|
|
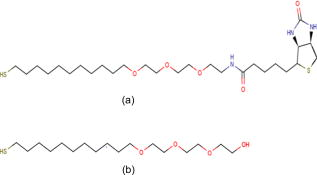
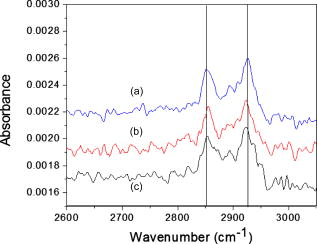
1.IntroductionThe need to identify rapidly and with high sensitivity different viral/bacterial pathogens, fungi, and toxins is one of the major forces driving extensive research addressing the development of biosensors. The conventional detection of viruses has been carried out with cell culture,1 immunological methods,2, 3, 4 and molecular methods including the polymerase chain reaction.5 These techniques, however, require much time and expertise.6, 7 The development of detection methods that are specific for targeted biomolecules and are easy to carry out is necessary for medical diagnostics, clinical analysis, or even field tests.8, 9, 10 Biosensors containing organic molecules offer promising solutions due to their speed, simplicity, and continuous monitoring capability.11 Such sensors could comprise antibodies immobilized via organic molecules on glass surface, e.g., of a microstructured fiber,12 the Au surface of a semiconductor surface plasmon resonance device,13 or a Si surface.10 Electrical properties of bacteria have also been taken advantage of in constructing an optoelectrical biosensor.14 Optical and electronic properties of III-V and II-VI semiconductor quantum well (QW) and quantum dot (QD) microstructures are also attractive for building biosensing devices because they can be used to detect miniscule perturbations of the semiconductor surface induced by selectively trapped biomolecules. For instance, the bright photoluminescence (PL) of a colloidal CdSe QD was investigated to develop fluorescent probes in sensing, imaging, immunoassay, and some other diagnostics applications.15, 16, 17, 18, 19, 20, 21, 22 Recently, we proposed that templates of epitaxial QDs, such as InAs QDs in a GaAs matrix, could be used to construct a family of innovative biosensors with a significant potential to address the rapid detection of numerous pathogens in parallel.23 Our interest in GaAs is driven both by its strong PL and its applications as a capping material for IR-emitting InAs QDs and some other quantum semiconductor microstructures. Consequently, the ability to immobilize specifically targeted biomolecules on the surface of GaAs is of high interest for the development of a proposed biosensor. We recently reported24 on the successful immobilization of avidin on the surface of GaAs. In this paper, we report on the specific immobilization of inactivated influenza A virus using an architecture based on antibody and a self-assembled monolayer (SAM) of polyethylene-glycol (PEG) thiol deposited on the GaAs (001) surface. 2.Experimental Methods2.1.MaterialsWafers of GaAs (001), series VW 10680-53 and 54, were bought from Wafer Technology Ltd. (Milton Keynes, United Kingdom). Polyclonal antibodies against the virus of influenza (H3N2) coupled with biotin or coupled with fluorescein isothiocyanate (FITC) were obtained from ViroStat, Inc. (Portland, Maine). Polyclonal antibodies against the hepatitis B and herpes simplex virus type 1 (HSV1) FITC conjugated were also obtained from ViroStat. PBS (phosphate buffered saline, pH 7.4) was bought from Sigma (Oakville, Canada). Neutravidin was obtained from Molecular Probes (Invitrogen, Burlington, Canada). Biotinylated PEG (polyethylene glycol) thiols and OH-terminated PEG thiols were obtained from Prochimia Surfaces (Gdansk, Poland). The molecular structure of these thiols is shown in Fig. 1 . Gamma ray inactivated viral particles of influenza A (rank 2, stock Texas ) were bought from Microbix Bio-Systems (Toronto, Canada). The inactivation of the virus is achieved due to the damage of its RNA genome.25 In contrast to conventional chemical methods of inactivation that use formaldehyde, this method leaves the virus outer shell intact. Nominally anhydrous ethanol (98% v/v) was bought from Commercial Alcohols, Inc. (Brampton, Canada). To remove residual oxygen, a flask filled with ethanol was purged for with a high-purity nitrogen stream ( , Praxair Canada Inc.). OptiClear, a solvent designed to remove impurities present at the surface of optical or electric compounds, was obtained from National Diagnostics (Mississauga, Canada). Acetone was bought at ACP (Montréal, Canada); isopropanol (2-propanol) was obtained from Fisher Scientific (Ottawa, Canada), acetic acid was obtained from Fisher Scientific, and ammonium hydroxide 28% was bought at Anachemia (Richmond, Canada). All the solvents were lab grade and all the products were used without additional purification. 2.2.Preparation of the SamplesA substrate of crystalline GaAs (001) was used to carry out the procedure of biofunctionalization. Samples of , obtained by cleaving GaAs (001) wafers, were cleaned sequentially during in an ultrasonic bath of undiluted solutions of OptiClear, acetone, and isopropanol, similar to a previously described procedure.24 The samples were, thereafter, dried using a flow of compressed nitrogen and etched with a solution of concentrated ammonium hydroxide for at room temperature to remove surface native oxides,26 such as , , and . The samples were then rinsed with freshly deoxygenized ethanol and immediately incubated for at room temperature in a mixture of biotinylated PEG thiol and OH-terminated PEG thiol (1:15) diluted in the deoxygenized ethanol to a final concentration of . The production of a biosensor using GaAs substrates primarily depends on our capacity to prepare the surface of this semiconductor so that it facilitates immobilization of targeted biomolecules. However, it is also important that the substrate remains stable and resists oxidation which could modify its optical and electric properties. The use of thiols helps to address this issue as it has been demonstrated that sulphuric inorganic compounds enable passivation of GaAs surface.27, 28, 29 Moreover, ethylene glycol groups, present on the thiol molecules used, provide an increased affinity of the antibodies for their antigens30 and decrease nonspecific association of the antibodies to certain molecules.31 The role of PEG thiols is also to prevent modification of the viability of the active site of the antibodies due to the steric hindrance effect8 that could occur when they are too close to the substrate. After thiolation, the samples were rinsed with isopropanol to get rid of superfluous thiol molecules physically adsorbed to the substrate. Thereafter, they were immersed for in a PBS buffer containing a concentration of of neutravidin. This step was followed by rinsing of the samples with the PBS buffer and then with deionized water. For the immobilization of polyclonal antibodies against the influenza A virus, samples having been exposed to neutravidin were immersed for in a solution of biotinylated antibodies diluted (1:25) to a final concentration of in the PBS buffer. Once the incubation was completed, the samples were placed for in a solution containing of inactivated viral particles. This was followed by rinsing the samples with the PBS solution and storing them in PBS for characterization or future processing. The procedure of biofunctionalization and the exposure of samples to the viral particles are schematically illustrated in Fig. 2 . A series of samples biofunctionalized with antibodies against influenza A were prepared by exposing them to inactivated influenza A viruses. Additional samples were prepared by immersing the influenza A virus exposed samples for in FITC-conjugated antibodies against influenza A, hepatitis B, or herpes simplex virus type 1 (HSV1) diluted in PBS buffer. These samples, after rinsing with PBS and deionized water to remove residual salts from the surface, were used for fluorescence microscopy experiments. Fig. 2Schematic illustration of the biofunctionalization steps applied for the immobilization of the influenza A virus on GaAs (001) substrate (a) that having been etched with was passivated using a solution of mixed PEG thiols (1:15) (b) and incubated in a solution of neutravidin (c). Biotinylated antibodies against influenza A were immobilized on the neutravidin-coated substrate (d), which enabled immobilizing virus particles from a solution (e).  2.3.Interface and Surface Characterization2.3.1.Fourier transform infrared (FTIR) spectroscopyTo verify the process of PEG-thiol SAMs formation on the GaAs (001) surface, FTIR absorption was investigated in the region characteristic of the stretching vibrations . The spectra were collected using an FTIR Bruker Optics Hyperion 2000 microscope coupled with a spectrometer (Bruker Optics Tensor 27). The analyzed area was approximately in diameter and the spectral resolution was . A sample that was etched and incubated in ethanol was used to determine a baseline for the spectroscopy measurements. 2.3.2.Atomic force microscopy (AFM)Surface morphology of processed samples was investigated using a Bioscope AFM (Veeco Metrology, Inc., California) operating in a contact mode. The biofunctionalized samples were never exposed to the atmosphere and their characterization was carried out in a PBS buffer solution. A MLCT-AUHW type tip (Veeco Metrology, Inc.) was used with a cantilever spring constant of . The AFM measurements of a reference GaAs sample (etched only) were carried out in an air ambient with contact mode using a Nanoscope E AFM (Veeco Metrology, Inc.). 2.3.3.Fluorescence microscopyThe fluorescence emitted by FITC fluorochrome attached to antibodies against influenza A was observed using fluorescence microscopy (Olympus IX71 inverted microscope with a DP71 digital camera). The excitation of the FITC fluorochrome was carried out with a blue light source emitting between 450 and . 3.Results and Discussion3.1.PEG Thiol SAM Formation on GaAs (001)The formation of PEG thiol SAMs on the surface of GaAs (001) was investigated by examining the spectral location and intensity of the FTIR peaks corresponding to stretching vibrations of molecules. Figure 3 shows the FTIR spectra obtained for three different samples fabricated under nominally the same conditions. For each sample investigated, two peaks are observed near 2924 and , which correspond respectively to the asymmetric and symmetric stretching mode vibrations of . The lack of spectral definition makes the peak locations difficult to specify precisely, and moreover, the absorption intensity is more than 2 times weaker than that observed in dodecanethiol SAMs, which have the same alkane number but that do form well-ordered SAMs.32, 33 A recent study has shown that an additional IR enhancement factor of up to 6 times applies for the case of highly ordered -alkane SAMs in transmission measurements.34 This carries the implication that SAMs having an increased degree of conformational disorder may belie the extent of the actual surface coverage by virtue of lower IR intensities observed, since the coverage and intensity will vary in a nonproportional manner. It is also expected that the PEG group will hinder ordering and surface coverage to some degree, but to just what extent is difficult to derive from our results for the reasons stated. More effort will result in a better characterization of the surface, but ultimately, the value of this interface will be determined by its functionality. 3.2.Surface Morphology and BiofunctionalizationThe surface morphology of an etched GaAs (001) sample is shown by an AFM micrograph in Fig. 4a . The root-mean-square roughness amplitude of the investigated area is . This value is expected for the high-quality GaAs (001) surface, and it is comparable to the results reported in literature.35 Figure 4b is an AFM micrograph of the GaAs (001) surface biofunctionalized with the influenza A antibody [as illustrated by the step represented in Fig. 2d]. The micrograph shows a granular microstructure composed of nanoparticles, each between 5 and in diameter. These could represent neutravidin, the largest size biomolecule present in the studied sample. However, we have observed that samples exposed exclusively to the PBS buffer solution exhibited qualitatively a similar surface morphology to that represented in Fig. 4b. This suggests that some PBS nanoparticles precipitate on the surface of samples studied by liquid-phase AFM, making it difficult to distinguish nanoparticles of different origin. An AFM micrograph of a fully biofunctionalized GaAs (001) sample that was exposed to the influenza A virus is shown in Fig. 4c. A significantly different surface morphology in this case is characterized by the presence of large nanoparticles, typically wide at the base and tall. It is known that the influenza virus is a quasispherical object, approximately in diameter.36, 37 Therefore, it seems reasonable to assume that the large nanoparticles in Fig. 4c represent either individual or clustered influenza virus particles. The tip convolution effect makes the viral particles detected appear larger than and a higher magnification experiment would have to be carried out to view details of the viral particle.38 The slightly reduced height of the viral particles observed in this experiment could be explained by the possible flattening occurring due to the pressure exercised by the AFM cantilever tip. Using AFM images, we have estimated that the average density of Influenza A particles covering the biofunctionalized GaAs (001) surface is approximately at 45 per . Fig. 4AFM images and cross-sectional scans of (a) a reference GaAs (001) sample, (b) the GaAs (001) sample biofunctionalized with PEG thiols and neutravidin, and (c) the sample that following the functionalization with the influenza A antibody was exposed to a solution of inactivated influenza A virus. 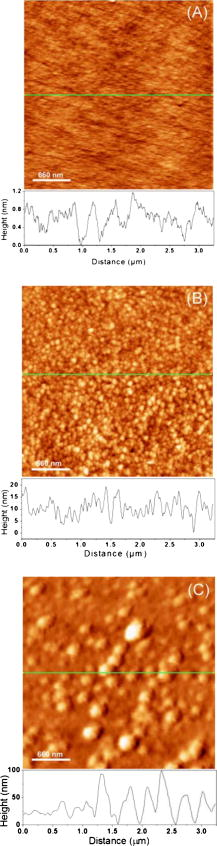 3.3.Fluorescence Tests on Virus Exposed SamplesAn example of the fluorescence microscopy image from the influenza A antibody biofunctionalized sample that was exposed to the inactivated influenza A virus and to the FITC-conjugated antibodies against influenza A is shown in Fig. 5 . A network of bright spots emitting at about can be seen in this image. In contrast, the similar experiment with samples exposed to hepatitis B and herpes simplex virus FITC-conjugated antibodies provided images free of green fluorescence. An example of the control fluorescence image observed in the experiment with the FITC-conjugated hepatitis B antibodies is shown in the inset of Fig. 5. This result corroborates our AFM experiments and it provides further evidence of the inactivated influenza A virus specifically immobilized at the surface of investigated GaAs (001) samples. The density of influenza A particles covering the surface has been estimated, according to the fluorescence measurements, at approximately 25 per . The reduced density of viral particles given by the fluorescence microscopy imaging in comparison to that obtained from the AFM experiments could be attributed to the lower binding efficiency of the FITC polyclonal antibodies to the viral particles. Indeed, it has been reported that biotin can be coupled to proteins, such as antibodies, with no significant loss of their binding affinity.39, 40 However, it has been observed that labeling antibodies with fluorescent molecules, such as FITC, often results in a significant loss of immunoreactivity.41 The spatial resolution of the fluorescence imaging is significantly inferior to that of the AFM technique. Thus, it is possible that more than one viral particle could be associated with an individual emission spot observed in Fig. 5. Regardless of this discrepancy, it seems reasonable to expect that the optimized process of immobilization could yield even greater concentrations of viral particles specifically attached to the surface of GaAs (001). Fig. 5Fluorescence microscopy image of a GaAs (001) sample that, following the specific immobilization of the influenza A virus, was exposed to FITC-conjugated antibodies against the influenza A virus. Inset shows a control fluorescence image observed if FITC-conjugated influenza A antibodies were replaced with FITC conjugated hepatitis B antibodies. 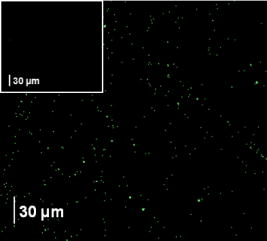 In conclusion, we investigated biofunctionalization of the GaAs (001) surface with the aim to immobilize influenza A virus. Samples with an architecture comprising PEG thiols, biotin, and neutravidin provided surface sites suitable for the attachment of biotinylated influenza A antibodies. Following this step, the samples were exposed to a solution comprising radiation-inactivated influenza A particles. With the AFM measurements, we identified the presence of viral nanoparticles on such samples. The surface density of immobilized particles was estimated as 45 per . Using fluorescence microscopy, we were able to verify the nature of these nanoparticles. Indeed, bright fluorescence images were observed following the exposure of samples with immobilized influenza A virus to FITC-conjugated influenza A antibodies. No measurable fluorescence was recorded for the samples with immobilized influenza A virus that were exposed to FITC-conjugated both herpes and hepatitis antibodies. Based on the fluorescence microscopy measurements, the density of influenza A particles specifically immobilized on the biofunctionalized GaAs (001) surface was estimated as at least 25 per . To the best of our knowledge, these results are the first evidence of controlled immobilization of viral particles on GaAs. We consider this achievement to be an important step toward demonstration of a III-V semiconductor-based photonic biosensor. AcknowledgmentsWe thank Gregory Marshall for FTIR measurements and helpful discussions. This research has been supported by the Canada Research Chair in Quantum Semiconductors Program and the joint NanoQuébec (NC)-Canadian Institute for Photonic Innovation (CIPI)-Canadian Space Agency (CSA) Support Program for Integrative Biosensor Research. ReferencesY. Amano and Q. Cheng,
“Detection of influenza virus: traditional approaches and development of biosensors,”
Anal. Bioanal. Chem., 381
(1), 156
–164
(2005). https://doi.org/10.1007/s00216-004-2927-0 1618-2642 Google Scholar
S. Y. Oh, B. Cornell, D. Smith, G. Higgins, C. J. Burrell, and T. W. Kok,
“Rapid detection of influenza A virus in clinical samples using an ion channel switch biosensor,”
Biosens. Bioelectron., 23
(7), 1161
–1165
(2008). https://doi.org/10.1016/j.bios.2007.10.011 0956-5663 Google Scholar
Y. Zhao, M. Zou, C. Li, P. Zhou, and C. Wang,
“On-site screening of avian influenza virus (AIV) by polystyrene microfluidic chip immunoassay,”
399
–402
(2007). Google Scholar
A. H. Peruski, L. F. Peruski Jr.,
“Immunological methods for detection and identification of infectious disease and biological warfare agents,”
Clin. Diagn. Lab Immunol., 10
(4), 506
–513
(2003). 1071-412X Google Scholar
M. Ma, N. Jin, Z. Wang, D. Fei, M. Zheng, K. Jin, Z. Xia, S. Ge, and M. Jin,
“Establishment of rapid test of AIV by RT-PCR,”
Gaoujishu Tongxin Chin. High Technol. Lett., 15
(5), 77
–81
(2005). Google Scholar
A. K. Bhunia and A. Lathrop,
“Pathogen detection, food-borne,”
McGraw-Hill 2003 Year Book of Science and Technology, 320
–323 McGraw-Hill Professional, New York
(2003). Google Scholar
C. Jae-Woo, A. Pu, and D. Psaltis,
“Bacteria detection in a microfluidic channel utilizing electromagnetic cellular polarization and optical scattering,”
Digest of IEEE LEOS Summer Topical Meetings, 17
–18
(2006) Google Scholar
T. Cao, A. Wang, X. Liang, H. Tang, G. W. Auner, S. O. Salley, and K. Y. S. Ng,
“Functionalization of AlN surface and effect of spacer density on Escherichia coli pili-antibody molecular recognition,”
Colloids Surf., B, 63
(2), 176
–182
(2008). https://doi.org/10.1016/j.colsurfb.2007.11.023 0927-7765 Google Scholar
A. Valsesia, P. Colpo, T. Meziani, P. Lisboa, M. Lejeune, and F. Rossi,
“Immobilization of antibodies on biosensing devices by nanoarrayed self-assembled monolayers,”
Langmuir, 22
(4), 1763
–1767
(2006). https://doi.org/10.1021/la0526333 0743-7463 Google Scholar
A. Balasubramanian, B. Bhuva, R. Mernaugh, and F. R. Haselton,
“Si-based sensor for virus detection,”
IEEE Sens. J., 5 340
–344
(2005). https://doi.org/10.1109/JSEN.2005.844543 1530-437X Google Scholar
F. Ricci, F. Caprio, A. Poscia, F. Valgimigli, D. Messeri, E. Lepori, G. Dall’Oglio, G. Palleschi, and D. Moscone,
“Toward continuous glucose monitoring with planar modified biosensors and microdialysis,”
Biosens. Bioelectron., 22
(9–10), 2032
–2039
(2007). https://doi.org/10.1016/j.bios.2006.08.041 0956-5663 Google Scholar
Y. Ruan, T. C. Foo, S. Warren-Smith, P. Hoffmann, R. C. Moore, H. Ebendorff-Heidepriem, and T. M. Monro,
“Antibody immobilization within glass microstructured fibers: a route to sensitive and selective biosensors,”
Opt. Express, 16
(22), 18514
–18523
(2008). https://doi.org/10.1364/OE.16.018514 1094-4087 Google Scholar
M. Bora, K. Celebi, J. Zuniga, C. Watson, K. M. Milaninia, and M. A. Baldo,
“Near field detector for integrated surface plasmon resonance biosensor applications,”
Opt. Express, 17
(1), 329
–336
(2009). https://doi.org/10.1364/OE.17.000329 1094-4087 Google Scholar
J. W. Choi, A. Pu, and D. Psaltis,
“Optical detection of asymmetric bacteria utilizing electro orientation,”
Opt. Express, 14
(21), 9780
–9785
(2006). https://doi.org/10.1364/OE.14.009780 1094-4087 Google Scholar
H. Mattoussi, J. M. Mauro, E. R. Goldman, G. P. Anderson, V. C. Sundar, F. V. Mikulec, and M. G. Bawendi,
“Self-assembly of CdSe–ZnS quantum dot bioconjugates using an engineered recombinant protein,”
J. Am. Chem. Soc., 122
(49), 12142
–12150
(2000). https://doi.org/10.1021/ja002535y 0002-7863 Google Scholar
C. A. Constantine, S. V. M. Kerim, M. Gattas-Asfura, G. Crespo, V. Rastogi, T.-C. Cheng, J. J. DeFrank, and R. M. Leblanc,
“Layer-by-layer biosensor assembly incorporating functionalized quantum dots,”
Langmuir, 19
(23), 9863
–9867
(2003). https://doi.org/10.1021/la035237y 0743-7463 Google Scholar
R. Wargnier, A. V. Baranov, V. G. Maslov, V. Stsiapura, M. Artemyev, M. Pluot, A. Sukhanova, and I. Nabiev,
“Energy transfer in aqueous solutions of oppositely charged core/shell quantum dots and in quantum dot-nanogold assemblies,”
Nano Lett., 4
(3), 451
–457
(2004). https://doi.org/10.1021/nl0350938 1530-6984 Google Scholar
M. A. Hahn, J. S. Tabb, and T. D. Krauss,
“Detection of single bacterial pathogens with semiconductor quantum dots,”
Anal. Chem., 77
(15), 4861
–4869
(2005). https://doi.org/10.1021/ac050641i 0003-2700 Google Scholar
X. D. Hoa, M. Martin, A. Jimenez, J. Beauvais, P. Charette, A. Kirk, and M. Tabrizian,
“Fabrication and characterization of patterned immobilization of quantum dots on metallic nano-gratings,”
Biosens. Bioelectron., 24
(4), 970
–975
(2008). https://doi.org/10.1016/j.bios.2008.07.069 0956-5663 Google Scholar
R. C. Stringer, D. Hoehn, and S. A. Grant,
“Quantum dot-based biosensor for detection of human cardiac Troponin I using a liquid-core waveguide,”
IEEE Sens. J., 8
(3), 295
–300
(2008). https://doi.org/10.1109/JSEN.2008.917489 1530-437X Google Scholar
I. L. Medintz and H. Mattoussi,
“Quantum dot bioconjugates for imaging, labelling and sensing,”
Nature Mater., 4
(6), 435
–446
(2005). https://doi.org/10.1038/nmat1390 1476-1122 Google Scholar
X. H. Gao, W. C. W. Chan, and S. M. Nie,
“Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding,”
J. Biomed. Opt., 7
(4), 532
–537
(2002). https://doi.org/10.1117/1.1506706 1083-3668 Google Scholar
J. J. Dubowski,
“Novel quantum dot based approach for biosensing,”
302
–303
(2006). Google Scholar
X. Ding, K. H. Moumanis, J. J. Dubowski, E. H. Frost, and E. Escher,
“Immobilization of avidin on (001) GaAs surface,”
Appl. Phys. A, 83
(3), 357
–360
(2006). https://doi.org/10.1007/s00339-006-3569-1 0947-8396 Google Scholar
E. E. Smolko and J. H. Lombardo,
“Virus inactivation studies using ion beams, electron and gamma irradiation,”
Nucl. Instrum. Methods Phys. Res. B, 236
(1–4), 249
–253
(2005). https://doi.org/10.1016/j.nimb.2005.04.055 0168-583X Google Scholar
C. Bryce and D. Berk,
“Kinetics of GaAs dissolution in solutions,”
Ind. Eng. Chem. Res., 35
(12), 4464
–4470
(1996). https://doi.org/10.1021/ie960278t 0888-5885 Google Scholar
F. S. Aguirre-Tostado, M. Milojevic, K. J. Choi, H. C. Kim, C. L. Hinkle, E. M. Vogel, J. Kim, T. Yang, Y. Xuan, P. D. Ye, and R. M. Wallace,
“S passivation of GaAs and band bending reduction upon atomic layer deposition of nanolaminates,”
Appl. Phys. Lett., 93
(6), 061907
–1
(2008). https://doi.org/10.1063/1.2961003 0003-6951 Google Scholar
O. S. Nakagawa, S. Ashok, C. W. Sheen, J. Martensson, and D. L. Allara, Surface Passivation Studies on GaAs with Octadecyl Thiol, 290
–292
(1991) Google Scholar
K. Moumanis, X. Ding, J. J. Dubowski, and E. H. Frost,
“Aging and detergent washing effects of the surface of (001) and (110) GaAs passivated with hexadecanethiol,”
J. Appl. Phys., 100
(3), 034702
(2006). https://doi.org/10.1063/1.2234538 0021-8979 Google Scholar
T. Hirase, T. Ishii, and Y. Nagasaki,
“Construction of poly(ethylene glycol) tethered chain surface possessing mercapto group at the distal end to achieve high protein sensing,”
Trans. Mater. Res. Soc. Jpn., 31
(3), 633
–636
(2006). 1382-3469 Google Scholar
Y. Nagasaki, H. Kobayashi, Y. Katsuyama, T. Jomura, and T. Sakura,
“Enhanced immunoresponse of antibody/mixed-PEG co-immobilized surface construction of high-performance immunomagnetic ELISA system,”
J. Colloid Interface Sci., 309
(2), 524
–530
(2007). https://doi.org/10.1016/j.jcis.2006.12.079 0021-9797 Google Scholar
X. Ding, K. Moumanis, J. J. Dubowski, L. Tay, and N. L. Rowell,
“Fourier-transform infrared and photoluminescence spectroscopies of self-assembled monolayers of long-chain thiols on (001) GaAs,”
J. Appl. Phys., 99
(5), 54701
–1
(2006). https://doi.org/10.1063/1.2178659 0021-8979 Google Scholar
R. G. Nuzzo, L. H. Dubois, and D. L. Allara,
“Fundamental studies of microscopic wetting on organic surfaces. I. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers,”
J. Am. Chem. Soc., 112
(2), 558
–569
(1990). https://doi.org/10.1021/ja00158a012 0002-7863 Google Scholar
G. M. Marshall, F. Bensebaa, and J. J. Dubowski, J. Appl. Phys., 105 094310
(2009). https://doi.org/10.1063/1.3122052 0021-8979 Google Scholar
Y. Ke, S. Milano, X. W. Wang, N. Tao, and Y. Darici,
“Structural studies of sulfur-passivated GaAs (100) surfaces with LEED and AFM,”
Surf. Sci., 415
(1–2), 29
–36
(1998). https://doi.org/10.1016/S0039-6028(98)00435-X 0039-6028 Google Scholar
S. R. Wickramasinghe, B. Kalbfu, A. Zimmermann, V. Thorn, and U. Reichl,
“Tangential flow microfiltration and ultrafiltration for human influenza A virus concentration and purification,”
Biotechnol. Bioeng., 92
(2), 199
–208
(2005). https://doi.org/10.1002/bit.20599 0006-3592 Google Scholar
T. Noda, H. Sagara, A. Yen, A. Takada, H. Kida, R. H. Cheng, and Y. Kawaoka,
“Architecture of ribonucleoprotein complexes in influenza A virus particles,”
Nature (London), 439
(7075), 490
–492
(2006). https://doi.org/10.1038/nature04378 0028-0836 Google Scholar
F. Kienberger, C. Stroh, G. Kada, R. Moser, W. Baumgartner, V. Pastushenko, C. Rankl, U. Schmidt, H. Müller, E. Orlova, C. LeGrimellec, D. Drenckhahn, D. Blaas, and P. Hinterdorfer,
“Dynamic force microscopy imaging of native membranes,”
Sens. Nanostruct., 97
(1–4), 229
–237
(2003). Google Scholar
K. N. Tyler and B. N. Fields,
“Pathogenesis of neurotropical infections,”
Handbook of Clinical Neurology, 12 25
–49 1989). Google Scholar
P. G. Abrams and A. R. Fritzberg,
“Pretargeted radioimmunotherapie of cancer,”
Radioimmunotherapy of Cancer, 169
–194 Informa Health Care, New York
(2000). Google Scholar
E. P. Diamandis and T. K. Christopoulos,
“Immunoassay configuration,”
Immunoassay, 227
–267 Academic Press, Ontario, Canada
(1996). Google Scholar
|