|
|
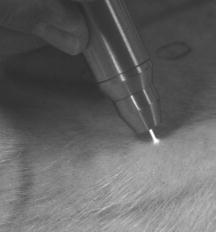
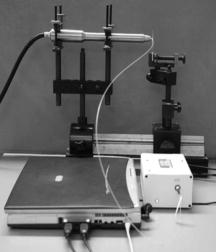
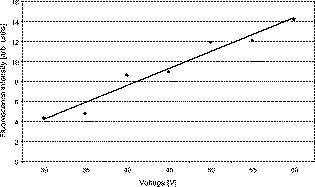
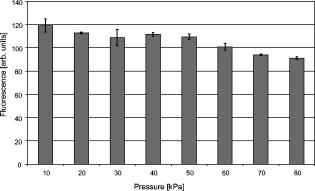
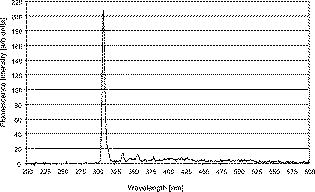
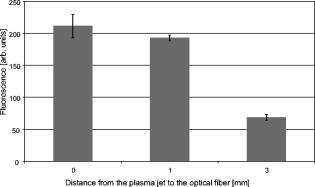
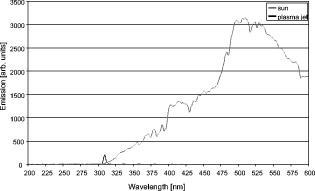
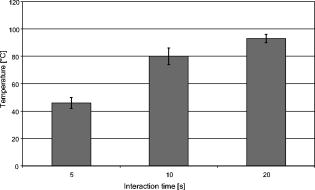
1.IntroductionThe life expectancy of the population in industrial countries is continuously increasing as a result of improved living conditions. Older people frequently suffer from chronic wounds caused by various diseases, for instance, venous dysfunction, diabetes mellitus, pressure ulcers, and arterial circulatory disorder.1 The treatment of these wounds is difficult, because of deep infections. Antisepsis is often insufficient under these conditions, due to the occurrence of scabbing and crusting of the wound.2 Under these conditions, the antiseptics do not penetrate efficiently into the tissue. Furthermore, they only attach to the outer cell wall without penetrating bacterial cells.3 Although iodine penetrates into cells,4, 5 in some conditions it shows cytotoxicity6, 7 but looses its activity in presence of 20% blood.8 In the past, electrical plasma discharges have frequently been used for disinfection and sterilization of nonliving materials.9 Such plasma systems usually have a temperature up to several hundred degrees. For so-called low-temperature plasma sterilization, the temperature is approximately . This technique would also avoid the problem of inducing antibiotic resistant strains. Recently, a plasma jet was introduced that can be applied in dermatology for antimicrobial treatment of the skin, especially in the case of chronic wounds. The plasma jet, reported in this paper, produces10 a “cold” plasma at a temperature of .10 In primary experiments, it was demonstrated that this plasma is well suited for the destruction of bacteria and fungi in cell structures.11 Prior to the application of the plasma-jet system on patients in vivo, a risk assessment must be performed. Potential risk factors are the electrical charge system, thermal damage of the tissue, and formation of UV radiation on the skin surface during treatment. In this paper, the optimal settings of plasma-jet parameters are determined; subsequently, a risk assessment is performed. 2.Materials and Methods2.1.Plasma JetIn this study, we used a plasma jet that was developed at the Institute of Plasma Physics, Greifswald,12 and manufactured at neoplas GmbH (product name “kinpen09®”). The used plasma jet principally fulfils the physical demands for an ideal interventional instrument in anti-infectious dermatotherapy. However, it must prove antibacterial efficacy against clinically relevant pathogens without substantially damaging vital tissue cells. The used compact miniaturized atmospheric plasma exhibits a very promising technological potential for different surface treatments. Basically, there are two features that make this jet unique: (1) the tool-like, small-size, and lightweight plasma generation unit enables fast and almost arbitrary 3-D movements and (2) the contracted and comparably cold plasma enables focused small-spot treatments, even on heat sensitive objects, such as tissue and cells with temperature loads to the surface between 30 and . The “kinpen09®” device passed CE-certification (electromagnetic compatibility), thus fulfilling the standards for electrical safety in humans. Argon gas was used as a discharge medium in the plasma jet. Figure 1 is a photograph of the plasma jet under working conditions. 2.2.Tissue SamplesOur investigations were performed on pig ear skin, which is a highly suitable model for human tissue.2 The unscalded pig ears had been freshly obtained from the butcher. The experiments performed on these tissue samples were carried out within after slaughtering. Approval for the experiments had been obtained from the Veterinary Board, District of Berlin-Koepenick. The pig ears were washed with cold water and dried with paper towels before being used in the experiments. 2.3.Sliced SkinSamples of sliced skin, with a thickness of and a sample size of , were obtained from the pig ears, using a Dermatom GA 148 (AESCULAP AG and Co. KG, Tuttlingen, Germany). 2.4.Tape StrippingTape stripping is a well-suited method for removal of the stratum corneum of the skin.13 Therefore, an adhesive film (Tesa Film No. 5529, Beiersdorf AG, Hamburg, Germany) was applied and by repeating this procedure continuously on the same tissue area, the stratum corneum could be removed sequentially, cell layer by cell layer. The first tape strips were investigated microscopically to ensure that they were completely covered with one layer of corneocytes. 2.5.Spectral Analysis of the PlasmaThe spectrum of the plasma was analyzed within a range of , using a fiber-based spectrometer EPP2000 (SI Scientific Instruments GmbH, Gliching, Germany). The optical quartz fiber (Laser-und Medizintechnologie GmbH, Berlin, Germany) contained an attached scattering body, which collects the surrounding light and transfers it to the fiber. The fiber was specifically developed for measurement of the spectral distribution of light, on and in different depths of tissue samples.14 In this study, the scattering body was fixed onto a quartz plate and positioned into the plasma stream. Additionally, the spectral characteristics of the plasma were measured behind one cell layer of corneocytes, removed by tape stripping, and behind the sliced skin samples. This experimental arrangement is shown in Fig. 2 . 3.Results3.1.Optimization of the Plasma-Jet Parameters3.1.1.VoltageThe influences of the applied voltage and the gas pressure on the parameters of the plasma stream were investigated. The emission intensity of the plasma increased continuously with increasing voltage from , which was the maximum voltage for application on the control unit (Fig. 3 ). 3.1.2.PressureThe argon gas pressure was changed from . The length of the plasma increased with increasing gas pressure (Fig. 4 ). At the same time, the diameter of the plasma stream decreased, which resulted in a decrease in the light emission of the plasma intensity at (Fig. 5 ). At values higher than , the gas stream used resulted in an uncomfortable sensation on the skin. This effect was tested on the forearm in vivo without a plasma discharge. 3.1.3.Spectral characteristicsTaking these results into consideration, a voltage of and a gas pressure at were determined as the optimal working conditions for the applied plasma jet. The spectrum of the plasma obtained under these conditions is presented in Fig. 6 . The spectrum consists of a band at a high intensity of and a series of significantly smaller bands in the spectral range from . Emission bands below were not observed. The plasma charge has a high stability. The emission intensity at changed during . When the plasma stream came into contact with the quartz plate or the tissue surface, the plasma-tissue interaction zone was approximately 5 times larger than the diameter of the plasma stream. The top of the plasma stream acted in the mode of a “brush” during the treatment of material. This means that the treated area is significantly larger than the diameter of the plasma stream. 3.1.4.Optimal distance of the nozzle of the plasma jet to the tissueUsing the optimal working conditions of the plasma jet, the influence of the distance of the nozzle to the quartz plate surface, regarding the distribution of the plasma in the plasma-material interaction zone of the plasma jet, was investigated. The results are demonstrated in Figs. 7 and 8 . Figure 7 shows diameter and distribution of the plasma emission in the plasma-material interaction zone. If the quartz plate was in direct contact with the nozzle of the plasma jet, an arc discharge inside the opening of the nozzle was observed. This arc discharge produced a nonhomogeneous distribution of the plasma in the cross section of the plasma stream [Fig. 7a]. The plasma is characterized by high emission intensity at (Fig. 8). However, when the distance of the plasma jet from the quartz plate surface was increased to , a homogeneous plasma charge was obtained in the cross section of the treated quartz surface [Fig. 7b], with a diameter of . This is greater than the diameter of the plasma stream itself (see Fig. 7). The increased size of the plasma-material interaction zone is caused by the “brush effect” of the plasma on the material surface. In this case, the intensity of the plasma emission was only slightly reduced in comparison to the contact regime. By further increasing the distance up to , the diameter of the cross section remained at a size of [Fig. 7c]. However, the intensity of the plasma emission at was reduced to 30% (Fig. 8). 3.2.Safety Assessment3.2.1.Plasma emissionThe intensity of the plasma emission measured behind the sliced skin was strongly reduced. Only less than 1% of the plasma radiation at a maximum of could be detected behind the sliced skin of the pig ear, at a depth of . Furthermore, tape strips were removed from the forearm of volunteers. By means of microscopic measurements, it was checked that these tape strips contained one layer of corneocytes, as demonstrated in Fig. 9 for three volunteers. Subsequently, the intensity of the plasma radiation was analyzed behind these tape strips. As seen in Fig. 10, approximately 25% of the incident radiation could be detected behind the first cell layer of corneocytes. Fig. 9First tape strips removed from the forearms of three different volunteers contained a single corneocyte layer.  Fig. 10Intensity of the plasma radiation behind the first tape strips, which each contained a single corneocyte layer. 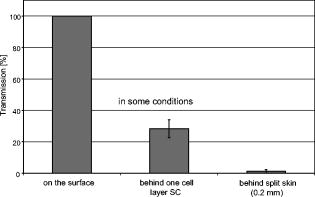 The same spectroscopic arrangement that was applied in the plasma experiment was used to measure the intensity of the sun radiation, on a sunny day in March at noon. Figure 11 compares the spectral intensity of the sun to the spectral distribution of the plasma jet. Comparing both spectra, it must be considered that the contact time of the plasma with the skin surface is usually less than , which corresponds to a moving velocity of the plasma stream on the skin surface at . Depending on the conditions of the outdoor experiments, the skin had to be exposed to sun radiation for more than to produce sunburn. This means that the radiation dose produced by the plasma jet at was an order of magnitude below the minimal erythema dose necessary for the formation of skin damage detectable as sunburn. 3.2.2.Temperature measurementsThe temperature of the skin surface was measured on the pig ear skin surface samples during different contact times with the plasma stream under optimal conditions. After a contact time of , the temperature increased to , after to , and after to . The results are presented in Fig. 12 . 4.Discussion4.1.Determination of the Optimal Parameters of the Plasma JetRegardless of the availability of several highly efficient commercialized antiseptics, the successful treatment of chronic wounds remains a problem.15, 16 The main reason for this problem is that chronic wounds usually become encrusted with scabs, which block the penetration of antiseptics into the upper layers of the tissue where microbes are located.17 Recently, it was demonstrated on excised skin and in cell cultures that the application of “cold” plasma produced in an electric discharge can be an effective tool for noninvasive antimicrobial treatment of tissue.10, 18 The mode of action of cold plasma seems to be based on the formation of a high amount of free radicals, which are able to destroy bacteria and fungi in the upper cell layers of the tissue.9 Based on the promising in vitro results, it is necessary to confirm these findings under in vivo conditions on patients. Therefore, a risk assessment is essential with regard to possible side effects of in vivo plasma application prior to the initiation of a clinical trial. Three major aspects must be addressed in such a risk assessment. One potentially dangerous aspect is the electrical properties of the discharge system, where electric shocks on the tissue must be avoided. In this study, the risk caused by electric charges could be ruled out, based on the construction of the applied plasma jet. The system was approved by the CE certification (electromagnetic compatibility) and also met the requirements for an approval by the German authorities regarding electrical safety. The two remaining safety aspects of the plasma jet—the influence of UV radiation and the temperature effect on the skin—were investigated in the presented study. Prior to evaluation of the safety aspects, the optimal plasma parameters concerning treatment efficacy of tissue were determined. For reasons of practicability the plasma stream should have a length of at least to enable the operator to use the plasma jet in a noncontact mode, i.e., in the same manner as using a brush, without the limitation of a strong fixed distance between the plasma nozzle and the tissue. On the tissue surface, the plasma should be homogeneously distributed in the induction zone, with the largest possible diameter. Our plasma-jet device has three adjustable parameters to optimize the plasma properties: (1) the applied gas, in which an electrical discharge takes place; (2) the voltage; and (3) the gas flow, determined by the applied gas pressure. The discharged electric circuit of the plasma jet was optimized for the application of argon as a discharge medium. A plasma stream was produced in the voltage range between 30 and . The highest plasma emission and the largest plasma stream were obtained at a maximum voltage of . The length of the plasma stream increased with the optimal voltage at proportionally to the gas pressure. On the other hand, the intensity of the plasma emission on the tissue surface was reduced with increasing gas pressure. This was a result of the changed discharge conditions and the reduction in the diameter of the plasma stream. Additionally, a high gas pressure led to an uncomfortable sensation on the skin, due to the mechanical stress produced by the gas stream. An excessive gas flow should be avoided to prevent splattering the wound fluid that is frequently present on the skin surface of chronic wounds. Wound fluid can contain a high amount of bacteria and fungi. The spreading of these microorganisms can contaminate the medical staff during treatment procedures. Therefore, the Ar gas pressure must be limited to for the presented plasma jet application. The optimal parameters ( , ) are related only to the special plasma jet investigated in this study. These parameters must be determined independently for all other types of plasma-jet systems. 4.2.Risk AssessmentUsing the optimal operation parameters of the plasma jet, the risk aspects were evaluated in terms of UV radiation and temperature effects. As seen in Fig. 7, the emission spectrum of the plasma consists of one intensive line at and several smaller bands between 325 and . The emission signal at is a nitrogen band, which results from the in the air. Using the described plasma-jet system with argon as a charge medium, no emission bands at wavelengths lower than could be detected. The UV light at is efficiently absorbed by the stratum corneum of the skin. As exhibited in Fig. 9, a single corneocyte layer absorbs approximately 25% of the plasma radiation. The stratum corneum of the human skin consists of 15 to 25 cell layers, depending on the body site. This means that almost no UV radiation reaches the living cells of the skin. This was also confirmed by the transmission measurements of sliced skin. Comparing the radiation dose of the plasma jet on the tissue and the dose of sun radiation under usual conditions, we demonstrated that the UV irradiation dose of the plasma was an order of magnitude below the minimal erythema dose.19 Thus, the potential risk of skin damage by UV radiation during treatment with the plasma jet under the present conditions can be ruled out. The temperature measurements on the skin surface showed, for an interaction time of , a moderate temperature increase to approx. . At higher plasma tissue exposure times, thermal damage of the tissue must be expected. Bearing in mind that the usual treatment velocity is in the range of , the contact time of the plasma with the tissue is less than . Under these realistic conditions, thermal damage can be disregarded. Summarizing the results obtained with the presented plasma jet system, it can be established that no major risk potentials are expected for the patient when the system is applied in vivo. Dangerous UV radiation and temperature effects during the plasma-tissue interaction can be neglected. On the other hand, it could be demonstrated in several in vitro studies that the plasma has a strong antimicrobial efficacy. AcknowledgmentsWe would like to thank the “Skin Physiology” Foundation of the Donor Association for German Science and Humanities for financial support. Also, our thanks go to the Ministry of Technology (BMBF) for their financial support. ReferencesT. Phillips,
“Current approaches to venous ulcers and compression,”
Dermatol. Surg., 27 611
–621
(2001). https://doi.org/10.1046/j.1524-4725.2001.00195.x 1076-0512 Google Scholar
N. Sekkat, Y. N. Kalia, and R. H. Guy,
“Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo,”
J. Pharm. Sci., 91 2376
–2381
(2002). https://doi.org/10.1002/jps.10220 0022-3549 Google Scholar
G. Müller and A. Kramer,
“Interaction of octenidine and chlorhexidine with mammalian cells and the resulting microbicidal effect (remanence) of the combinations,”
GMS Krankenhaushyg., 2 46
–49
(2007). Google Scholar
H. Below, W. Behrens-Baumann, C. Bernhardt, H. Volzke, A. Kramer, and P. Rudolph,
“Systemic iodine absorption after preoperative antisepsis using povidone-iodine in cataract surgery—an open controlled study,”
Dermatology, 212
(Suppl. 1), 41
–46
(2006). https://doi.org/10.1159/000089198 Google Scholar
F. Hansmann, H. Below, A. Kramer, G. Muller, and G. Geerling,
“Prospective study to determine the penetration of iodide into the anterior chamber following preoperative application of topical 1.25% povidone-iodine,”
Graefe's Arch. Clin. Exp. Ophthalmol., 245 789
–793
(2007). https://doi.org/10.1007/s00417-006-0320-8 0721-832X Google Scholar
A. Kramer, V. Adrian, P. Rudolph, S. Wurster, and H. Lippert,
“Explant test with skin and peritoneum of the neonatal rat as a predictive test of tolerance of local anti-infective agents in wounds and body cavities,”
Chirurg, 69 840
–845
(1998). https://doi.org/10.1007/s001040050498 0009-4722 Google Scholar
G. Muller and A. Kramer,
“Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity,”
J. Antimicrob. Chemother., 61 1281
–1287
(2008). https://doi.org/10.1093/jac/dkn125 0305-7453 Google Scholar
H. P. Werner,
“Die mikrobiozide Wirksamkeit ausgewählter Antiseptika,”
Hyg. Med., 17 51
–59
(1992). Google Scholar
T. v. Woedtke, A. Kramer, and K. D. Weltmann,
“Plasma sterilization: what are the conditions to meet this claim?,”
Plasma Processes Polym., 5 534
–539
(2008). https://doi.org/10.1002/ppap.200800013 1612-8850 Google Scholar
K. D. Weltmann, T. v. Woedtke, E. Kindel, and R. Brandenburg,
“Atmospheric pressure plasma jet for medical therapy: plasma parameters and risk estimation,”
Google Scholar
K. D. Weltmann, R. Brandenburg, T. v. Woedtke, R. F. J. Ehlbeck, M. Stieber, and E. Kindel,
“Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs),”
J. Phys. D: Appl. Phys., 41 194008
(2008). https://doi.org/10.1088/0022-3727/41/19/194008 0022-3727 Google Scholar
R. Foest, E. Kindel, H. Ohl, M. Stieber, and K. D. Weltmann,
“Non-thermal atmospheric pressure discharges for surface modification,”
Plasma Phys. Controlled Fusion, 47 B525
–B536
(2005). https://doi.org/10.1088/0741-3335/47/12B/S38 0741-3335 Google Scholar
J. Lademann, U. Jacobi, C. Surber, H. J. Weigmann, and J. W. Fluhr,
“The tape stripping procedure—evaluation of some critical parameters,”
Eur. J. Pharm. Biopharm., 72 317
–323
(2009). https://doi.org/10.1016/j.ejpb.2008.08.008 0939-6411 Google Scholar
J. Lademann, U. Jacobi, H. Richter, N. Otberg, H. J. Weigmann, H. Meffert, H. Schaefer, U. Blume-Peytavi, and W. Sterry,
“In vivo determination of UV-photons entering into human skin,”
Laser Phys., 14 234
–237
(2004). 1054-660X Google Scholar
R. J. White, R. Cooper, and A. Kingsley,
“Wound colonization and infection: the role of topical antimicrobials,”
Br. J. Nurs., 10 563
–578
(2001). 0966-0461 Google Scholar
M. J. Alfa, P. Degagne, N. Olson, and T. Puchalski,
“Comparison of ion plasma, vaporized hydrogen peroxide, and 100% ethylene oxide sterilizers to the ethylene oxide gas sterilizer,”
Infect. Control Hosp. Epidemiol., 17 92
–100
(1996). https://doi.org/10.1086/647252 0899-823X Google Scholar
T. J. Phillips,
“Successful methods of treating leg ulcers. The tried and true plus the novel and new,”
Postgrad Med., 105 159
–161
(1999). 0032-5481 Google Scholar
P. A. Martens, V. Galliani, G. Graham, and R. A. Caputo,
“Sterilization of medical products using gas plasma technology,”
Sterilization of Drugs and Devices, 157
–196 Interpharm Press, Buffalo, NY
(1998). Google Scholar
J. Lademann, A. Rudolph, U. Jacobi, H. J. Weigmann, H. Schaefer, W. Sterry, and M. Meinke,
“Influence of nonhomogeneous distribution of topically applied UV filters on sun protection factors,”
J. Biomed. Opt., 9 1358
–1362
(2004). https://doi.org/10.1117/1.1805557 1083-3668 Google Scholar
|