|
|
1.IntroductionAn estimated 278 million people worldwide are living with disabling hearing impairment (at least moderate hearing loss in the better hearing ear), and this number is rising, mainly due to a growing global population and longer life expectations.1 For hearing, sound pressure waves from the environment enter the outer ear, pass through the middle ear ossicular chain, and form sound pressure waves within the fluids of the inner ear. The auditory portion of the inner ear, the cochlea, transduces sound pressure waves into electrical signals that are then passed to the brain via the auditory nerve. The above-mentioned mechano-electrical transduction takes place in the sensory cells of the inner ear, the so-called hair cells. The deflection of their apical stereocilia under the fluid movements in the inner ear causes the hair cells to depolarize. The mechanical induced electrical signal is then transmitted to the dendrites of the spiral ganglion cells, the first neuron of the auditory pathway. There are two types of hair cells: inner hair cells and outer hair cells. The outer hair cells serve to modulate the vibrations within the cochlea,2 and their loss results in varying degrees of sensorineural hearing loss but not deafness. The inner hair cells serve as the main afferent input into the central auditory system. Loss of inner hair cells results in complete deafness.3 Traditionally, electrical neural stimulation has been used to bypass the nonfunctional peripheral sensory organ, the cochlea, to reasonably restore some auditory function, such as speech perception.4 Mechanical energy, either through acoustic amplification or direct vibration transmitted to the inner ear, has also been effectively used for hearing aids.5 However, the aided hearing performance is dramatically reduced in noisy environments and for more complex sounds of daily life (e.g., multiple talkers). In part, this limited performance has been attributed to the lack of localized sensorineural activation across different frequency regions with these devices. Therefore, alternative stimulation strategies and technologies need do be developed to enable more appropriate and specific activation, especially for varying conditions associated with sensorineural hearing loss. Laser light, as a source of energy, can be focused in a controlled manner and thus may be a promising technology for frequency specific activation of the cochlea. Light has been used as a tool for tissue activation for more than ,6 and laser light has been used as early as 1971.7 The first nonablative laser application into the inner ear, the cochlea, was reported by Wenzel in 2004.8 The authors showed that laser irradiation of the cochlea with a pulsed dye laser can change collagen organization within the basilar membrane, a method that may be used to modulate cochlear mechanics and induce changes in cochlear tuning. The first report on auditory nerve activation with mid-infrared light as an alternative to electrical stimulation was reported by Izzo in 2006.9 The authors showed that it is possible to stimulate the auditory nerve with optical radiation of a Holmium:YAG laser, with a wavelength of , a pulse duration of , operating at . The stimulation threshold was measured as . No neural damage could be detected even for hours of continual stimulation.10 In addition, the immunohistochemical staining for the transcription factor c-FOS, further demonstrated that optical stimulation can provide spatial selectivity of stimulation.11 Richter 12 demonstrated that in chronically deaf animals, optically evoked cochlear action potential (CAP) thresholds were correlated with the number of surviving spiral ganglion cells and the optical parameters that were used for stimulation. The mechanism responsible for the neuronal activation with mid-infrared laser pulses has been suggested to be photothermal.9, 10, 11, 12, 13 The approaches using infrared laser light as a stimulation method for peripheral nerve activation target patients with severe to profound sensorineural hearing loss (i.e., significant loss of functional hair cells). However, a large proportion of hearing impaired individuals have residual hearing and functional inner hair cells that can still be activated. Unfortunately, many do not receive sufficient sound information from conventional hearing aids, which is partially due to the lack of specific activation of different cochlear regions and/or functional outer hair cells (i.e., the cochlear amplifier responsible for frequency tuning). In these individuals, an alternative technology that could enable specific cochlear activation while preserving and using residual hearing could provide significant improvements in overall hearing performance. It has become increasingly evident that combining residual hearing with cochlear implant stimulation provides dramatic hearing improvements,14 arguing for a technology that preserves and enhances rather than replaces the residual function of the cochlea. This may be achieved by inducing controlled vibration within the cochlea to selectively activate the residual functioning inner hair cells. Encouragingly, it has been recently shown that basilar membrane vibration is possible through application of an -wavelength laser.15 However, light-evoked responses appeared vulnerable to repeated exposure in which there was a decline in cochlear sensitivity and mechanical activation over time. These undesirable effects may have been caused by the excessive thermal effects of repeated pulse stimulation of cochlear tissue. Considering that we are interested in stimulating the remaining functional sensory cells as physiologically normal as possible and for an indefinite period of time in hearing impaired-patients with residual hearing, we sought to determine if controlled activation of the cochlea without significant functional damage due to heating could be achieved using laser light. Depending on the laser wavelength, pulse duration, and intensity, it is possible to induce a brief and localized thermal expansion of tissue that results in an acoustic transient within the so-called stress confinement regime (i.e., the laser pulse duration is shorter than the time the acoustic wave needs to cross the irradiated tissue volume) with minimal heating effects.16, 17 As a proof of principle study, we initially assessed whether laser light with a short pulse duration could be used to effectively and reliably activate the cochlea in a guinea pig model. We selected this laser wavelength to have less absorption by water and through this to also minimize thermal effects on the sensory cells within the organ of Corti. If this could be achieved, then further investigations as to the actual mechanism as well as frequency-specific nature of green laser light activation of the cochlea would be justified. 2.Material and Methods2.1.Animal ModelPigmented guinea pigs (Charles River Laboratories, Solingen, Germany) of either sex were used for our study according to the guidelines of The Animal Care and Use Committee of the Medical University of Hannover and Lower Saxony. They were initially anesthetized with of ketamine (Ketanest, Albrecht, Aulendorf/Württemberg, Germany) and xylazine (Rompun, Bayer Health Care, Leverkusen, Germany) and maintained with of the initial dosage every to maintain an areflexive state. We also provided of the anticholinergic agent Robinul (Riemser Arzneimittel, Greifswald-Insel Riems, Germany) intramuscularly, of the analgesic Rimadyl (Pfizer, Karlsruhe, Germany), and Ringer solution subcutaneously. Throughout the experiment, the body temperature was maintained at using a water heating pad. 2.2.Surgical TechniqueWe performed a retroauricular incision to expose and open the left tympanic bulla to visualize the round window (RW) membrane. After stabilizing the head with a custom-made holder, we inserted a core-diameter optical fiber into the bulla using a micromanipulator (H.Saur, Reutlingen, Germany). The fiber was positioned near the RW membrane and directed toward the basilar membrane (BM) and osseous spiral lamina (OSL) (Fig. 1 ). In additional experiments, we also opened the RW membrane and inserted the fiber into the cochlea pointing towards the BM and OSL. Fig. 1Diagram of the experimental setup. (a) Retroauricular incision in a guinea pig and positioning of the recording electrodes at the vertex (V), mastoid (M), and ground (G). (b) Magnified view of the left ear with the optical fiber (F) positioned in the round window (RW) niche and directed toward the basilar membrane (BM) of the basal turn (BT), which is anchored medially to the osseous spiral lamina (OSL). Tympanic membrane (T); outer ear canal (E); and second turn of the cochlea (ST). (c) Further magnification of a section through the cochlear duct. Three rows of outer hair cells and one row of inner hair cells reside on the BM within the organ of Corti. The BM is fixed at the OSL medially and the spiral ligament laterally. Auditory nerve fibers connect the hair cells with the central auditory pathways. The path of the laser beam onto the basilar membrane is shown (white area). 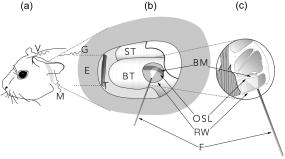 2.3.Laser StimulationFor stimulation, we used a Nd:YAG laser (Quantel Brilliant BW, France) that delivers pulses with a repetition rate of . We recorded optically induced auditory brainstem responses (OABRs) to varying energy levels (radiant exposure /pulse, 500 repetitions/average) and compared them to acoustically driven auditory brainstem responses (AABRs) recorded preoperatively [Fig. 2a ]. Fig. 2ABRs to varying levels of acoustic and optical stimulation. (a) Level series of ABRs to acoustic click stimulation, and (b) a similar trend of activity to optical radiation energy. The arrows point to the measured wave V magnitude. (c) Input–output functions for laser stimulation (thin lines: each individual animal, solid line: average of the animals) and for acoustic stimulation SPL (inset at the bottom-right of the graph) based on wave V of the ABR curves. For better visualization of the data, we have normalized each optical curve by the maximum magnitude value across levels for each animal. 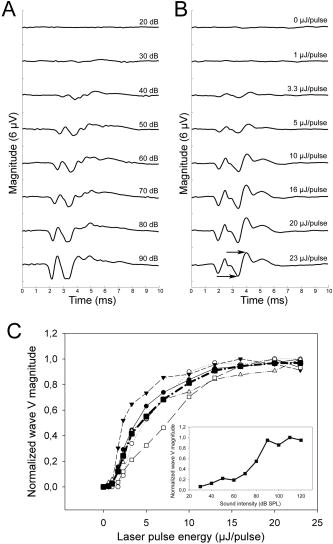 2.4.Measurement of ABRThe acoustic stimuli were delivered monaurally through polyurethane foam ear tips connected via plastic tubes to calibrated transducers (TIP-300 Tubal Insert Phone, Nicolet Biomedical, Inc., Fitchburg, Wisconsin). Since the AABRs were initially used to confirm normal hearing thresholds in our animals, we stimulated with varying levels from SPL in steps for clicks ( duration, alternating polarity). The contralateral (right) ear was masked with white noise below stimulus level for the left ear. All recordings were obtained in an electrically shielded and sound attenuated chamber using the Nicolet Viking IV system (Nicolet Biomedical, Inc.). Subdermal needle electrodes (Subdermal EMG Needle Electrodes, , Medtronic Xomed, Jacksonville, Florida) were placed at the vertex (reference), at the right and left mastoids (signals), and in the neck muscles (ground). Each recorded signal was filtered between 300 and and averaged across 500 trials. Threshold was defined as the lowest stimulus level that generated a visually detectable waveform. For acoustic stimulation, thresholds were considered normal if they were below SPL for click stimuli. 2.5.Deafening ProcedureTo assess if OABRs resulted from direct activation of the cochlea or the auditory nerve, we stimulated deafened guinea pigs (i.e., those without functional hair cells). For deafening, we administered a single intraperitoneal injection of body weight kanamycin (American Pharmaceutical Partners, Inc., Schaumburg, Illinois) followed later by an intravenous injection of body weight ethacrynic acid (Merck & Co., Inc., Whitehouse Station, New Jersey). Acoustic thresholds were measured before deafening, one week after the deafening procedure, and before the animals were used for the experiment. The lack of an AABR response at sound input was selected as the criterion for a successful deafening procedure. To ensure a functional auditory nerve in these deafened guinea pigs, we also obtained ABRs to electrical stimulation. Electrical stimulation was performed with a monopolar ball electrode inserted into the cochlea through the round window and a ground electrode placed into the neck muscle. We presented single biphasic pulses at a rate of . The pulse level varied from in steps. We used a total of 18 guinea pigs for this study. The first 10 animals were used to develop the irradiation technique and surgical approach. All presented data are based on the findings across the remaining 8 animals. 3.Results3.1.Laser Stimulation of Hearing AnimalsIn response to pulses applied at round window level, optical-induced auditory brainstem responses (OABRs) could be recorded [Fig 2b]. All OABRs exhibited the classical Jewett wave shape similar to the one obtained from acoustic stimulation except for a shorter latency of about (Figs 2a and 2b; Figs. 3a and 3c . Although the OABR peak amplitudes varied slightly across animals, in all cases they increased with increasing energy levels, generally reaching saturation around [Fig. 2c]. This demonstrates that cochlear activation can be systematically modulated with different levels of laser intensity, which is essential for an auditory prosthesis. When normalizing the wave V magnitude versus level curve by the maximum magnitude value across levels for each animal, the shape of those curves became quite similar, demonstrating the consistency of laser stimulation at the RW across animals [Fig. 2c]. The OABRs also remained consistent to stimulation over time, including stimulation at /pulse and for [Fig. 3c], indicating minimal or no damage within the cochlea due to our repeated laser stimulation. These findings are encouraging as to the feasibility of laser stimulation for a new type of auditory prosthesis. Fig. 3ABRs to acoustic (AABR), optical (OABR), and electrical (EABR) stimulation in normal hearing and chronically deafened animals. (a) ABR and OABR examples showing similar amplitudes between activation at SPL and /pulse, respectively. The two last curves present the control recordings to of energy and /pulse in the soft tissue underneath the bulla, which demonstrate that the laser needs to irradiate an absorbing structure that can transmit vibrations to the cochlea in order to activate it. (b) The first and second recordings present responses to acoustic stimulation of the animal before and after deafening, respectively. The last three curves demonstrate that optical stimulation did not elicit brainstem responses (OABR), while electrical stimulation did elicit activity (EABR) in the deafened animal, excluding a direct neural activation mechanism for optical stimulation as well as proving the viability of spiral ganglion cells after deafening. (c) Stimulation of the cochlea with over did not cause any electrophysiologically apparent damage, as demonstrated by the constant amplitudes of the responses throughout the stimulation period. 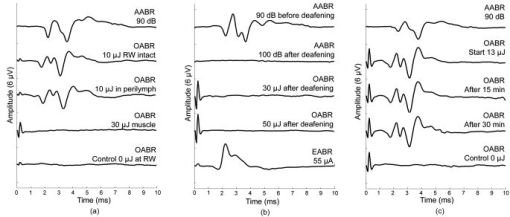 The activity was quite similar whether we stimulated through the intact RW or inserted the fiber through the RW, demonstrating that the OABRs were not a consequence of optical-induced vibration of the RW membrane [Fig 3a], but the result of optical-induced activation within the cochlea. Since the activity degraded and fluctuated over time after opening the RW and performing multiple manipulations within the perilymph, most likely through temperature and pressure changes within the cochlea, we focused our analyses on the effects of stimulation through the intact RW. In performing this study, we hypothesized that laser stimulation would induce optoacoustic waves within the cochlea that would vibrate the organ of Corti and activate the hair cells. As a control measurement, we stimulated the muscle surrounding the bulla, which did not elicit any OABRs [Fig. 3a], indicating that the activity is not induced by an electromagnetic or acoustic artifact created by the laser pulses irradiating any structure in close proximity to the cochlea. We also could not record any OABR at /pulse, in which only the flash lamp of the laser system was active, confirming that the cochlear activation was not elicited through flash lamp irradiation and acoustic artifacts [Fig. 3a]. 3.2.Laser Stimulation of Deafened AnimalsTo examine the possible mechanism in which the laser elicits brainstem responses, we further performed experiments in deafened guinea pigs that were void of a functional organ of Corti through the administration of ethacrynic acid and kanamycin. When we optically stimulated the cochlea, we could not elicit any OABRs [Fig. 3b]. However, when we stimulated the cochlea with electrical current, thus stimulation of the nerve fibers, we were able to elicit electrical brainstem responses (EABR). These findings suggest that green laser light with our parameters predominantly activates the organ of Corti rather than direct activation of auditory nerve fibers. 4.DiscussionsA new stimulation method for improved frequency-specific activation of the peripheral auditory system is needed to achieve better speech perception by hearing impaired individuals. Classical hearing aids do not ensure controlled mechanical activation of specific cochlear regions, while current cochlear implants exhibit significant spread of electrical activation throughout the cochlea. In contrast, laser light can be delivered in a more focused manner and thus may serve as an alternative energy source for providing precise cochlear activation. As described in Sec. 1, we investigated whether green laser light with a short pulse duration could elicit effective and safe activation of the cochlea. We demonstrated that the cochlea can be activated with these irradiation parameters. One encouraging result was that we obtained consistent OABRs across animals that increased with laser pulse energy up to a saturation level around [Fig. 2c]. This demonstrates the reliability of cochlear activation with green laser light as well as the ability to systematically control the overall level effects, and thus likely loudness precepts, by adjusting laser energy along these input–output functions. Another encouraging result was the ability to elicit stable response over time. The main issue of a previous report of mechanical activation of the cochlea was the decrease in responses during stimulation.15 In our experiments, stimulation with /pulse and for [Fig. 3c] elicited stable ABRs, indicating minimal or no damage within the cochlea due to our repeated laser stimuli. Laser stimulation at the RW level as well as within the perilymph activated the cochlea, inducing acoustic-like ABRs. Figure 3a demonstrates that the activation mechanism is not dependent on the presence or absence of the RW membrane and appears to be related to laser-induced mechanisms, most likely optoacoustic-induced vibrations within the cochlea. The fact that no OABRs could be recorded in chronically deafened animals demonstrates that the activation mechanism is dependent on an at least partially functional organ of Corti [Fig. 3b]. We believe that the mechanism of activation is through laser-induced vibrations of the OSL, the bony rim to which the BM is medially anchored. It is possible that a certain amount of energy may pass through the organ of Corti and reach the roof of the cochlear duct, where it would induce further vibrations. However, calculating the energy dissipation for our fiber with a numerical aperture , where is the divergence angle of the laser beam from the fiber, the estimated laser intensity at a distance of from the fiber in water would only be 0.04 of the original intensity. Therefore, it is likely that the major vibratory component is the OSL, since it is a bony structure. The absorption spectra of human bone and its two major constituents (collagen and apatite) are Ref. 18, matching our laser. Another possible absorber for green laser light within the scala media could be the stria vascularis. This structure forms the lateral wall of the scala media and contains a network of blood vessels as well as melanin. Both are good absorbers of green light and could result in vibrations of the fluid within the scala media that would induce the depolarization of the hair cells. Also the BM could be a possible absorber, which has on its side facing the scala tympani single isolated venules that could absorb some of the laser light. However, it is unlikely that such absorbtion could be sufficient for activating the residual hair cells. Further studies are needed to clarify these different absorption possibilities as well as whether the entire organ of Corti also might be absorbing part of the laser light. Although we expect that the laser is activating the cochlea through an optoacoustic effect, other mechanisms may also be involved that still need to be identified. One possible mechanism could be photochemical activation of the inner hair cells through photosensitive ion channels or activation of photoreceptors if existent on the hair cells.19 Also it cannot be excluded that part of the activation is due to laser stimulation of the dendrites of the spiral ganglia (i.e., those synapsing onto the hair cells) not covered by bone that would degenerate after the deafening procedure. However, since no OABRs could be recorded in the chronically deafened animals [Fig. 3b], activation of remaining spiral ganglia covered by bone does not appear to be a mechanism for cochlear activation with our laser parameters. Overall, our data demonstrate that green laser light in the stress confinement regime can effectively and consistently activate the cochlea. Therefore cochlear activation in hearing-impaired patients with residual hearing using green laser light stimulation seems plausible. If we can demonstrate that this cochlear activation is frequency-specific, a new type of auditory prosthesis can be developed that may provide improvements over current hearing aids and cochlear implants. Additionally, recent improvements in cochlear implant electrodes and surgery have made it possible to achieve hearing preservation during implantation of classical cochlear implant devices.14 It will be crucial to further develop surgical technologies and techniques to ensure an atraumatic insertion of the optical cochlear implant. In a later stage of hearing impairment when the last sensory cells are no longer functional, it may also be possible to use the same laser fibers to transmit mid-infrared light and directly stimulate the nerve fibers as described in previous studies.9, 10, 11, 12, 13 5.ConclusionsOur study presents a novel stimulation method using green laser light to effectively activate the cochlea and may serve as the basis for a new cochlear implant system. Further studies to determine the optimal laser parameters and fiber placement locations for localized and tonotopic activation as well as analyzing the exact mechanism underlying this activation are in progress. AcknowledgmentsWe would like to thank the German Research Foundation (Transregio 37) for funding our project, Isaac Ayala for the illustration in Fig. 1, and Peter Erfurt for technical support. References, Primary Ear and Hearing Care Training Resource CD-ROM,
(2006) Google Scholar
L. Robles and M. A. Ruggero,
“Mechanics of the mammalian cochlea,”
Physiol. Rev., 81
(3), 1305
–1352
(2001). 0031-9333 Google Scholar
A. Brandt, J. Striessnig, and T. Moser,
“CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells,”
J. Neurosci., 23
(34), 10832
–10840
(2003). 0270-6474 Google Scholar
R. Klinke and R. Hartmann,
“Physiological basis for a cochlear prosthesis [author’s transl],”
Arch. Otolaryngol., 223
(1), 77
–137
(1979). 0003-9977 Google Scholar
R. Hausler, C. Stieger, H. Bernhard, and M. Kompis,
“A novel implantable hearing system with direct acoustic cochlear stimulation,”
Audiol. Neuro-Otol., 13
(4), 247
–256
(2008). 1420-3030 Google Scholar
A. D. Arsonval,
“La fibre musculaire est directement excitable par la lumiere,”
C. R. Soc. Biol., 43 318
–320
(1891). Google Scholar
R. L. Fork,
“Laser stimulation of nerve cells in aplysia,”
Science, 171
(974), 907
–908
(1971). https://doi.org/10.1126/science.171.3974.907 0036-8075 Google Scholar
G. I. Wenzel, B. Pikkula, C. H. Choi, B. Anvari, and J. S. Oghalai,
“Laser irradiation of the guinea pig basilar membrane,”
Lasers Surg. Med., 35
(3), 174
–180
(2004). https://doi.org/10.1002/lsm.20091 0196-8092 Google Scholar
A. D. Izzo, C. P. Richter, E. D. Jansen, J. T. Walsh Jr.,
“Laser stimulation of the auditory nerve,”
Lasers Surg. Med., 38
(8), 745
–753
(2006). https://doi.org/10.1002/lsm.20358 0196-8092 Google Scholar
A. D. Izzo, J. T. Walsh Jr., E. D. Jansen, M. Bendett, J. Webb, H. Ralph, and C. P. Richter,
“Optical parameter variability in laser nerve stimulation: a study of pulse duration, repetition rate, and wavelength,”
IEEE Trans. Biomed. Eng., 54
(6 Pt 1), 1108
–1114
(2007). https://doi.org/10.1109/TBME.2007.892925 0018-9294 Google Scholar
A. D. Izzo, E. Suh, J. Pathria, J. T. Walsh, D. S. Whitlon, and C. P. Richter,
“Selectivity of neural stimulation in the auditory system: a comparison of optic and electric stimuli,”
J. Biomed. Opt., 12
(2), 021008
(2007). https://doi.org/10.1117/1.2714296 1083-3668 Google Scholar
C. P. Richter, R. Bayon, A. D. Izzo, M. Otting, E. Suh, S. Goyal, J. Hotaling, J. T. Walsh Jr.,
“Optical stimulation of auditory neurons: effects of acute and chronic deafening,”
Hear. Res., 242
(1–2), 42
–51
(2008). 0378-5955 Google Scholar
A. D. Izzo, J. T. Walsh Jr., H. Ralph, J. Webb, M. Bendett, J. Wells, and C. P. Richter,
“Laser stimulation of auditory neurons: effect of shorter pulse duration and penetration depth,”
Biophys. J., 94
(8), 3159
–3166
(2008). https://doi.org/10.1529/biophysj.107.117150 0006-3495 Google Scholar
T. Lenarz, T. Stover, A. Buechner, G. Paasche, R. Briggs, F. Risi, J. Pesch, and R. D. Battmer,
“Temporal bone results and hearing preservation with a new straight electrode,”
Audiol. Neuro-Otol., 11
(Suppl 1), 34
–41
(2006). https://doi.org/10.1159/000095612 1420-3030 Google Scholar
A. Fridberger and T. Ren,
“Local mechanical stimulation of the hearing organ by laser irradiation,”
NeuroReport, 17
(1), 33
–37
(2006). https://doi.org/10.1097/01.wnr.0000195665.22714.ee 0959-4965 Google Scholar
A. A. Oraevsky, S. L. Jacques, and F. K. Tittel,
“Measurement of tissue optical properties by time-resolved detection of laser-induced transient stress,”
Appl. Opt., 36
(1), 402
–415
(1997). https://doi.org/10.1364/AO.36.000402 0003-6935 Google Scholar
M. F. Kornel, P. Köstli, H. P. Weber, G. Paltauf, and H. Schmidt-Kloiber,
“Optoacoustic infrared spectroscopy of soft tissue,”
J. Appl. Phys., 88
(3), 1632
–1637
(2000). https://doi.org/10.1063/1.373864 0021-8979 Google Scholar
J. Behari, S. K. Guha, and P. N. Agarwal,
“Absorption spectra of bone,”
Calcif. Tissue Res., 23
(2), 113
–114
(1977). https://doi.org/10.1007/BF02012774 0008-0594 Google Scholar
P. J. Reece, K. Dholakia, R. C. Thomas, and G. A. Cottrell,
“Green laser light activates a chloride current in the C1 neuron of Helix aspersa,”
Neurosci. Lett., 433
(3), 265
–269
(2008). https://doi.org/10.1016/j.neulet.2008.01.017 0304-3940 Google Scholar
|

