound. 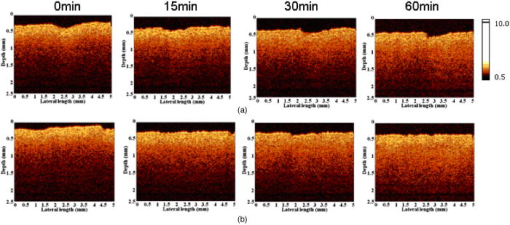 Fig. 4OCT intensity profiles of (a) native and 60% PEG200 and (b) native and 60% PEG200 in combination of ultrasound-treated in vitro porcine skin. 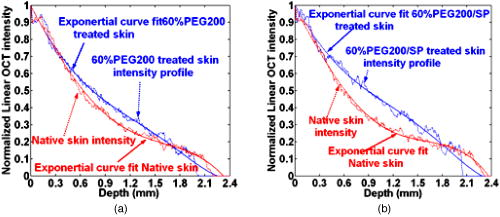 Figure 5 summarizes the relative light penetration depth value for 60% Gly, Gly/SP, 60% PEG200, and PEG200/SP at . The depth increased by roughly 40%, 56%, 29%, and 56%, respectively. Consistent with the total transmittance data from spectroscopy measurement, results from OCT imaging that measures least scattering photons demonstrate that the PEG200-ultrasound combination is much more effective than PEG200 alone and exhibits the same effect as glycerol-ultrasound combination. Fig. 5Dynamic changes of light penetration depth into in vitro optically cleared porcine skin with 60% glycerol, Gly/SP, 60% PEG 200, and PEG200/SP at . 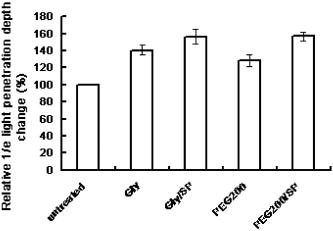 One of the major protective functions of the skin is derived from its uniquely low permeability. The excellent diffusion resistance of the SC makes the transdermal delivery of OCA and water loss through skin difficult. Subsequently, the optical clearing effect of intact skin is limited with topical application of OCA onto the surface of epidermis.13 Smaller substances are used as OCAs for their ability to penetrate the skin more easily.14 In addition, if an OCA has more hydroxyl groups, it penetrates the skin more easily due to hydrophilic nature of the epidermis.14 Hence, without the aid of ultrasound, glycerol with smaller MW and three hydroxyl groups is more effective into the skin than PEG200 with two hydroxyl groups, as reported in Ref. 11 [Fig. 2a and Fig. 5a]. The constantly increasing interest in transdermal drug delivery has led to the development of innovative technologies in attempt to increase transdermal drug delivery. Among the noninvasive methods, ultrasound has demonstrated an enhancing transdermal mass transport effect when applied topically. Based on the preceding results, we hypothesize that, due to ultrasound penetration enhancing and convection effects, more alcohol can penetrate the skin and/or alcohol can penetrate more quickly to achieve more refractive index matching in the skin, therefore increasing light transmittance in the skin.7, 8, 9 Furthermore, sonophoretic delivery is effective in enhancing permeability for chemicals with MW from 76 to 400 and the enhanced effect is not dependent on solution osmolarity (Table 1). With sonophoretic delivery, the optical clearing effect may not be directly related to the number of hydroxyl groups in the alcohol structure, or MW, or osmolarity. Although MW of PEG200 is almost twice that of glycerol, the osmolarity of 60% PEG200 is about twice less of 60% glycerol, PEG200 has one hydroxyl group less than glycerol (Table 1), and 60% PEG200 with the aid of ultrasound exhibits the same clearing effect as 60% glycerol-ultrasound. After the OCA is topically applied onto the surface of epidermis and allowed to diffuse into the dermis, the optical clearing effect probably depends on the quantity of an agent reaching the derma. Ultrasound is likely to drive same quantity of PEG200 and glycerol into the skin no matter the different osmolarity and MW between the two solutions. In addition, a similar effect was achieved by PEG200/SP as glycerol/SP only after exposure. Obviously, ultrasound accelerates PEG200 penetration into the skin, as was reported for glycerol earlier.7, 8 It should be noted that, in general, the stratum corneum in the fresh porcine skin obtain from an accredited abattoir may have been removed. Although we have already demonstrated the glycerol-ultrasound effect on in vivo human skin with stratum corneum in Ref. 8, if a skin sample with stratum corneum was used in this study, the results would be more pervasive and significant. Polyethylene glycol is nontoxic, odorless, neutral, lubricating, nonvolatile, and nonirritating and is used in a variety of pharmaceuticals and in medications as a solvent, dispensing agent, ointment and suppository base, vehicle, and tablet excipient. PEG is characterized by less toxicity and less skin irritation.15 There is no damage in case of contact with skin or lips. It is the basis of many skin creams and sexual lubricants, frequently combined with glycerol. In addition, PEG200 and PEG400 have been used as penetration enhancers in transdermal drug delivery, and the effect is same as oleic acid for some drugs.16 Recently, the enhanced effect of penetration enhancers such as oleic acid,2 azone,3 and sodium lauryl sulfate (SLS)9 on skin optical clearing have been demonstrated. We have reported the combined effect of ultrasound and the penetration enhancers with topical application of glycerol.7, 9 Hence, it can be expected that the coadministration of the mixture of PEG200 with glycerol under ultrasound-assistance may be a more effective approach in intact skin clearing as PEG200 can serve as both an optical clearing agent and penetration enhancers at the same time. 4.ConclusionsIt was demonstrated that simultaneous application of ultrasound and alcohols led to markedly increase in light transmission and imaging depth through in vitro porcine skin. Ultrasound-assisted enhancement was not related to the number of hydroxyl groups in the chemical structure and osmolarity of OCA solutions. PEG200 with ultrasound exhibited the highest enhancement among the six alcohols. It can be expected that an ultrasound-mediated mixture of glycerol and PEG200 should be a better solution for skin clearing based on the nature of PEG200 as a clearing agent and a penetration enhancer. AcknowledgmentsThis research was supported by research grants from the National Natural Science Foundation of China (30470426) and the Zhejiang Provincial Natural Science Foundation (X206958). ReferencesM. Khan, S. Chess, B. Choi, K. M. Kelly, and J. S. Nelson,
“Can topically applied optical clearing agents increase the epidermal damage threshold and enhance therapeutic efficacy?,”
Lasers Surg. Med., 35 93
–95
(2004). https://doi.org/10.1002/lsm.20078 0196-8092 Google Scholar
J. Jiang and R. K. Wang,
“Comparing the synergistic effects of oleic acid and dimethyl sulfoxide as vehicles for optical clearing of skin tissue in vitro,”
Phys. Med. Biol., 49 5283
–5294
(2004). https://doi.org/10.1088/0031-9155/49/23/006 0031-9155 Google Scholar
X. Xu and Q. Zhu,
“Evaluation of skin optical clearing enhancement with azone as a penetration enhancer,”
Opt. Commun., 279 223
–228
(2007). https://doi.org/10.1016/j.optcom.2007.06.055 0030-4018 Google Scholar
V. V. Tuchin, G. B. Altshuler, A. A. Gavrilova, A. B. Pravdin, D. Tabatadze, J. Childs, and I. V. Yaroslavsky,
“Optical clearing of skin using flash lamp–induced enhancement of epidermal permeability,”
Lasers Surg. Med., 38 824
–836
(2006). https://doi.org/10.1002/lsm.20392 0196-8092 Google Scholar
O. Stumpp, B. Chen, and A. J. Welch,
“Using sandpaper for noninvasive transepidermal optical skin clearing agent delivery,”
J. Biomed. Opt., 11 041118
(2006). https://doi.org/10.1117/1.2340658 1083-3668 Google Scholar
J. Yoon, T. Son, E. H. Choi, B. Choi, J. S. Nelson, and B. Jung,
“Enhancement of optical skin clearing efficacy using a microneedle roller,”
J. Biomed. Opt., 13 02113
(2008). https://doi.org/10.1117/1.2907483 1083-3668 Google Scholar
X. Xu and Q. Zhu,
“Feasibility of sonophoretic delivery for effective skin optical clearing,”
IEEE Trans. Biomed. Eng., 55
(4), 1432
–1437
(2007). https://doi.org/10.1109/TBME.2007.912416 0018-9294 Google Scholar
X. Xu and Q. Zhu,
“Sonophoretic delivery for contrast and depth improvement in skin optical coherence tomography,”
IEEE J. Sel. Top. Quantum Electron., 14
(1), 56
–61
(2008). https://doi.org/10.1109/JSTQE.2007.912900 1077-260X Google Scholar
X. Xu, Q. Zhu, and C. Sun,
“Combined effect of ultrasound-SLS on skin optical clearing,”
IEEE Photonics Technol. Lett., 20
(24), 2117
–2119
(2008). https://doi.org/10.1109/LPT.2008.2006987 1041-1135 Google Scholar
S. Mitragotri,
“Sonophoresis: a journey,”
Drug Discov. Today, 9 735
–736
(2004). Google Scholar
Z. Mao, D. Zhu, Y. Hu, X. Wen, Z. Han, and Q. Luo,
“Influence of alcohols on the optical clearing effect of skin in vitro,”
J. Biomed. Opt., 13
(2), 021104
(2008). 1083-3668 Google Scholar
B. Choi, L. Tsu, E. Chen, T. S. Ishak, S. M. Iskandar, S. Chess, and J. S. Nelson,
“Determination of chemical agent optical clearing potential using in vitro human skin,”
Lasers Surg. Med., 36 72
–75
(2005). https://doi.org/10.1002/lsm.20116 0196-8092 Google Scholar
V. V. Tuchin,
“A clear vision for laser diagnostics (review),”
IEEE J. Sel. Top. Quantum Electron., 13
(6), 1621
–1628
(2007). https://doi.org/10.1109/JSTQE.2007.911313 1077-260X Google Scholar
S. Scheindlin,
“Transdermal drug delivery: past, present, future,”
Mol. Interv., 4
(6), 308
–312
(2004). https://doi.org/10.1124/mi.4.6.1 Google Scholar
V. O. Sheftel, Indirect Food Additives and Polymers: Migration and Toxicology, 1114
–1116 Lewis Publishers, Boca Raton, FL
(2000). Google Scholar
D. L. Wise, Handbook of Pharmaceutical Controlled Release Technology, Marcel Dekker Press, New York
(2000). Google Scholar
|

