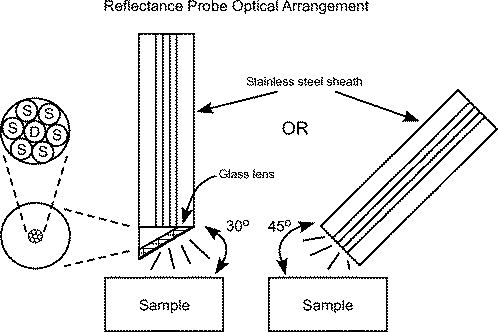
|
|
1.IntroductionBlood cultures, used for the determination of sepsis in febrile patients, have been in use since the 1940’s1 and gained momentum in their usage in the late 1970’s.2 By the 1990’s a number of automated blood culture analyzers were beginning to be used routinely in the clinical laboratory. These automated systems increased the clinical laboratory’s productivity and decreased the turn-around time for ascertaining blood culture results for improved patient care. Sepsis (which includes bacteremia and fungemia) is currently the leading cause of death in the United States and its prevalence is increasing annually.3 Sepsis in the United States afflicts 750,000 people per year with a mortality rate of and an annual incurred total cost of $16.7 billion.4 In 2001, 50% of the patients seen in hospital emergency departments (325,000 patients) had samples collected for blood cultures and the incidence of sepsis has been rising steadily every year (4). In fact, by 2007 approximately 570,000 patients were treated for severe sepsis in the hospital emergency department.4 One can only assume that with the aging population of the U.S., the increase in the number of immunocompromised patients, and the increased use of invasive devices, that the prevalence of bacterimia will continue to rise along with a concomitant rise in the use of automated blood culture methods. While the clinical laboratory has made significant advances in microbial identification methods including the use of molecular approaches such as polymerase chain reaction (PCR), the techniques developed for the detection of bacterial and fungal pathogens in blood culture have changed little since their inception. Early automated blood culture systems relied upon either radiometric or photo detection of within the blood culture vial.5 Later, non-radiometric methods were developed through the use of an immobilized sensor within the blood culture vial. The sensor was designed to measure changes in the pH within the blood culture vial due to the production of metabolic byproducts of microbial growth such as .6 The time to detection (TTD) of a positive blood culture is dependent on the sensitivity of the measurement technique, the time required to reach the minimum detectable concentration, and the transport and reaction time constants for a given system. The rate of growth and, therefore, the rate of production of metabolic depends on the type of microorganisms, the initial concentration of organisms (CFU/mL of blood) and on the growth conditions (growth media, temperature, etc.). Existing detection systems require between for a positive signal of microbial growth in a blood culture sample.7 Today, the most common automated blood culturing systems are based on the measurement of microbial growth and respiration under aerobic and anaerobic conditions using either a chemical indicator (colorimetric, fluorometric measurement) or directly measuring gas pressure changes within the head space of the culture bottle.8, 9 While these systems have become fixtures within the clinical laboratory for the diagnosis of sepsis they are not without their limitations. Current systems have concentrated on modifications of the growth media within the culture bottles to enable more rapid growth of target organisms for earlier detection of positive samples. However, these systems still require the bacterial growth to reach concentrations as high as prior to signal detection due to the lack of sensitivity of the indicator system to the gas consumption/production of bacterial growth. Their limitations not withstanding, continuous automated monitoring of blood culture vials has had a major impact in the efficiency in the diagnosis of sepsis in the U.S. as approximately 89% of positive blood cultures are detected within the first .10 Further, the rapid detection of sepsis provides the physician with actionable information for greatly improved patient outcome and decreased healthcare expenditures through shortened hospital stays.11, 12, 13 Due to the critical importance of blood culture results, every hour of earlier detection is crucial to improved clinical outcome. A novel approach based on the utilization of the intrinsic properties of blood for the detection of positive blood cultures overcomes the disadvantages of current blood culture techniques by detecting microbial growth in the absence of additional indicators or dyes. The approach uses the changes in the physical and chemical properties of blood that result from microbial activity. These changes can be direct, (e.g., particle counts) and/or indirect (e.g. hemoglobin composition). It is well established that microorganisms, either through their direct oxidation-reduction systems or through other metabolic functions and/or by-products, act on oxyhemoglobin to convert it into deoxyhemoglobin and, under the appropriate conditions, into other forms of hemoglobin.14 These forms of hemoglobin have significant differences in their optical properties that can be captured using spectroscopic techniques.15, 16 This new approach takes advantage of the fact that hemoglobin is present in large concentrations, is well-dispersed through the culture media, either free in solution or contained within the RBCs and its optical spectrum changes dramatically as a function of ambient conditions. The diffuse reflectance measurements of blood cultures can be quantitatively interpreted with a theoretical deconvolution model.17 This model is based on the rigorous wavelength dependent solutions to photon diffusion problem and Mie scattering theory and it has been shown that the accurate implementation of these theories can successfully extract quantitative estimates of optically relevant parameters such as volume fraction and size of particles, the chemical composition of the particles and media from measured diffuse reflectance spectra of blood cultures.17 The computations are nearly instantaneous enabling real-time continuous monitoring for early detection of microbial growth. The novel method reported herein utilizes multiwavelength reflectance-based approach to detect the presence of microorganisms in blood cultures without the use of an external indicator system or the monitoring of gas pressure within the culture vials. This method advances the determination of the presence of microorganisms in blood culture as compared to conventional systems in use today. In this paper the model of Serebrennikova 200817 was applied to a series of simulated blood culture experiment conducted with various microorganisms to validate the practical application of this novel approach. 2.Materials and Methods2.1.Simulated Blood Culture PreparationSeveral clinically relevant aerobic microorganisms were chosen for use in this study. The microorganisms were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Microbiological media was obtained from BD-BBL (Franklin Lakes, NJ). Laboratory grown microbial cultures were prepared using standard techniques. Blood samples from healthy individuals were provided by Florida Blood Services (St. Petersburg, FL). Seeded blood cultures were prepared with the following steps. Twenty or thirty milliliters of venous blood was mixed with approximately of microorganisms. Next, the contaminated blood was distributed between two or three blood culture bottles, of the blood and bacteria mixture was injected into each bottle. The prepared bottles were immediately placed into both the reflectance monitoring system and the reference detection system (BacT/ALERT 3D® bioMerieux, Inc Hazelwood, MO). The seeded bottles were incubated at until they were detected positive by both systems. Measurements of the actual concentrations of the inocula were performed by sub culturing of the bacterial suspension on tryptic soy agar prior to placing the blood culture vials into the instruments. Non-seeded control blood culture samples were prepared by inoculating of venous blood into each blood culture bottle. A maximum five day incubation protocol was performed for non-seeded control sample. 2.2.MeasurementThe schematic of the diffuse reflectance measurement set up is illustrated with Fig. 1a . A USB-4000 spectrometer characterized by linear CCD detector, signal to noise ratio of 300:1, and aperture was used. The spectrometer was equipped with either standard or angled reflectance QR400-Vis-NIR probe. Each probe had seven radius optical fibers in a stainless steel ferrule. One detection fiber was placed in the middle of the bundle and was surrounded by six illumination fibers as shown in Fig. 1b. The probes were characterized by source-detector distance of and numerical aperture of 0.22. LS-1 or HL-2000 Tungsten Halogen lamps with dynamic range of were used as isotropic light sources. The spectrometer, probes, and light sources were purchased from Ocean Optics Inc. (Dunedin, FL). The optical readings were taken by placing the surface of a reflectance probe at 90° angle to the surface of a blood culture vial as shown in Fig. 1b. Blood culture vials were incubated with continuous agitation to ensure that the samples were well mixed. The spectra were recorded at a constant time interval, which ranged between 2 and depending on the experiment. The diffuse reflectance was calculated by scaling the measured reflected intensity of a sample to that of used as a 100% reflectance reference and subtracting the dark intensity measured when there was no light according to Eq. 1. The reference intensities were measured at the beginning and the end of each blood culture experiment. 2.3.Theory and AnalysisThe obtained wavelength dependent diffuse reflectance was deconvoluted in terms of optically relevant parameters such as volume fraction, number density and mean volume of scattering elements, total concentration of hemoglobin, hemoglobin concentration within red blood cells, hemoglobin concentration in solution (i.e., from lysed red blood cells), and fractions of three hemoglobin forms (oxyhemoglobin, deoxyhemoglobin, and methemoglobin) as described in Serebrennikova 2007.17 The deconvolution processes consisted of a theoretical prediction of the diffuse reflectance spectra with photon diffusion and Mie scattering theories followed by fitting of the measured and corresponding theoretical spectra in the Least Squares sense with a choice of the appropriate optimization algorithm.17 In general terms, the theoretically predicted reflectance intensity for an arbitrary detector radius , the source radius , and source-detector distance can be expressed as shown in Equations 2, 3.18, 19 The terms of the Equations 2, 3 are defined as:
Radius of the incident photon beam
Radius of the detector
Source-detector separation distance ;
Photon diffusion constant defined as and and first-order modified Bessel functions of first kind and and first-order modified Bessel, functions of second kind
The eigenvalue of the Green’s function defined by
Eigenvalue normalization constant
Radius of observation
Depth of the sample
The term of the dependence series of distance along the axis of incident light
Phase function
Bessel function parameter of the order
Macroscopic absorption cross-section
Macroscopic scattering cross-section
Total macroscopic cross-section
Reduced macroscopic scattering cross-section
Asymmetry parameter As described in17 the independent optically relevant parameters of blood culture system are number density and mean volume of the particle populations (red blood cells and bacteria cells), hemoglobin concentration within red blood cells , fractions of hemoglobin forms , and concentration of hemoglobin dissolved in the media of a sample . Of them, , , and directly appear in the formulations of the macroscopic cross-sections in Equations 4, 5, 6. The and , appear in the formulation of the complex refractive index of the red blood cell population as shown in Equation 7. For the deconvolution of the measured diffuse reflectance spectra, the theoretical spectra were calculated with the values of the independent parameters being iterated. At each iteration step the residual sum of squares for the difference between measured and calculated spectra was calculated and the iteration procedure was continued until the minimal residual sum of squares was achieved. Therefore, the deconvoluted parameters of the model were those corresponding to the last iteration of the fitting procedure. From the estimated independent blood culture parameters, other parameters relevant to the description of the processes taking place in blood culture can be derived. These include, volume fraction of each particle population (i.e., red blood cells and bacteria), which are products of the corresponding number density and mean volume , and the fraction of lysed red blood cells , which is a function of the sample volume , concentration of hemoglobin dissolved in the media , volume fraction of red blood cells and hemoglobin concentration within red blood cells as shown in Equation 9. The relevant parameters for blood cultures were obtained from the deconvolution of the diffuse reflectance spectra collected at each time point during the blood culture experiments. The progressions of these estimated parameters were tracked over the course of the experiments. When the values of the parameters estimated for seeded blood culture bottles changed and significantly deviated from those of uncontaminated control blood cultures, the seeded cultures were declared positive. The earliest time point of the experiment when this occurred was denoted as TTD.3.ResultsSeveral of the most common aerobic organisms were utilized for the evaluation of the application of the reflectance based quantitative interpretation model to blood cultures. Figure 2 reveals the changes over time in the measured reflectance spectra for a blood culture sample contaminated with . coli at an initial concentration of approximately in of whole blood. The measured spectra were collected over a period of . During this time the major fractions of hemoglobin (oxy-and deoxyhemoglobin) change, as seen in the spectral signature, due to the metabolic processes of the microorganisms primarily, the consumption of oxygen and the production of carbon dioxide. The shifting composition of the various hemoglobin fractions that comprise the reflectance spectral signature over time can be seen clearly with selected representative spectra in Fig. 2b. Fig. 2(a) diffuse reflectance spectra in the spectral range collected during a blood culture experiments with E. coli. (b) Selected spectra from the same experiment that depict the distinct spectral shift of hemoglobin signatures from oxy- (1) to deoxy- (3) hemoglobin over . 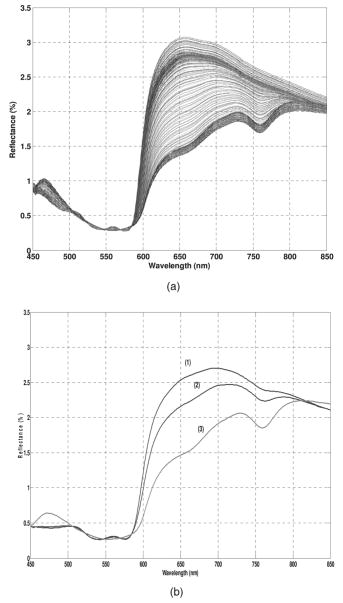 The deconvoluted estimates for the fractions of three major forms of hemoglobin as well as other optically relevant parameters for the spectra shown in Fig. 2b are summarized in Table 1 . Note, that the residuals of the deconvolution are minimal attesting to the accuracy of the deconvolution model. Table 1Relevant parameters of blood culture components obtained from the deconvolution of the three diffuse reflectance spectra shown in Fig. 1b.
Figure 3a shows the temporal evolution of the blood culture parameters including the fractions of three hemoglobin forms as well as the total volume fraction of scattering elements (i.e. RBCs and bacteria), and the fraction of lysed RBCs. Estimates of these parameters were obtained through the deconvolution of each spectrum measured during the course of blood culture experiment. This figure demonstrates that the fractions of lysed RBCs and methemoglobin were relatively constant and negligible while the fractions of oxy- and deoxyhemoglobin changed significantly over the time course of the experiment. During the first four hours of the experiment the fractions of oxy- and deoxyhemoglobin remained fairly constant with values of 0.96 and 0.03, respectively. As time progressed, the fraction of oxyhemoglobin began to decrease while the fraction of deoxyhemoglobin increased reaching the values of and respectively, at the end of the experiment [Fig. 3a]. The timing for the dramatic change in hemoglobin composition was from the start of the experiment. Fig. 3(a) Estimated fraction of oxy-, deoxy-, and methemoglobin, total volume fraction of scatterers, fraction of lysed red blood cells over the time course of a positive blood culture sample containing E. coli. (b) Calculated deoxy/oxy ratio over time. 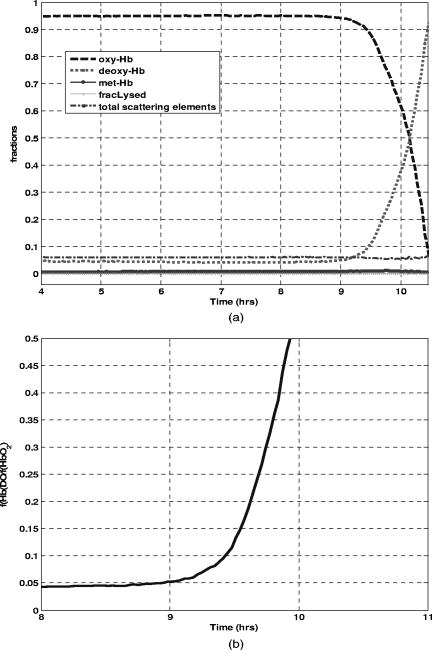 Given our knowledge of the process taking place in blood culture,16 these changes were indicative of the growth of microorganisms and the impact of their metabolic processes, i.e. uptake of oxygen and production of , on the hemoglobin composition of the blood culture vial. The shift in the chemical composition of hemoglobin can be enhanced by tracking the ratio of the fraction of deoxyhemoglobin to that of oxyhemoglobin as shown in Fig. 3b. In addition, the total volume fraction of scatterers increased by the end of the experiment reflecting the recognizable contribution of the bacterial population to the diffuse reflectance signal. The results of the blood culture experiments conducted with a variety of other clinically relevant microorganisms are consistent with those of the E. coli experiment. For instance, Fig. 4 illustrates the diffuse reflectance spectra recorded during blood culture experiments with S. aureus, E. faecalis, P. aeruginosa, and E. cloacae. In these experiments, the initial concentrations of contaminating microorganisms were 1–25 CFU per of blood and the diffuse reflectance spectra were recorded for . Fig. 4Representative diffuse reflectance spectra in the spectral range collected during blood culture experiments with two representative gram positive cocci (a) S. aureus and (b) E. faecalis and two representative gram negative rods (c) P. aeruginosa and (d) E. cloacae.  These experiments demonstrated the same pattern of temporal changes in the spectral features from the initial diffuse reflectance spectra of oxyhemoglobin to those of deoxyhemoglobin at the end. The consistency of the diffuse reflectance spectra of blood cultures contaminated with different microorganisms indicates that the spectral features are determined by the optically relevant blood culture parameters but not by the choice of a contaminating microorganism. The choice of a specific microorganism influences only timing of the changes occurring in the reflectance spectra of the blood cultures. In the illustrated blood culture experiments with S. aureus, E. faecalis, P. aeruginosa, and E. cloacae the primary change was the shift in the chemical composition of hemoglobin namely between its oxygenated to deoxygenated forms. Figure 5 shows the corresponding temporal progressions of the ratios of the fractions of deoxyhemoglobin and oxyhemoglobin calculated for the above blood culture experiments. The ratios from two parallel blood cultures for each microorganism are demonstrated and compared to those of non-seeded control blood cultures [black lines in Fig. 5]. The distinct pattern of the temporal changes in hemoglobin composition of blood cultures occurring when microbial contaminants are present is apparent. The deoxy- to oxyhemoglobin ratios of non-seeded control blood cultures remained fairly constant over time of the experiments (Fig. 5]. Fig. 5Calculated ratios of the fractions of deoxyhemoglobin (f(HbDO)) and oxyhemoglobin over the time courses of duplicate blood culture experiments with (from left to right) E. faecalis, E cloacae, S. aureus and P. aeruginosa and experiments with uncontaminated negative blood cultures. 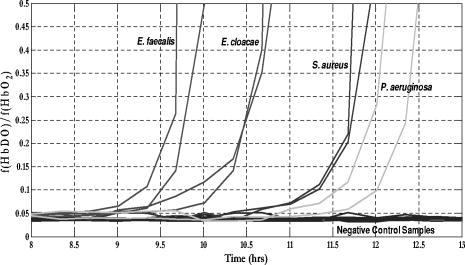 The timing of the dramatic changes in the deoxy- to oxy-hemoglobin ratios is specific for each organism and is a function of the initial concentration of the microorganisms in a blood culture. Thus, for the purpose of comparison between our method and the existing reference system parallel samples were always used. The time point of the clear shift in hemoglobin composition was denoted as the TTD of the new method. Table 2 compares the TTD of the new method to that of the reference system (BacT/ALERT 3D®) for several aerobic microorganisms. It is apparent that the new method allowed for faster detection of the presence of the contaminating microorganisms than the reference system and the improvement in TTD ranged from (22–35% improvement). Table 2Summary of blood culture experiments with a variety of clinically relevant microorganisms including the values of initial inoculum, TTD achieved with the new method, time to detection with a reference method (BacT/ALERT), and calculated percentage of the improvement in TTD. The experiments with first five microorganisms are discussed in detail in the text.
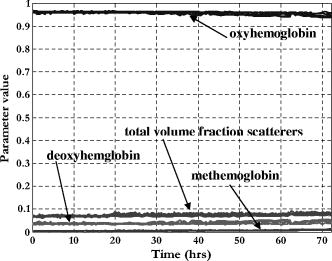
To support the claim of the cause-effect relationship between the metabolic activity of microbial contaminants and the changes in the chemical composition of hemoglobin the experiments with non-seeded control blood cultures were conducted. The temporal progressions of the deconvoluted blood culture parameters such as fractions of three hemoglobin forms and total volume fraction of scatterers from these experiments showed negligible changes in the values over [Fig. 6 ]. Thus, the distinction between positive and negative blood cultures can be made. With long incubation times for non-seeded control blood cultures a number changes in blood parameters will occur; a slight decrease in the fraction of oxyhemoglobin due to formation of methemoglobin and alteration in the size and shape of red blood cells (not shown). Both processes result from the ageing of the red blood cells at elevated temperature and in blood culture media are predictable and occur far beyond the time frame needed for the detection of microbial growth in blood culture. 4.DiscussionEffective implementation of deconvolution techniques17 to the measured diffuse reflectance spectra allows for the extraction of the physical and chemical properties of blood culture components. Further, the changes in the estimated properties can be related to the microbial metabolic processes and can therefore serve as indicators of the presence of microorganisms in blood cultures.16 Several characteristics of the blood culture vial may affect the values and dynamics of the optically relevant blood culture parameters used as indicators of microbial growth. Examples of such factors could be the composition of growth media and composition of the gas phase. Blood culture media have different compositions that may have an effect on the physical and/or chemical properties of blood. An example of these can be the presence of lysing reagents that would break red blood cells and release hemoglobin into the solution. Other media factors impacted by its chemical composition would be osmolarity and pH. However, standard blood culture vials typically have physiological osmolarity, are buffered to sustain constant pH values, and have no lysing reagents unless specifically added. The gas composition of the vials, in particular, the partial pressure of oxygen, would affect the hemoglobin’s level of oxygenation. The partial pressure of oxygen in standard aerobic blood culture bottles is which is sufficient for hemoglobin to reach an equilibrium oxygenation level of 95-97% and sustain this level over a long period of time as was demonstrated with non-seeded control blood culture experiments [Fig. 6]. Over the time course of any blood culture experiment, two or four distinct time intervals where changes in the hemoglobin composition and other blood culture properties can be identified:
The rate of change of hemoglobin composition in blood culture during the third identified interval is a function of hemoglobin concentration in the sample as well as the rate of growth (type) of microorganisms present. Smith 200716 and Garcia-Rubio (unpublished data) demonstrated that chemical composition of hemoglobin can be quantitatively related to the metabolic rates (i.e., respiration) of microorganisms with numerical simulations and analyses of experimental data. These results allow for determination of microbial growth rates from the measurements of diffuse reflectance from blood cultures. Additional chemical/physical changes in the properties of blood cultures induced by microbial activity include the formation of methemoglobin, other hemoglobin forms and derivatives, and partial or full lysis of red blood cells. These processes were found to be specific to certain groups of microorganisms. Most of the blood culturing systems on the market today rely on the detection of metabolically produced gases with external indicators as a mean to determine microbial growth (5,6). For instance, the BacT/ALERT 3D® system employs the pH-sensitive disks fixed at the bottom of blood culture vials. As pH changes due to metabolic production of , the disk changes color (7). Although this system is relatively simple, it is limited by the gas transport and reaction constants. Hemoglobin appears to be much sensitive indicator of the changes in the gas composition of blood culture bottles than external indicators. Moreover, while the shift from-oxy to deoxy-hemoglobin is most rapid at the point when the microorganisms are growing in the late exponential phase, by applying a detection model that tracks the changes in multiple blood culture parameters it is possible to determine microbial growth even earlier. This is achieved because the distinct spectral differences among the forms of hemoglobin allow for great sensitivity in terms of quantification of small changes in the optically relevant blood culture parameters. These results lead to detection of microbial contamination on average 30% faster when compared to the BacT/ALERT 3D®. Effective performance of this method has been demonstrated for a variety of clinically relevant microorganisms including both gram negative and gram positive bacteria and fungi (Table 2). 5.ConclusionsThis paper provides a report on a pilot study of a new method for the detection of microorganisms in blood culture. The method utilizes diffuse reflectance measurements over a broad wavelength range and a quantitative deconvolution of the measured spectral signals in terms of optically relevant blood culture parameters. Further, the changes in these parameters over time are related to the metabolic processes occurring in the blood cultures. Perceptible changes in those parameters are indicative of microbial growth. Key parameters include the changes in the composition of hemoglobin and volume fractions of cell populations in a given blood culture vial. The approach described herein takes advantage of the fact that hemoglobin is present in large concentrations, is well-dispersed through culture media and its spectrum changes as a function of ambient conditions. The distinct spectral differences between the forms of hemoglobin allow for greater detection sensitivity by enabling small changes in hemoglobin chemical composition to be quantified. This method is quantitative, robust and spectra can be collected using a fully automated instrument requiring a minimum amount of operator time. It overcomes the disadvantages of current blood culture detection techniques by allowing detection to occur in the absence of additional indicators or dyes. Furthermore and most significantly, it provides a substantial improvement in the time to detection of microbial contamination in blood cultures. ReferencesH. Fox and J. S. Forrester,
“Clinical blood cultures: an analysis of over 5,000 cases,”
Am. J. Clin. Pathol., 10 493
–504
(1940). 0002-9173 Google Scholar
C. Bryan,
“Clinical implications of positive blood culture,”
Clin. Microbiol. Rev., 329
–353
(1989). 0893-8512 Google Scholar
D. C. Angus, W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky,
“Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care,”
Crit. Care Med., 29 1303
–1310
(2001). https://doi.org/10.1097/00003246-200107000-00002 0090-3493 Google Scholar
H. E. Wang, N. I. Shapiro, D. C. Angus, and D. M. Yealy,
“National estimates of severe sepsis in United States emergency departments,”
Crit. Care Med., 35
(8), 1928
–1936
(2007). https://doi.org/10.1097/01.CCM.0000277043.85378.C1 0090-3493 Google Scholar
J. A. Washinton,
“Blood Cultures: An Overview,”
Eur. J. Clin. Microbiol. Infect. Dis., 8
(9), 803
–806
(1989). https://doi.org/10.1007/BF02185852 0934-9723 Google Scholar
M. J. Calandra, J. E. Turner, T. C. Thorpe, J. L. DiGuiseppi, R. C. Driscoll, and C. S. Moody,
(1992) Google Scholar
T. C. Thorpe, M. L. Wilson, J. E. Turner, J. L. DiGuiseppi, M. Willert, S. Mirrett, and L. B. Reller,
“BacT/Alert: an automated colorimetric microbial detection system,”
J. Clin. Microbiol., 28 1608
–1612
(1990). 0095-1137 Google Scholar
K. W. Berndt,
“Blood culture sensor station utilizing two distinct light sources,”
(1995). Google Scholar
K. W. Berndt,
“Method and apparatus for detecting bacteria using a blood culture froth,”
(1998). Google Scholar
R. Ziegler, I. Johnscher, P. Martus, D. Lenhardt, and H. M. Just,
“Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections,”
J. Clin. Microbiol., 36
(3), 657
–661
(1998). 0095-1137 Google Scholar
J. Valles, J. Rello, A. Ochagavia, J. Garnacho, and M. A. Alcala,
“Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival,”
Chest, 123 1615
–1624
(2003). https://doi.org/10.1378/chest.123.5.1615 0012-3692 Google Scholar
J. Barenfanger, C. Drake and G. Kacich,
“Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing,”
J. Clin. Microbiol., 37 1415
–1418
(1999). 0095-1137 Google Scholar
G. V. Doern, R. Vautour, M. Gaudet, and B. Levy,
“Clinical impact of rapid in vitro susceptibility testing and bacterial identification,”
J. Clin. Microbiol., 32 1757
–1762
(1994). 0095-1137 Google Scholar
J. Prag, J. Jensen, and K. Lebech,
“Darkening of haemoglobin in simulated, continuously agitated aerobic blood cultures: an early indicator of bacterial growth,”
Acta Pathol. Microbiol. Scand., 99 1083
–1088
(1991). 0365-5555 Google Scholar
W. G. Zijlstra, A Manual of Reflection Oximetry,
(1958) Google Scholar
J. M. Smith, Y. M. Serebrennikova, D. E. Huffman, G. F. Leparc, and L. H. Garcia-Rubio,
“A new method for the detection of microorganism in blood cultures: Part I. theoretical analysis and simulation of blood culture processes,”
Can. J. Chem. Eng., 86
(5), 947
–959
(2009). https://doi.org/10.1002/cjce.20095 0008-4034 Google Scholar
Y. M. Serebrennikova, J. M. Smith, D. E. Huffman, G. F. Leparc, and L. H. Garcia-Rubio,
“Quantitative interpretations of visible-NIR reflectance spectra of blood,”
Opt. Express, 16 18215
–18229
(2008). https://doi.org/10.1364/OE.16.018215 1094-4087 Google Scholar
L. C. Reynolds, C. Johnson, and A. Ishimaru,
“Diffuse reflectance from a finite blood medium: applications to the modeling of fiber optic catheters,”
Appl. Opt., 15 2059
–2067
(1976). https://doi.org/10.1364/AO.15.002059 0003-6935 Google Scholar
A. Ishimaru, Wave Propagation and Scattering in Random Media, Academic Press, New York
(1978). Google Scholar
M. Kerker, The Scattering of Light and Other Electromagnetic Radiation, Academic Press, New York
(1969). Google Scholar
|