|
|
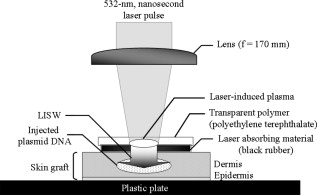
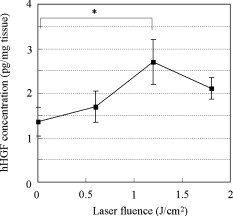
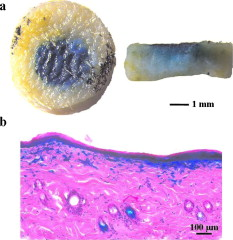
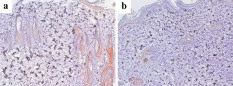
1.IntroductionGene therapy has recently received much attention as a new strategy for improving the outcome of skin transplantation, based on the fact that a number of growth factors related to wound healing have been identified and extensively investigated as described in the following. For gene transfer to skin tissue, viral vectors, such as adenoviruses,1 adeno-associated viruses,2, 3 and retroviruses,4 have been used widely, but the use of nonviral methods, including naked plasmid DNA injection,5, 6, 7 liposomal transfection,8, 9 and electroporation,10, 11 is becoming increasingly important due to their safety, easy handling, and higher targeting characteristics. However, there still remain requirements for further improvements in efficiency, flexibility, and targeting characteristics of nonviral gene transfer methods. We have been investigating nonviral gene transfer based on laser-induced stress waves (LISWs) or photomechanical waves12, 13, 14 that are generated by irradiating a solid material with nanosecond laser pulses. On the basis of this technique, we demonstrated that reporter genes can be transferred to skins in rats and central nervous systems in mice in vivo, as well as to various types of cell lines in vitro.15, 16, 17 The most important advantage of this method is its high spatial controllability of transfection due to the nature of a laser beam, i.e., highly controllable, well-defined optical energy. In addition, deep-located tissue can be treated by LISWs, since an LISW is a kind of acoustic wave and it can therefore be efficiently propagated through tissue. Furthermore, laser energy can be transmitted through an optical fiber. Thus, catheter-based or endoscopic gene transfer may come into practical use, which would considerably improve the flexibility of the gene transfer process. Skin transplantation is essential for treatment of full-thickness skin injuries and defects such as severe burns, and its outcome is determined by adhesion of grafted tissues, which is completed through a spontaneous wound healing process since vascular anastomosis is not applied. Wound healing is a complex and dynamic process that follows an orderly sequence of events: inflammation, cell migration, angiogenesis, granulation tissue formation, collagen deposition, and reepithelialization.18 Proper balance of these biological reactions is important for efficient progress of wound healing. It has been revealed that wound healing is regulated by a number of growth factors, such as basic fibroblast growth factor (bFGF), transforming growth factor- (TGF- ), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF).19 Adhesion of transplanted tissue, which is completed through a wound healing process, is initiated by angiogenesis, and accelerated angiogenesis can therefore effectively improve the outcome of transplantation; earlier adhesion is important to decrease the risk of infection. Since VEGF, bFGF, and HGF are known to enhance angiogenesis,20, 21, 22 the secretion of these growth factors that are up-regulated by gene transfection is a promising strategy for accelerating the wound healing process and hence adhesion of skin grafts and skin flaps.23, 24, 25, 26, 27 In our previous study, we attempted to deliver the human HGF (hHGF) gene to rat skin grafts by the use of LISWs ex vivo; autografting was performed using the grafts with the objective of accelerating angiogenesis in the grafted tissue.28 At after grafting, it was found that the density of CD31-positive cells was significantly greater for the grafted tissue with hHGF gene transfer by LISWs than the densities in three control groups: normal grafting without any treatment, grafting with injection of hHGF gene (no LISWs), and grafting with application of LISWs after control plasmid vector injection. However, further investigation is needed to validate the efficacy of LISW-based hHGF gene delivery for improving the adhesion of grafted skin; evaluation of reperfusion, cellular proliferation, and reepitheliarization in grafted skin is essential. In the present study, we performed experiments to validate LISW-based delivery of a therapeutic vector construct carrying the hHGF gene to rat free skin grafts for accelerating their adhesion. We first optimized laser fluence to maximize the concentration of hHGF expressed in the grafted skins. Distribution of gene expression in the grafted tissue was also assessed by using the LacZ gene to analyze the outcome of gene expression. With the grafts transfected under optimum conditions, we performed autografting and analyzed angiogenesis, cell proliferation, and reepithelialization based on immunohistochemistry for the grafted tissue and evaluated recovery of blood perfusion by laser Doppler imaging (LDI). The results were compared with those obtained for five control groups: grafting with hHGF gene injection alone, grafting with control plasmid vector injection alone, grafting with LISW application alone, grafting with LISW application after control plasmid vector injection, and normal grafting. 2.Materials and MethodsThe protocols in this study were approved by the Committee on Ethics of Animal Experiments of the National Defense Medical College. 2.1.Construction of Plasmid DNAHuman HGF (hHGF) expression plasmid vector was constructed by insertion of hHGF cDNA 21 into the NotI site of pcDNA3.1 (Invitrogen Corp., Carlsbad, California). A cDNA clone coding for hHGF was provided by AnGes MG, Inc. (Japan). The vector was driven by a cytomegalovirus (CMV) promoter. The vector used as a control vector was a CMV vector plasmid not containing HGF cDNA (Invitrogen Corp.). Plasmid DNA encoding -galactosidase (E1081) was purchased from a commercial source (Promega, Madison, Wisconsin). The -galactosidase expression vector was driven by an SV40 promoter. Each plasmid was transformed into Escherichia coli competent cells and then amplified and purified on a Qiagen column (Qiagen, Inc., Hilden, Germany) according to the manufacturer’s manual. 2.2.Measurement of hHGF Protein Expressed in Grafted SkinWe examined the expression of hHGF in the grafted rat skins to determine the optimum gene transfer conditions. We used Sprague-Dawley rats weighing . They were anesthetized by intraperitoneal injection of pentobarbital sodium ( animal weight), and their dorsal hairs were clipped and depilated. Dorsal skin of a rat, measuring , was exsected for use as a graft, and its subcutaneous fat was removed; four grafts were obtained from one rat. Each graft was placed upside-down on a -thick plastic plate, and hHGF expression vector plasmid DNA ( , ) was injected into the graft from the top, hence the reverse side, using a syringe (80601, Hamilton Company, Reno, Nevada) with a needle (Terumo, Tokyo, Japan). A -thick black natural rubber disk was used as the laser target; a -thick transparent polyethylene terephthalate sheet was bonded to the top surface of the target to confine the laser-induced plasma. The laser target was placed on the gene-injected site, and the target was irradiated with three , (FWHM) laser pulses at a laser fluence of 0.6, 1.2, and to generate LISWs (Fig. 1 ). The laser spot diameter on the target was . After application of LISWs, autografting was performed, i.e., the graft was transplanted onto the donor site of the rat. At after the operation, punch biopsy of samples of in diameter was performed for gene-transferred grafted skins. Five grafts were analyzed at each fluence: 0, 0.6, 1.2, and . Tissue samples obtained from the sites of gene injection into the grafts were homogenized in an hHGF extract solution containing phenylmethylsulfonyl fluoride (Institute of Immunology, Tokyo, Japan) for . The homogenate was centrifuged at for at . The concentration of hHGF protein expressed in the tissue sample was measured by an enzyme-linked immunosorbent assay (ELISA) using an anti-human-HGF monoclonal antibody (Institute of Immunology, Tokyo, Japan). With this assay, no cross-reaction with rat HGF is expected. 2.3.Histological Analysis of -Galactosidase ExpressionTo analyze sites of -galactosidase expression, punch biopsy of samples of in diameter was performed for gene-transferred grafted skins at after application of LISWs. The excised samples were fixed in cold 4% paraformaldehyde in phosphate-buffered saline (PBS) for , washed in PBS for , and then stained in X-gal 5-bromo-4-chloro-3-iindol-b-d-galactopyranoside solution ( -gal staining kit, Invitorogen, Carlsbad, California) at overnight. The stained samples were embedded in optical coherence tomography (OCT) compound (Sakura Finetek USA, Inc., Torrance, California) in liquid nitrogen, cut into -thick axial sections, and counterstained with hematoxylin and eosin (HE). 2.4.Delivery of hHGF Gene to Skin Grafts by LISWs and AutograftingWe delivered the hHGF gene to rat skin grafts by using LISWs with the objective of enhancing their adhesion after autografting. This experiment was performed using the optimum experimental conditions described earlier, i.e., at a laser fluence of . Dorsal skin of a rat, measuring , was exsected for use as a graft, and its subcutaneous fat was removed; two grafts were obtained from one rat. Human HGF expression plasmid vector ( , ) was injected into the graft; the gene was injected at the four corners and the center (five points) of the graft. LISWs, which were generated by irradiating a laser target with three laser pulses, were applied to each gene-injected site. After application of LISWs, the graft was transplanted onto the donor site of the rat (hHGF plus LISWs). As controls, autografting was performed under five other conditions: (1) hHGF plasmid was injected into the grafts but no LISWs were applied (hHGF alone); (2) control vector (CV) was injected into the grafts but no LISWs were applied (CV alone); (3) after CV injection, LISWs were applied to the grafts (CV plus LISWs); (4) LISWs were applied to the grafts without any plasmid injection (LISWs alone); and (5) neither plasmid injection nor LISWs was applied (normal grafting). In the back of each rat, grafting was performed at two sites symmetric to the axis of vertebrae. To protect the grafted skins and to avoid infection, the back was covered with nonadhering dressing (ADAPTIC, Johnson and Johnson, Gargrave, Skipton, UK), and the dressing was replaced every day. During the experiment, rats were singly caged with free access to food and water at an ambient temperature in the range of . 2.5.Measurement of Blood Flow by Laser Doppler ImagingBlood flow within the grafted skins was examined by laser Doppler imaging (Periscan System, Primed, Sweden); blood flow signals can be correlated with the density of neovascularities in the grafted tissue. From day 1 to day 7 after transplantation, measurement was performed for a area on the grafted skin. Integrated blood flow signals were also used to assess reperfusion in the grafted skins. More than six grafts were examined in each condition. 2.6.Immunohistochemical AnalysisAnti-rat CD31/PECAM-1 (platelet endothelial cell adhesion molecule-1) antibody was used to investigate vasculogenesis in the grafted skin.29 Specimens were excised from the grafted skins, and they were fixed in ethanol and embedded in paraffin blocks. Deparaffinized sections of the specimens were incubated overnight with anti-rat CD31 (BD Biosciences, Franklin Lakes, New Jersey) at a dilution of 1:5 and then incubated for with biotinylated rabbit anti-mouse immunoglobulins (DAKO, Inc., Tokyo, Japan) at a dilution of 1:200. For digital histological images, CD31-positive pixels were discriminated on the basis of RGB values, and the total number of CD31-positive pixels in the grafted skin was counted. For each condition, three rats were examined. A tissue slice with a full width of the graft was obtained, and its portion at the middle was analyzed; 12 to 15 digital images were combined to form an image of one tissue slice. To analyze the cell proliferation activity and reepithelialization in the grafted skin, anti-rat Ki-67 antibody30 and anti-human antibody31 were used. Specimens were excised from the grafted skins, and they were fixed in neutral buffered formalin and embedded in paraffin blocks. Deparaffinized sections of the grafts were incubated overnight with anti-rat Ki-67 (DAKO, Inc., Tokyo, Japan) at a dilution of 1:250 and anti-human (DAKO, Inc.) at a dilution of 250 and then incubated for with biotinylated rabbit anti-mouse immunoglobulins (LSAB-2 rat kit, DAKO, Inc.) against Ki-67 and peroxidase-conjugated goat anti-mouse and rabbit immunoglobulins (Rat MAX MULTI, Nichirei, Inc., Tokyo, Japan) against . 3.Results3.1.Optimal Laser Irradiation Conditions for LISW-Based Gene Transfection into Skin GraftsFigure 2 shows the protein level of hHGF expressed in the grafted skin at after LISW application. At a laser fluence of , i.e., with plasmid injection only, hHGF concentration in the graft was as low as tissue. A twofold increase in hHGF concentration was obtained at a laser fluence of . No further increase in hHGF concentration was observed at a higher fluence . The protein level and its fluence dependence were similar to those observed in our previous experiment on hHGF gene delivery to rat native skin (not graft).28 On the basis of these results, we determined the optimal laser fluence for the following experiments to be . 3.2.Distribution of -Galactosidase Expression in Grafted SkinFigure 3 shows the expression of lacZ gene in the grafted skins at after grafting. Intense gene expression was observed in an area of approximately in diameter [Fig. 3a]. This area corresponded to a laser spot on the target, demonstrating the capability of our method for highly site-specific transfection. A cross section of the grafted skin stained with hematoxylin and eosin shows that the gene was efficiently expressed not only in the epidermal layer but also in the upper layer of the dermis and the hair follicles [Fig. 3b]. In the case of hHGF alone, limited gene expression was observed along with the track of a needle (data not shown). 3.3.Histological Analysis of Vascular Endothelial Cells in Grafted SkinFigure 4 (A) shows cross-sectional images of the grafted skins with immunohistochemical staining using anti-rat CD31/PECAM-1 antibody on day 3 after grafting. The results obtained with skin grafts treated under the four different conditions (hHGF plus LISWs, hHGF alone, CV alone, and normal grafting) were compared. The grafted skin with hHGF gene plus LISWs showed a much larger number of CD31-positive cells than did those of the three other conditions; many stained vascular structures were observed in the graft with hHGF gene plus LISWs. Figure 4(B) shows the averaged distributions of CD31-positive pixel numbers, i.e., CD31-positive cells in the horizontal direction for three rats . A considerably greater distribution of CD31-positive cells was observed over the whole range of the graft of hHGF gene plus LISWs compared with those of the three control groups; there were significant differences in the total number of CD31-positive cells over the graft between hHGF gene plus LISWs and the three control groups [ , Fig. 4(C)]. Fig. 4Results of evaluation of angiogenesis for grafted skins in rats under different graft treatment conditions: (a) hHGF plus LISWs, (b) hHGF alone, (c) control vector (CV) alone, and (d) normal grafting. (A) Cross sections of the grafted skins with immunohistochemical staining using anti-rat CD31/PECAM-1 antibody at after transplantation. Scale bars indicate . (B) Averaged distributions of CD31-positive cells in the horizontal direction for the grafted skins; the depth-integrated CD31-positive cells number at each point is shown . (C) Area- and depth-integrated CD31-positive cell numbers for the grafted skins, derived from the distributions in Fig. 4(B). Values are expressed as error . 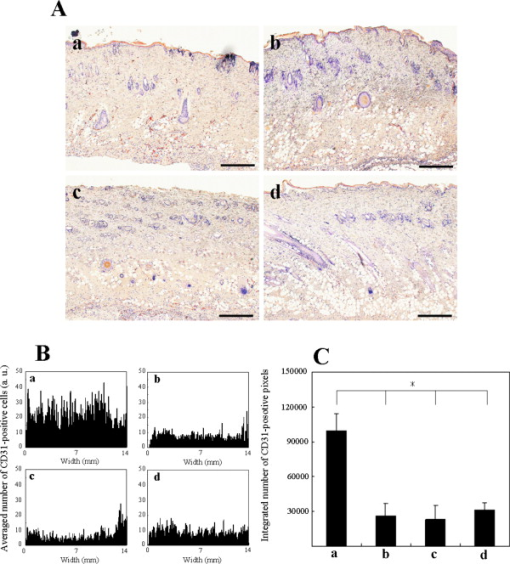 3.4.Evaluation of Reperfusion in Grafted skin by Laser Doppler ImagingFigure 5 (A) shows laser Doppler images of the grafted skin with hHGF gene transfer by LISWs (hHGF plus LISWs) and the grafted skins of the five controls (hHGF alone, CV alone, CV plus LISWs, LISWs alone, and normal grafting) on days 2, 3, and 4 after transplantation. Before day 3, blood flow signal was low in all cases, while on day 4, the signal for the graft with hHGF gene plus LISWs was much higher than those of the five other conditions. Figure 5(B) shows the increased ratio of the blood flow signal on day 4 to that on day 3 for 6 to 9 rats in each condition; there was a significant difference in the increased ratio between hHGF gene plus LISWs and the five other conditions . As described in the following, no infection was observed for any of the grafted skins during the experiment, but partial or total necrosis was observed for grafted skins with delayed reperfusion. Fig. 5Results of laser Doppler imaging for grafted skins in rats under the different graft treatment conditions: (a) hHGF plus LISWs, (b) hHGF alone, (c) control vector (CV) alone, (d) CV plus LISWs, (e) LISWs alone, and (f) normal grafting. (A) Laser Doppler images at 2, 3, and after transplantation. Laser Doppler images of a area of the grafted skins are shown. The level of blood flow signal was subdivided into 28 sections, for which different colors were allocated. The section of the lowest signal level is displayed in dark blue, whereas the section of the highest signal level is displayed in red. (B) Increased ratio of blood flow signal within the grafted skins from day 3 to day 4 ( to 9). Values are expressed as error ( , , hHGF plus LISWs versus others). (Color online only.) 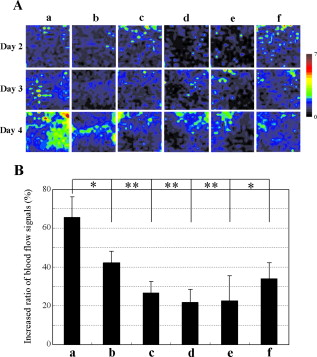 3.5.Histological Analysis of Cell Proliferation in Grafted SkinFigure 6 shows the expression of Ki-67 antigen in the grafted skin with hHGF gene plus LISWs and that of normal grafting on day 3 after transplantation; the tissues in both images correspond to the edge portions of the grafted skins. No evident cell-proliferative activity was observed in the normal grafted skin, while cell proliferation was considerably activated in the grafted skin with hHGF gene plus LISWs, especially in the hair follicles. 3.6.Histological Analysis of Reepitheliarization in Grafted SkinFigure 7 shows the expressions of cytokeratin in cross sections of grafted skins on days 3, 5, and 7 after transplantation. There was no discernible difference in reepitherialization between the groups on day 3 [Figs. 7a, 7d, 7g, 7j, 7m, 7p]. On day 5, however, more efficient reepithelialization occurred on the edge of the grafted skin with hHGF gene plus LISWs [Fig. 7b] compared with that in the five control groups [Figs. 7e, 7h, 7k, 7n, 7q]. The grafted skin with hHGF gene plus LISWs showed almost completed reepithelialization on day 7 [Fig. 7c]; the newly formed epidermis had more than five cell layers. On the other hand, epidermis in the five control groups was deficient and a scab formation was partially observed [Figs. 7f, 7i, 7l, 7o, 7r]. Fig. 7Expressions of keratins in the grafted skins with immunohistochemical staining using antikeratin antibodies at 3, 5, and after transplantation: (a–c) hHGF plus LISWs; (d–f) hHGF alone; (g–i) control vector (CV) alone; (j–l) CV plus LISWs; (m–o) LISWs alone; and (p–r) normal grafting (3 and : edge of grafted skin; : center of grafted skin). Dotted lines indicate the boundary between the graft and recipient: left side is graft, and right side is recipient. Scale bars indicate . 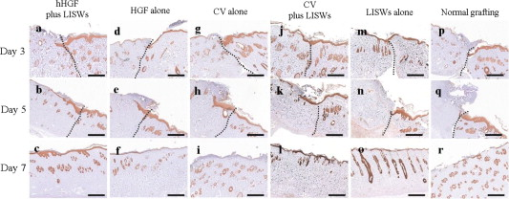 3.7.Measurement of Weights of Rats after GraftingWeights of the rats (four rats in each condition) were measured for after grafting. Although about 30% of the rats showed a weight reduction of more than 10%, with a maximum reduction of 18% until after grafting, the weights recovered to their initial levels within one week after grafting in most cases. Since dependence of weight reduction on experimental conditions was not observed, the weight reductions seemed to be caused by invasion due to grafting, not due to additional treatments such as gene transfer. No infection was observed during the period of experiments under all conditions. 4.DiscussionIn the present study, we delivered a therapeutic vector construct carrying the hHGF gene to free skin grafts of rats by applying LISWs; autografting was performed with the grafts. We observed acceleration of both the reperfusion of blood flow and reepithelialization for the grafted skins with LISW-based hHGF gene delivery compared with that for the grafted skins of the five control groups. For efficient adhesion of grafted skins, earlier recovery of blood flow and reepithelialization are essential, since transplanted tissue should be provided with nutrients and oxygen by blood flow and infection should be avoided by wound closure. Thus, the results obtained in this study demonstrate that our gene delivery method is valid for accelerating adhesion of skin grafts. In this study, we delivered a gene to skin grafts by applying LISWs ex vivo, while a gene can be delivered to a wound bed (subcutaneous tissue) or tissue around the wound in vivo with the objective of accelerating grafted skin adhesion or wound healing. For example, Meuli reported that a high level of gene expression in mice was achieved by simply injecting a plasmid into tissue around the wound.32 However, the present study showed that efficient gene expression in the grafted skin was not achieved by only injecting a plasmid into the grafts (Fig. 2), and no significant acceleration of graft adhesion was observed in this case (Figs. 4, 5, 7). This is presumably associated with reduced tissue viability of the skin grafts in our ex vivo case. However, ex vivo gene transfer can provide a unique advantage for skin transplantation; gene delivery can be spatially and temporally separated from the recipients, considerably increasing the flexibility of treatment and operation. It should also be noted that there is a difference in sites of efficient gene expression between the in vivo case described earlier and our ex vivo case. The group mentioned earlier reported efficient gene expression mainly in adipocytes,32 while we observed strong gene expression in the epidermis, fibroblasts, and hair follicles in the tissue after grafting (Fig. 3). It is known that activation, proliferation, and migration of keratinocytes both in the epidermis and hair follicles are important for epidermal regeneration after skin transplantation.33, 34, 35 Hair follicles have a complex structure with many cell types, and stem cells reside in the bulge, their permanent upper portion.36, 37 Participation of follicular cells in reepithelialization has been known for many years. Ito reported that stem cells from the bulge quickly ascend the follicle in response to wounding and ultimately comprise about 30% of the new cells in the healed wound.38 Since efficient gene expression was observed in both the epidermal layer and hair follicles in the present study (Fig. 3), accelerated adhesion of grafted skin is attributable to the activation of epithelial cells and endothelial cells, which are both targeted cells of HGF. Hair follicles are considered to be an ideal target for accelerated skin regeneration by gene therapy, since they can produce many kinds of cytokines and proteins. Thus, our gene delivery technique might be useful not only for accelerating adhesion of grafted skins but also for healing various types of wounds. HGF is known to be a mitogen for endothelial cells and keratinocytes; its effect on angiogenesis has been reported for rabbit, rat, and mouse ischemic hindlimb models and even human patients with peripheral arterial disease.22, 39, 40, 41 In this study, early recovery of blood flow after grafting) was observed for the grafted skins with LISW-based delivery of hHGF gene compared with that for the grafted skins of the five control groups (Fig. 5). This indicates that blood perfusion was recovered even in the top layer of the grafted skins with hHGF gene delivery as early as after grafting, since the measurement depth of the LDI used in this study was only about . On the other hand, newly formed vessels with CD31-positive vascular endothelial cells were observed in the depth range of on the same postgrafting day for the grafted skin with hHGF gene delivery [Fig. 4(A)]. This suggests that connection of neovascularities formed in the bottom part of the grafted skin with remaining native blood vessels in the grafts contributes to recovery of blood perfusion. Importantly, there was no significant difference either in reperfusion or in reepithelialization between the grafted skin with control vector injection and normal grafted skin. Although it is known that unmethylated CpG motifs in bacterial DNA trigger an inflammatory response that inhibits gene expression,42 we observed neither increase nor decrease in therapeutic effect. This suggests that therapeutic effects obtained in the present study, i.e., accelerated angiogenesis and reepithelialization, resulted from production of hHGF proteins by gene transfer, not from natural immunity by macrophages and dendritic cells. Characteristics of LISWs are similar to those of shock waves, and it has recently been reported that shock waves accelerate angiogenesis or reperfusion.43, 44, 45 Actually, we observed in our previous study that application of LISWs alone (without gene transfer) improved angiogenesis in the grafted skin to some extent.28 In the present study, however, we did not observe any significant effects by applying LISWs alone (CV plus LISWs and LISWs alone in Figs. 5 and 7). This also supports our claim that therapeutic effects obtained in the present study were due to production of hHGF proteins by gene transfer. The effect of LISWs on angiogenesis is likely to be sensitive to experimental conditions such as pressure value. Further study is needed to evaluate the effect of LISWs on angiogenesis. In general, angiogenesis or increase in vascular formation occurs after HGF gene transfection.40, 46 The reason why we observed enhanced angiogenesis as early as after grafting is not clear, but it is possible that the expression of HGF and its receptor c-met was increased in response to wounding.47 It is also possible that the level of rat endogenous HGF is up-regulated by the effect of exogeneous hHGF gene transfection.23, 48 In the present study, we demonstrated the effect of accelerated adhesion for autografts by LISW-based hHGF gene transfection. Although autografting is ideal for early adhesion because of the absence of rejection, its application is limited for large-area severe skin injuries such as extended burns. Thus, allografts and cultured skin substitutes are used for immediate coverage of the wounds.49, 50 The use of allografts is expanding due to the establishment of skin bank networks. However, since skin is a highly immunogenic organ, allografting causes immune reaction, resulting in defluxion of grafts. Continuous administration of calcineurin inhibitors is used to suppress such reaction after transplantation, but the use of these agents has been shown to increase the risk of infection and to sometimes have serious side-effects, including occurrence of a malignant tumor. Therefore, an alternative strategy for immunosuppression is needed, and HGF gene transfer has recently received much attention as a possible alternative strategy. HGF ameliorates the progression of experimental autoimmune myocarditis, a Th1-type dominant immune response, inducing production of Th2 cytokines.51 In addition, it has been reported that HGF suppresses the development of Th2-type immune responses as well,52, 53, 54 and Yamamura reported that HGF reduced acute and chronic rejection of cardiac allografts.55 Thus, our HGF gene transfer technique might also be useful for controlling rejection in skin allografting. Cultured skin substitutes with genetic modification have also been developed as high-performance bioengineered skin; in most cases, a growth factor gene is transferred to keratinocytes in the cultured skin substitutes by using a retrovirus vector.56, 57, 58 The use of lipofectamine-mediated gene transfection has also been reported for the same purpose.59 However, these methods require a time-consuming process for culturing transfected keratinocytes on a fibrin matrix before grafting. Our gene transfection method might be applicable to cultured skin substitutes immediately before grafting, enabling much more efficient preparation of high-performance bioengineered skins. Nonviral gene delivery, including our LISW-based method, is free from serious virus-related side effects, such as mutagenicity, immunogenicity, and carcinogenicity. In addition, the transgene is not inserted into genomic DNA by nonviral methods, and the gene is therefore expressed transiently. This characteristic is desirable for transplantation and wound healing since no gene expression is needed after completion of the wound healing process; continuous gene expression would have detrimental effects. There are also advantages in our LISW-based method when compared with conventional physical methods, such as electropolation and ultrasound. Much higher site specificity of transfection can be achieved by our method due to the excellent spatial controllability of laser energy, reducing the risk of side effects. Our method seems to be minimally invasive; for skin tissues, we observed neither apoptosis nor inflammation under typical conditions for gene transfer (data not shown). For clinical application, however, we need much more data on interaction of LISWs with tissues. Investigation on the effect of LISWs at gene and protein levels is an important part of our future works; the possible accelerated angiogenesis by LISWs (without gene transfer) described earlier suggests the effect of LISWs either at gene or protein level. Long-term functional or behavioral analysis should also be conducted, especially when LISWs directly interact with a central nerve system. In conclusion, we have demonstrated for a rat autograft model that delivery of the hHGF gene to skin grafts by using laser-induced stress waves ex vivo results in significant acceleration of reperfusion of blood and reepithelialization. This suggests that our gene delivery method is useful for accelerating adhesion of transplanted tissue and hence improving the outcome of skin transplantation. In recent years, automated high-speed laser scanning for arbitrary-shaped objects has been established in industry; this technology can be beneficially applied to gene delivery to various types and shapes of grafts and bioengineered tissues as well as wounds both ex vivo and in vivo. ReferencesK. W. Liechty, M. Nesbit, M. Herlyn, A. Radu, N. S. Adzick, and T. M. Crombleholme,
“Adenoviral-mediated overexpression of platelet-derived growth factor-B corrects ischemic impaired wound healing,”
J. Invest. Dermatol., 113 375
–383
(1999). https://doi.org/10.1046/j.1523-1747.1999.00705.x 0022-202X Google Scholar
B. Deodato, N. Arsic, L. Zentilin, M. Galeano, D. Santoro, V. Torre, D. Altavilla, D. Valdembri, F. Bussolino, F. Squadrito, and M. Giacca,
“Recombinant AAV vector encoding human VEGF165 enhances wound healing,”
Gene Ther., 9 777
–785
(2002). https://doi.org/10.1038/sj.gt.3301697 0969-7128 Google Scholar
M. Galeano, B. Deodato, D. Altavilla, G. Squadrito, P. Seminara, H. Marini, F. S. d’Alcontres, M. Colonna, M. Calo, P. L. Cascio, V. Torre, M. Giacca, F. S. Venuti, and F. Squadrito,
“Effect of recombinant adeno-associated virus vector-mediated vascular endothelial growth factor gene transfer on wound healing after burn injury,”
Crit. Care Med., 31 1017
–1025
(2003). https://doi.org/10.1097/01.CCM.0000059435.88283.C2 0090-3493 Google Scholar
R. Quinonez and R. E. Sutton,
“Lentiviral vectors for gene delivery into cells,”
DNA Cell Biol., 21 937
–951
(2002). https://doi.org/10.1089/104454902762053873 1044-5498 Google Scholar
J. C. Vogel,
“Nonviral skin gene therapy,”
Hum. Gene Ther., 11 2253
–2259
(2000). https://doi.org/10.1089/104303400750035780 1043-0342 Google Scholar
D. Sawamura, S. Ina, K. Itai, X. Meng, A. Kon, K. Tamai, K. Handa, and I. Hashimoto,
“In vivo gene introduction into keratinocytes using jet injection,”
Gene Ther., 6 1785
–1787
(1999). https://doi.org/10.1038/sj.gt.3301002 0969-7128 Google Scholar
U. Hengge, B. Dexling, and A. Mirmohammadsadegh,
“Safety and pharmacokinetics of naked plasmid DNA in the skin: studies on dissemination and ectopic expression,”
J. Invest. Dermatol., 116 979
–982
(2001). https://doi.org/10.1046/j.1523-1747.2001.01341.x 0022-202X Google Scholar
X. Gao and L. Huang,
“Cationic liposome-mediated gene transfer,”
Gene Ther., 2 710
–722
(1995). 0969-7128 Google Scholar
M. G. Jeschke and D. Klein,
“Liposomal gene transfer of multiple genes is more effective than gene transfer of a single gene,”
Gene Ther., 11 847
–855
(2004). https://doi.org/10.1038/sj.gt.3302229 0969-7128 Google Scholar
L. Zhang, E. Nolan, S. Kreitschitz, and D. P. Rabussay,
“Enhanced delivery of naked DNA to the skin by noninvasive in vivo electroporation,”
Biochim. Biophys. Acta, 1572 1
–9
(2002). Google Scholar
F. Andre and L. M. Mir,
“DNA electrotransfer: its principles and an updated review of its therapeutic applications,”
Gene Ther., 11 S33
–S42
(2004). https://doi.org/10.1038/sj.gt.3302367 0969-7128 Google Scholar
S. E. Mulholland, S. Lee, D. J. McAuliffe, and A. G. Doukas,
“Cell loading with laser-generated stress waves: the role of the stress gradient,”
Pharm. Res., 16 514
–518
(1999). https://doi.org/10.1023/A:1018814911497 0724-8741 Google Scholar
G. K. Menon, N. Kollias, and A. G. Doukas,
“Ultrastructural evidence of stratum corneum permeabilization induced by photomechanical waves,”
J. Invest. Dermatol., 121 104
–109
(2003). https://doi.org/10.1046/j.1523-1747.2003.12302.x 0022-202X Google Scholar
A. G. Doukas and N. Kollias,
“Transdermal drug delivery with a pressure wave,”
Adv. Drug Delivery Rev., 56 559
–579
(2004). https://doi.org/10.1016/j.addr.2003.10.031 0169-409X Google Scholar
M. Terakawa, S. Sato, H. Ashida, K. Aizawa, M. Uenoyama, Y. Masaki, and M. Obara,
“In vitro gene transfer to mammalian cells by the use of laser-induced stress waves: effects of stress wave parameters, ambient temperature, and cell type,”
J. Biomed. Opt., 11 014026
(2006). https://doi.org/10.1117/1.2160407 1083-3668 Google Scholar
M. Ogura, S. Sato, K. Nakanishi, M. Uenoyama, T. Kiyozumi, D. Saitoh, T. Ikeda, H. Ashida, and M. Obara,
“In vivo targeted gene transfer in skin by the use of laser-induced stress waves,”
Lasers Surg. Med., 34 242
–248
(2004). https://doi.org/10.1002/lsm.20024 0196-8092 Google Scholar
Y. Satoh, Y. Kanda, M. Terakawa, M. Obara, K. Mizuno, Y. Watanabe, and S. Endo,
“Targeted DNA transfection into the mouse central nervous system using laser-induced stress waves,”
J. Biomed. Opt., 10 060501
(2005). https://doi.org/10.1117/1.2128432 1083-3668 Google Scholar
A. B. Wysocki,
“Wound management,”
Int. J. Dermatol., 35 82
–91
(1996). https://doi.org/10.1111/j.1365-4362.1996.tb03266.x 0011-9059 Google Scholar
J. Slavin,
“The role of cytokines in wound healing,”
J. Pathol., 178 5
–10
(1996). https://doi.org/10.1002/(SICI)1096-9896(199601)178:1<5::AID-PATH443>3.0.CO;2-W 0022-3417 Google Scholar
N. N. Nissen, P. J. Polverini, A. E. Koch, M. V. Volin, R. L. Gamelli, and L. A. DiPietro,
“Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing,”
Am. J. Pathol., 152 1445
–1452
(1998). 0002-9440 Google Scholar
A. Baird and P. A. Walicke,
“Fibroblast growth factors,”
Br. Med. Bull., 45 438
–452
(1989). 0007-1420 Google Scholar
R. Morishita, S. Nakamura, S. Hayashi, Y. Taniyama, A. Moriguchi, T. Nagano, M. Taiji, H. Noguchi, S. Takeshita, K. Matsumoto, T. Nakamura, J. Higaki, and T. Ogihara,
“Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy,”
Hypertension, 33 1379
–1384
(1999). 0194-911X Google Scholar
K. Nakanishi, M. Uenoyama, N. Tomita, R. Morishita, Y. Kaneda, T. Ogihara, K. Matsumoto, T. Nakamura, A. Maruta, S. Matsuyama, T. Kawai, T. Aurues, T. Hayashi, and T. Ikeda,
“Gene transfer of hepatocyte growth factor into rat skin wounds mediated by liposomes coated with the Sendai virus (hemagglutinating virus of Japan),”
Am. J. Pathol., 161 1761
–1772
(2002). 0002-9440 Google Scholar
R. Gurunluoglu, R. Meirer, M. Shafighi, G. M. Huemer, B. Yilmaz, and H. Piza-Katzer,
“Gene therapy with adenovirus-mediated VEGF enhances skin flap prefabrication,”
Microsurgery, 25 433
–441
(2005). https://doi.org/10.1002/micr.20142 0738-1085 Google Scholar
P. Lubiatowski, C. K. Goldman, R. Gurunluoglu, K. Carnevale, and M. Siemionow,
“Enhancement of epigastric skin flap survival by adenovirus-mediated VEGF gene therapy,”
Plast. Reconstr. Surg., 109 1986
–1993
(2002). https://doi.org/10.1097/00006534-200205000-00031 0032-1052 Google Scholar
P. J. Taub, J. D. Marmur, W. X. Zhang, D. Senderoff, P. D. Nhat, R. Phelips, M. L. Urken, L. Silver, and H. Weinberg,
“Locally administered vascular endothelial growth factor cDNA increases survival of ischemic experimental skin flaps,”
Plast. Reconstr. Surg., 102 2033
–2039
(1998). https://doi.org/10.1097/00006534-199811000-00034 0032-1052 Google Scholar
A. Nakagawa, H. Makino, M. Aoki, T. Miyake, S. Shiraya, T. Nakamura, T. Ogihara, Y. Kimota, and R. Morishita,
“Improvement of survival of skin flaps by combined gene transfer of hepatocyte growth factor and prostacyclin synthase,”
J. Gene Med., 9 1087
–1094
(2007). https://doi.org/10.1002/jgm.1105 Google Scholar
M. Terakawa, S. Sato, D. Saitoh, H. Tsuda, H. Ashida, H. Okano, and M. Obara,
“Enhanced angiogenesis in grafted skins by laser-induced stress wave–associated gene transfer of hepatocyte growth factor,”
J. Biomed. Opt., 12 034031
(2007). https://doi.org/10.1117/1.2745313 1083-3668 Google Scholar
H. M. DeLisser, M. Christofidou-Solomidou, R. M. Strieter, M. D. Burdick, C. S. Robinson, R. S. Wexler, J. S. Kerr, C. Garlanda, J. R. Merwin, J. A. Madri, and S. M. Albelda,
“Involvement of endothelial PECAM-1/CD31 in angiogenesis,”
Am. J. Pathol., 151 671
–677
(1997). 0002-9440 Google Scholar
C. Gerlach, M. Golding, L. Larue, M. R. Alison, and J. Gerdes,
“Ki-67 immunoexpression is a robust maker of proliferative cells in the rat,”
Lab. Invest., 77 697
–698
(1997). 0023-6837 Google Scholar
R. Moll, W. W. Franke, and D. I. Schermer,
“The catalog of human cytokeratins: patterns of expression in normal epithelia, tumor, and cultured cells,”
Cell, 31 11
–24
(1982). https://doi.org/10.1016/0092-8674(82)90400-7 0092-8674 Google Scholar
M. Meuli, Y. Liu, D. Liggitt, M. Kashani-Sabet, S. Knauer, C. Meuli-Simmen, M. R. Harrison, N. S. Adzick, T. D. Heath, and R. J. Debs,
“Efficient gene expression in skin wound sites following local plasmid injection,”
J. Invest. Dermatol., 116 131
–135
(2001). https://doi.org/10.1046/j.1523-1747.2001.00139.x 0022-202X Google Scholar
T. Argyris,
“Kinetics of epidermal production during epidermal regeneration following abrasion in mice,”
Am. J. Pathol., 83 329
–340
(1976). 0002-9440 Google Scholar
G. Taylor, M. S. Lehrer, P. J. Jensen, and R. M. Lavker,
“Involvement of follicular stem cells in forming not only the follicle but also the epidermis,”
Cell, 102 451
–461
(2000). https://doi.org/10.1016/S0092-8674(00)00050-7 0092-8674 Google Scholar
M. I. Morasso and M. Tomic-Canic,
“Epidermal stem cells: the cradle of the epidermal determination, differentiation, and wound healing,”
Biol. Cell, 97 173
–183
(2005). https://doi.org/10.1042/BC20040098 0248-4900 Google Scholar
G. Cotsarelis, T. T. Sun, and R. M. Laver,
“Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis,”
Cell, 61 1329
–1337
(1990). https://doi.org/10.1016/0092-8674(90)90696-C 0092-8674 Google Scholar
E. Fuchs, T. Tumbar, and G. Guasch,
“Socializing with the neighbors: stem cells and their niche,”
Cell, 116 769
–778
(2004). https://doi.org/10.1016/S0092-8674(04)00255-7 0092-8674 Google Scholar
M. Ito, Y. Liu, Z. Yang, J. Nguyen, F. Liang, R. J. Morris, and G. Cotsarelis,
“Stem cells in the hair follicle bulge contribute to wound healing repair but not to homeostasis of the epidermis,”
Nat. Med., 11 1351
–1353
(2005). https://doi.org/10.1038/nm1328 1078-8956 Google Scholar
Y. Taniyama, R. Morishita, M. Aoki, H. Nakagawa, K. Yamamoto, K. Yamazaki, K. Matsumoto, T. Nakamura, Y. Kaneda, and T. Ogihara,
“Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hind limb ischemia models: preclinical study for treatment of peripheral arterial disease,”
Gene Ther., 8 181
–189
(2001). https://doi.org/10.1038/sj.gt.3301379 0969-7128 Google Scholar
R. Morishita, M. Sakaki, K. Yamamoto, S. Iguchi, M. Aoki, K. Yamasaki, K. Matsumoto, T. Nakamura, R. Lawn, T. Ogihara, and Y. Kaneda,
“Impairment of collateral formation in Lp(a) transgenic mice: therapeutic angiogenesis induced by human hepatocyte growth factor gene,”
Circulation, 105 1491
–1496
(2002). https://doi.org/10.1161/01.CIR.0000012146.07240.FD 0009-7322 Google Scholar
R. Morishita, M. Aoki, N. Hashiya, H. Makino, K. Yamasaki, J. Azuma, Y. Sawa, H. Matsuda, Y. Kaneda, and T. Ogihara,
“Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease,”
Hypertension, 44 203
–209
(2004). https://doi.org/10.1161/01.HYP.0000136394.08900.ed 0194-911X Google Scholar
L. Qin, Y. Ding, D. R. Pahud, E. Chang, M. J. Imperiale, and J. S. Bromberg,
“Promoter attenuation in gene therapy: interferon- and tumor necrosis factor- inhibit transgene expression,”
Hum. Gene Ther., 8 2019
–2029
(1997). https://doi.org/10.1089/hum.1997.8.17-2019 1043-0342 Google Scholar
G. M. Huemer, R. Meirer, R. Gurunluoglu, F. S. Kamelger, K. M. Dunst, S. Wanner, and H. Piza-Katzer,
“Comparison of the effectiveness of gene therapy with transforming growth factor-beta or extracorporal shock wave therapy to reduce ischemic necrosis in an epigastric skin flap model in rats,”
Wound Repair Regen, 13 262
–268
(2005). https://doi.org/10.1111/j.1067-1927.2005.130308.x 1067-1927 Google Scholar
C. J. Wang, F. S. Wang, K. D. Yang, L. H. Weng, C. C. Hsu, C. S. Huang, and L. C. Yang,
“Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits,”
J. Orthop. Res., 21 984
–989
(2003). https://doi.org/10.1016/S0736-0266(03)00104-9 0736-0266 Google Scholar
F. S. Wang, C. J. Wang, Y. J. Chen, P. R. Chang, Y. T. Huang, Y. C. Sun, H. C. Huang, Y. J. Yang, and K. D. Yang,
“Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave–stimulated osteoblasts,”
J. Biol. Chem., 279 10331
–10337
(2004). https://doi.org/10.1074/jbc.M308013200 0021-9258 Google Scholar
M. Aoki, R. Morishita, Y. Taniyama, I. Kida, A. Moriguchi, K. Matsumoto, T. Nakamura, Y. Kaneda, J. Higaki, and T. Ogihara,
“Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets,”
Gene Ther., 7 417
–427
(2000). https://doi.org/10.1038/sj.gt.3301104 0969-7128 Google Scholar
A. J. Cowin, N. Kallincos, N. Hatzirodos, J. G. Robertson, K. J. Pickering, J. Couper, and D. A. Belford,
“Hepatocyte growth factor and macrophage stimulating protein are upregulated during excisional wound repair in rats,”
Cell Tissue Res., 306 239
–250
(2001). https://doi.org/10.1007/s004410100443 0302-766X Google Scholar
N. Tomita, R. Morishita, Y. Taniyama, H. Koike, M. Aoki, H. Shimizu, K. Matsumoto, T. Nakamura, Y. Kaneda, and T. Ogihara,
“Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1,”
Circulation, 107 1411
–1417
(2003). https://doi.org/10.1161/01.CIR.0000055331.41937.AA 0009-7322 Google Scholar
B. S. Atiyeh, S. W. Gunn, and S. N. Hayek,
“State of the art in burn treatment,”
World J. Surg., 29 131
–148
(2005). https://doi.org/10.1007/s00268-004-1082-2 0364-2313 Google Scholar
R. E. Horch, J. Kopp, U. Kneser, J. Beier, and A. D. Bach,
“Tissue engineering of cultured skin substitutes,”
J. Cell. Mol. Med., 9 592
–608
(2005). https://doi.org/10.1111/j.1582-4934.2005.tb00491.x Google Scholar
H. Futamatsu, J. Suzuki, S. Mizuno, N. Koga, S. Adachi, H. Kosuge, Y. Maejima, K. Hirao, T. Nakamura, and M. Isobe,
“Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines,”
Circ. Res., 96 823—830
(2005). https://doi.org/10.1161/01.RES.0000163016.52653.2e 0009-7330 Google Scholar
K. Okunishi, M. Dohi, K. Nakagome, R. Tanaka, S. Mizuno, K. Matsumoto, J. Miyazaki, T. Nakamura, and K. Yamamoto,
“A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function,”
J. Immunol., 175 4745
–4753
(2005). 0022-1767 Google Scholar
W. Ito, A. Kanehiro, K. Matsumoto, A. Hirano, K. Ono, and H. Maruyama,
“Hepatocyte growth factor attenuates airway hyperresponsiveness, inflammation, and remodeling,”
Am. J. Respir. Cell Mol. Biol., 32 268
–280
(2005). https://doi.org/10.1165/rcmb.2004-0058OC 1044-1549 Google Scholar
K. Okunishi, M. Dohi, K. Fujio, K. Nakagome, Y. Tabata, and T. Okasora,
“Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice,”
J. Immunol., 179 5504
–5513
(2007). 0022-1767 Google Scholar
K. Yamamura,
“Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation,”
Circulation, 110 1650
–1657
(2004). https://doi.org/10.1161/01.CIR.0000143052.45956.71 0009-7322 Google Scholar
H. Rakhorst, G. G. Krueger, and J. R. Morgan,
“FGF-7 expression enhances the performance of bioengineered skin,”
Mol. Ther., 10 76
–85
(2004). https://doi.org/10.1016/j.ymthe.2004.04.013 Google Scholar
K. E. Hamoen and J. R. Morgan,
“Transient hyperproliferation of a transgenic human epidermis expressing hepatocyte growth factor,”
Cell Transplant, 11 385
–395
(2002). 0963-6897 Google Scholar
P. M. Vogt, S. Thompson, C. Andree, P. Liu, K. Breuing, D. Hatzis, H. Brown, R. C. Mulligan, and E. Eriksson,
“Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis,”
Proc. Natl. Acad. Sci. U.S.A., 91 9307
–9011
(1994). https://doi.org/10.1073/pnas.91.20.9307 0027-8424 Google Scholar
M. D. Rio, F. Larcher, A. Meana, J. C. Segovia, A. Alvarez, and J. L. Jorcano,
“Nonviral transfer of genes to pig primary keratinocytes. Induction of angiogenesis by composite grafts of modified keratinocytes overexpressing VEGF driven by a keratin promoter,”
Gene Ther., 6 1734
–1741
(1999). https://doi.org/10.1038/sj.gt.3300986 0969-7128 Google Scholar
|