|
|
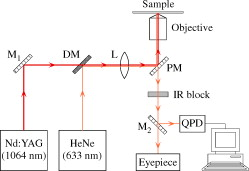
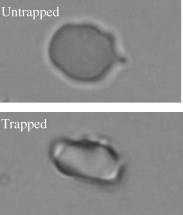
1.IntroductionMalaria is one of the most severe human diseases, responsible for half a billion cases of infection and over one million deaths each year.1 It is caused by the protozoan Plasmodium (P). There are four species of Plasmodium that can infect human red blood cells (RBCs); of these P. falciparum and P. vivax are the most common, with the former resulting in the more deadly form of malaria. Indeed, malaria induced by P. falciparum can be considered as the most widespread and deadly parasitic disease in humans. Malarial infection starts when mosquitoes inject parasites (sporozoites), usually into the subcutaneous tissue, from where they travel to the liver. The disease state occurs when the parasite leaves the liver and invades RBCs. The developmental stages of the parasite inside the RBC are broadly classified as: (i) ring stage, with characteristic thin-ring features whose formation commences at from the time of penetration; (ii) trophozoite stage, with irregular bulges or knobs at the surface of the parasite-infected cell appearing at about after invasion; and (iii) the schizont stage, with nuclear division of the parasite (resulting in the multiplication of the number of parasites) occurring some after invasion. It is well known that the deployment of parasite proteins in the infected RBC membrane results in a change in its shape from the original biconcave shape for the healthy RBC (see, for example, the review of structure evolution during these stages given by Miller 1). Indeed, observing such morphological changes in the RBC is the first and most widespread method to detect the disease. In this work, we have developed a technique for studying the changes in the RBC due to malarial infection. The technique relies on the measurement of the Brownian fluctuations of a single RBC held in an optical-tweezers trap, which in turn depends on the structural properties of the RBC. Because the parasite is known to cause significant stiffening of the RBC2 and a change in its biomechanical properties,3, 4 we expect the trap properties to differ between normal and infected cells. We have therefore studied the progressive changes in trap properties of infected RBCs at different developmental stages of P. falciparum, from early ring to late trophozoite. 2.Experimental TechniqueOptical tweezers5, 6 are now a standard tool for the manipulation of microscopic objects. The technique uses a laser beam that is focused to a near-diffraction-limited spot, usually through a high numerical aperture (NA) microscope objective. Micron-sized objects (having a refractive index higher than the surrounding medium) are then trapped at the intensity maximum of the focused laser beam. The technique finds diverse applications in biology,7, 8, 9, 10, 11, 12 ranging from the studies of single cells to macromolecules. For example, the elastic properties of a single deoxyribonucleic acid molecule can be studied by attaching it to a polystyrene bead and using optical tweezers to manipulate the bead.13 Similarly, molecular forces in single motor-protein molecules (such as kinesin) are studied by attaching them to polystyrene beads.14, 15 The strength of the optical-tweezers trap is an important experimental parameter in these studies. It of course increases with the power of the trapping laser, but also depends on physical properties of the trapped particle, such as its refractive index, shape, and size. The trap strength can be determined using a variety of techniques; one of the standard methods is to measure the power spectrum of thermal fluctuations (Brownian motion) in the position of the trapped particle.15 Standard thermodynamic analysis16 shows that the power spectrum of the fluctuations has a Lorentzian distribution given by where is Boltzmann’s constant, is the temperature of the surrounding medium, is Stoke’s viscous drag coefficient, and is called the corner frequency. The trap strength is related to these parameters according to . Thus, for two particles in the same medium, the experimentally measurable corner frequency will differ if the physical characteristics of the particles are different.Our experimental strategy is now clear. We want to measure the corner frequency for a trapped RBC and see whether it changes between healthy and infected cells. The experimental schematic is shown in Fig. 1 . The heart of the setup is a Zeiss inverted microscope with a , oil-immersion objective. The trapping laser is a diode-pumped Nd:YAG laser operating at with a maximum power of . The beam size is adjusted using a lens so that it fills the back aperture of the objective. The output from a HeNe laser is mixed with the primary trapping beam and used for detecting the position of the trapped particle. The backscattered light from the HeNe laser is detected by a quadrant photodetector (QPD) whose output is analyzed to determine the position of the particle. The typical sensitivity of the QPD is . The RBCs, which have a size of , were separated from blood by centrifugation and suspended in phosphate buffered saline (PBS). RBCs infected with P. falciparum were synchronized according to the procedure given in Ref. 17. Thus, both the normal and infected RBCs were trapped in the same kind of PBS medium. 3.Results and DiscussionThe first experiment was to verify that the corner frequency gives a reliable measure of the trap strength. Corner frequency measurement is a well-established technique for spherical objects (such as polystyrene beads) but is not that well understood for the biconcave shape of RBCs. It is well known that RBCs reorient themselves in an optical-tweezers trap, and malaria-infected RBCs have been recently shown to rotate inside the trap.18 In Fig. 2 , we show photographs of how an RBC from an infected sample reorients itself when trapped. We therefore wanted to verify that the measured value of gave a reasonable value of the trap strength for an RBC and that the values increased linearly with trapping laser power. The value of was measured as follows.19 The output of the QPD was first sampled at for a total data length of 100,000 points. A fast Fourier transform of the data set gave its (complex) Fourier spectrum. From this, the (real) power spectrum was calculated and plotted on a log-log plot. A typical spectrum for a normal RBC taken at a trapping power of is shown in Fig. 3 . The measured spectrum is fitted to a Lorentzian [Eq. 1] in the range of to obtain the value of . In this case, the value of is with a fit error of . However, by repeating the measurement several times and varying the fit range, we estimate the error to be . For an infected RBC, the spectrum is similar but is more flat for frequencies below . More importantly, the corner frequency from the Lorentzian fit is higher at . We have also verified that the spectrum without any particle trapped (shown in the inset of Fig. 3) is flat except for a few spikes due to line frequency noise at and its harmonics. Fig. 3Spectrum of Brownian fluctuations for normal and infected RBCs held in the optical-tweezers trap with a laser power of . The solid lines are Lorentzian fits to the range of . The inset shows the featureless spectrum when no particle is trapped. 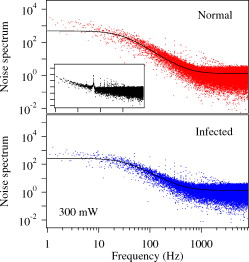 Figure 3 shows that the value of the corner frequency is for an input power of . From this value of , we can find the trap strength as,15 where is the viscosity of the PBS at room temperature and is the typical size of the RBC. This yields a trap strength of , which is comparable to a strength of measured by us under similar conditions for a polystyrene bead in water.The measured values of for powers ranging from are shown in Fig. 4 . Measurements at laser powers of have larger errors; thus, we concentrate on this range. Note that this is the power measured at the mouth of the laser. Losses in the intervening optics (including the microscope objective) and reflection at the sample holder give a power at the trap location of of this input power. Measuring the power directly at the trap is very difficult, but it will always be some fraction of the input power and therefore linearly related to it. Fig. 4Power dependence of . The plot shows the variation of with trapping laser power for normal and infected cells. The solid lines are linear fits showing the expected behavior with no zero offset. 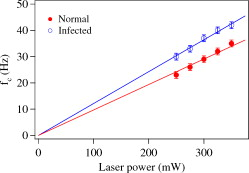 Figure 4 shows that the values of increase linearly as the laser power is increased, for both normal and infected samples. The linear fits, constrained to pass through the origin with no offset, give a (reduced) of 1.9 for the normal sample and 0.67 for the infected sample. Thus, within the error bars, the trap strength goes to zero as the laser power goes to zero, as expected. But the more striking feature is that the slope of the fit is significantly higher for the infected sample. This means that the value of at a given power is higher for infected RBCs, which is the basis of our work. However, because the infected cell is just one specimen from a sample of infected blood with a parasitemia count of 5–10%, it is possible that the particular cell being measured does not actually host the parasite. Therefore, we have measured the corner frequency for an ensemble of cells from an infected sample, containing cells that actually host the parasite and those that do not. A histogram of independently measured frequencies from 43 cells at a laser power of is shown in Fig. 5 , for both normal (control) and infected samples. Note the clear increase in the peak of the distribution for cells from the infected sample, and the large increase in the width. Fig. 5Statistical distribution of . The histograms show the change in the distribution for 43 independent cells from normal and infected samples. 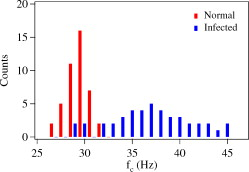 Because we use the same concentration PBS solution for suspending both normal and infected cells, the difference in corner frequency can be attributed to changes in the cell and not the medium. More interestingly, the parasitemia count of 5–10% means that it is likely that only a few of the cells being measured (chosen randomly) actually host the parasite. Thus, it appears that even the cells that do not host the parasite but are in a pool of infected cells undergo structural changes and hence contribute to the observed increase in . This is a surprising finding, and we call it the “bystander effect.” One possible explanation is that it occurs due to structure-changing exochemicals released by the parasite. To verify this hypothesis, we have taken an infected sample, removed all the RBCs, injected normal healthy RBCs into this spent medium, incubated them for , and then measured the corner frequency for these “normal” cells in a PBS solution. We find that the corner frequency shows 80% of the increase as that for the infected sample. We hence conclude that exochemicals play a major role in changing the structure of the cell. It is interesting to note that a previous study has also seen alteration of the properties of uninfected RBCs in a P. falciparum culture.20 A recent study comparing deformability of normal and P. falciparum–infected RBCs found a trans-effect of parasitized cells on the uninfected cells,21 giving further support to our observation. Because the mechanical properties of the RBC depend on the age and storage medium of the sample, we wanted to ensure that the bystander effect was not due to healthy controls being exposed to cell-culture conditions. We therefore prepared a sample of normal RBCs using the same procedure for incubating the cells as for the infected sample. Measurements on an ensemble of the cultured cells gave a mean corner frequency within 5% of the value for the uncultured cells. In order to improve the statistical significance of our results and to ensure a random sampling of the cells, we repeated the measurements several times over a period of a few weeks and confirmed the above findings. We then took independent measurements each for normal cells, ring stage of infection, and trophozoite stage of infection. All the measurements were done at a laser power of . The results were plotted as a histogram, and we verified that they had the expected Gaussian distribution. A Gaussian fit yielded the mean and standard deviation as follows:
Our final experiment was to see if the change depended on the duration of the infection. We therefore repeated the measurements every from the onset of the ring and trophozoite stages. Each value was obtained by taking measurements on 25 individual cells, plotting the results as a histogram, and fitting a Gaussian to the histogram to extract the mean and standard deviation. The results are shown in Fig. 6 . The vertical error bars represent the error in calculating the mean, as discussed above. Within the error bars, the mean corner frequency for the infected samples does not show any significant dependence on time and shows the aforementioned increase of 25% over normal cells. More importantly, the increase is easily measurable with a sample size of 25 cells because the difference is more than ten times the error bars. Fig. 6Time dependence of for normal and infected cells, measured after the onset of each stage. The time for the trophozoite stage is offset by for clarity. The vertical error bars represent the error in calculating the mean. 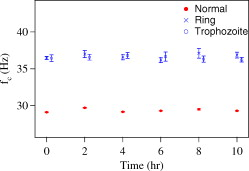 We thus see that value of increases significantly with infection and that the change is independent of the stage or duration of infection. The standard deviation of measurements over an ensemble of cells show us that the change can be measured accurately (i.e., without false positives) with a sample size as small as 25 cells. Each individual measurement of takes only min; hence, the process can be finished in . The statistical nature of the change also makes this technique robust. Small errors in individual measurements of will not significantly affect the distribution over many cells. Finally, the procedure for measuring is straightforward and can be automated. Thus, this method could have potential applications in reliable detection of malaria. 4.ConclusionIn summary, we have demonstrated a method for studying structural changes in RBCs due to malarial infection. We measure the Brownian fluctuations of single RBCs suspended in PBS and held in an optical-tweezers trap, and find that the power spectrum shows a statistically significant increase in corner frequency from normal (control) cells to those from a P. falciparum culture. The corner frequency changes because of changes in physical properties of the cell induced by infection, but the change appears to be independent of the stage of infection. Because the parasitemia count is , it appears that even the uninfected cells in a pool of infected cells contribute to the observed increase. This so-called bystander effect lends support to other observations that infected cells can influence the mechanical properties of uninfected cells. Further studies with blood samples from infected patients (where the parasitemia count is typically ) might shed more light on this phenomenon. We also plan to address issues such as the specificity of the observed change to malarial infection versus other kinds of blood infection (e.g., septicemia due to bacterial or fungal infection), and whether the method works only for the species P. falciparum, because P. falciparum infects erythrocytes while P. vivax prefers reticulocytes. AcknowledgmentsThis work was supported by the Board of Research in Nuclear Sciences (DAE), the Life Sciences Research Board (DRDO), and the Department of Biotechnology, India. ReferencesL. H. Miller, D. I. Baruch, K. Marsh, and O. K. Doumbo,
“The pathogenic basis of malaria,”
Nature, 415 673
–679
(2002). https://doi.org/10.1038/415673a 0028-0836 Google Scholar
M. Marinkovic, M. Diez-Silva, I. Pantic, J. J. Fredberg, S. Suresh, and J. P. Butler,
“Febrile temperature leads to significant stiffening of Plasmodium falciparum parasitized erythrocytes,”
Am. J. Physiol.: Cell Physiol., 296 C59
–64
(2009). https://doi.org/10.1152/ajpcell.00105.2008 0363-6143 Google Scholar
F. K. Glenister, R. L. Coppel, A. F. Cowman, N. Mohandas, and B. M. Cooke,
“Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells,”
Blood, 99 1061
–1063
(2002). https://doi.org/10.1182/blood.V99.3.1060 0006-4971 Google Scholar
S. Suresh, J. Spatz, J. Mills, A. Micoulet, M. Dao, C. Lim, M. Beil, and T. Seufferlein,
“Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria,”
Acta Biomat., 1 15
–30
(2005). https://doi.org/10.1016/j.actbio.2004.09.001 Google Scholar
A. Ashkin,
“Acceleration and trapping of particles by radiation pressure,”
Phys. Rev. Lett., 24 156
–159
(1970). https://doi.org/10.1103/PhysRevLett.24.156 0031-9007 Google Scholar
A. Ashkin, J. M. Dziedzic, J. E. Bjorkholm, and S. Chu,
“Observation of a single-beam gradient force optical trap for dielectric particles,”
Opt. Lett., 11 288
–290
(1986). https://doi.org/10.1364/OL.11.000288 0146-9592 Google Scholar
A. Ashkin and J. M. Dzeidzic,
“Optical trapping and manipulation of single cells using infrared laser beams,”
Science, 235 1517
–1520
(1987). https://doi.org/10.1126/science.3547653 0036-8075 Google Scholar
A. Ashkin, J. M. Dzeidzic, and T. Yamane,
“Optical trapping and manipulation of single cells using infrared laser beams,”
Nature, 330 769
–771
(1987). https://doi.org/10.1038/330769a0 0028-0836 Google Scholar
A. Ashkin, K. Schütze, J. M. Dziedzic, U. Euteneuer, and M. Schliwa,
“Force generation of organelle transport measured in vivo by an infrared laser trap,”
Nature, 348 346
–348
(1990). https://doi.org/10.1038/348346a0 0028-0836 Google Scholar
K. Svoboda and S. M. Block,
“Biological applications of optical forces,”
Annu. Rev. Biophys. Biomol. Struct., 23 247
–285
(1994). https://doi.org/10.1146/annurev.bb.23.060194.001335 1056-8700 Google Scholar
L. Wilson and P. Matsudaira, Laser tweezers in cell biology, 55 Academic Press, New York
(1998). Google Scholar
K. C. Neuman and S. M. Block,
“Optical trapping,”
Rev. Sci. Instrum., 75 2787
–2809
(2004). https://doi.org/10.1063/1.1785844 0034-6748 Google Scholar
S. B. Smith, Y. Cui, and C. Bustamante,
“Overstretching b-dna: the elastic response of individual double-stranded and single-stranded DNA molecules,”
Science, 271 795
–799
(1996). https://doi.org/10.1126/science.271.5250.795 0036-8075 Google Scholar
S. M. Block, L. S. B. Goldstein, and B. J. Schnapp,
“Bead movement by single kinesin molecules studied with optical tweezers,”
Nature, 348 348
–352
(1990). https://doi.org/10.1038/348348a0 0028-0836 Google Scholar
K. Svoboda and S. M. Block,
“Force and velocity measured for single kinesin molecules,”
Cell, 77 773
–784
(1994). https://doi.org/10.1016/0092-8674(94)90060-4 0092-8674 Google Scholar
P. M. Chaikin and T. C. Lubensky, Principles of condensed matter physics, Cambridge University Press, Cambridge, United Kingdom
(1995). Google Scholar
C. Lambros and J. P. Vanderberg,
“Synchronization of plasmodium falciparum erythrocytic stages in culture,”
J. Parasitol., 65 418
–420
(1979). https://doi.org/10.2307/3280287 0022-3395 Google Scholar
J. Dharmadhikari, S. Roy, A. Dharmadhikari, S. Sharma, and D. Mathur,
“Torque-generating malaria-infected red blood cells in an optical trap,”
Opt. Express, 12 1179
–1184
(2004). https://doi.org/10.1364/OPEX.12.001179 1094-4087 Google Scholar
J. Joykutty, V. Mathur, V. Venkataraman, and V. Natarajan,
“Direct measurement of the oscillation frequency in an optical-tweezers trap by parametric excitation,”
Phys. Rev. Lett., 95 193902
(2005). https://doi.org/10.1103/PhysRevLett.95.193902 0031-9007 Google Scholar
D. Sabolovic, B. Canque, N. Berbiguier, and L. Galey,
“Stage-dependent alteration of negative charges of uninfected erythrocytes in plasmodium falciparum culture,”
In Vitro Cell. Dev. Biol.: Anim., 27 595
–596
(1991). https://doi.org/10.1007/BF02631099 1071-2690 Google Scholar
K. Bambardekar, A. K. Dharmadhikari, J. A. Dharmadhikari, D. Mathur, and S. Sharma,
“Measuring erythrocyte deformability with fluorescence, fluid forces, and optical trapping,”
J. Biomed. Opt., 13 064021
(2008). https://doi.org/10.1117/1.3037342 1083-3668 Google Scholar
|