|
|
|
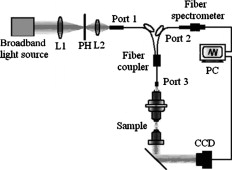
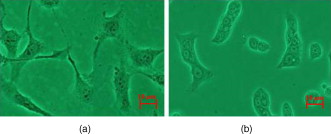
The rapid development of new optical technology has provided much better tools for the study of living cells and for performing pathological diagnosis in vivo.1, 2 Elastic light scattering is one of the properties that can be used to investigate interaction between light and organic tissue. For example, the light scattering signals from a nucleated cell contain the size and morphological information.3, 4 Understanding light scattering in living cells is crucial to many modern diagnostic technologies, such as optical coherent technology (OCT),5 elastic scattering spectroscopy,6 and confocal microscopy.7 The size, refractive index of cells, and nuclei are closely related to intensity, angular distribution, and wavelength responses of light scattering.8, 9 If it is possible to obtain optical backscattering signals from the cell or nuclei, then spectral analysis will be able to differentiate between cell sizes and their indices of refraction. Light scattering spectroscopy (LSS)10 is a system that measures the scattering spectrum related to an angle to obtain quantitative information of cell morphology. Wu and Qu used the elastic scattering spectrum to study light scattering changes of uterine cancer cells treated with acetic acid.11 Liu reported an elastic backscattering spectroscopy technique that can obtain both the backscattering spectrum and microscopic image on one pixel simultaneously.12 Fang developed a new technique, namely confocal light absorption and scattering spectroscopic (CLASS) microscopy, which can observe in-vivo submicron cell structure.13 CLASS microscopy been used to monitor organelles in live cells with no exogenous labels.14 We describe a new technique called fiber confocal backscattering (FCBS) spectroscopy. It aims to measure single-cell backscattering spectra to study normal and cancerous cells. The FCBS spectrometer can be used without biomarkers, because the information of cellular structure and components come from the backscattering light scattered by the subcellular organs. The performance of the system can be further improved by using small particles as biomarkers to distinguish different types of cell organelles that are of particular diagnostic interests.15 The FCBS spectrometer has combined fiber confocal technology and light scattering spectroscopy (LSS). The backscattering light signal at the focal point is collected by the FCBS spectrometer, so the scattering light is just about single scattering light. Multiple scattering is obstructed by the confocal system of the FCBS spectrometer. Thus different cellular structures within the confocal volume of the living cell will exhibit different spectra obtained by the FCBS spectrometer. A schematic illustration of the FCBS spectrometer is shown in Fig. 1 . The FCBS spectrometer contains a broadband light source with a special focusing system [B&W Tek Incorporated (Newark, Delaware), BPS120, ], the light from which is coupled to a port of the multimode fiber coupler with a port and 50:50 splitting ratio, via the SMA-905 standard interface. The fiber with a diameter of and a NA of 0.22 works as a light source and point detector, in which the fiber coupler works as an optical splitter to separate the light of illumination and that of the signal.16 Moreover, the fiber coupler port 3 replaces the confocal system pinhole as well. The light is coupled by port 3 to the optic probe, which is made up of an achromatic objective ( , ) as the collimator and an achromatic objective ( , ) as the objective. The lateral and axial resolutions of this architecture are 5 and , respectively. The light from cell scattering in the objective NA angle range is all received by the optic probe and transmitted through port 2. The backscattering light is split by the coupler and then transmitted through the SMA-905 interface of port 2 to a charge-coupled device (CCD) spectrograph (B&W Tek, BRC112), with a spectral resolution of . To ensure that the object in the field of view is a cell and not an impurity, the FCBS offers an observation function to help view and locate the cell. Illuminating light is given by the same transmitted light through the same optic probe. GES-1 is a human normal gastric epithelial cell line, as shown in Fig. 2 . NCI-N87 is a human gastric carcinoma cell line, as shown in Fig. 2 (The Cell Bank of Type Culture Collection, Shanghai Institute of Cell Biology, Chinese Academy of Sciences). The two cell lines were cultured at , 5% , and in RPMI-1640 medium [1640, GIBCO (Invitrogen, Carlsbad, California) 31800] containing 10% fetal bovine serum [FBS, HyClone (Thermo Scientific, Logan, Utah) SV30087.02]. Both cell lines were cultured with low passage number on a petric dish with a diameter of . The cell lines were planted for about . When they were steady and adhered to the petric dish, after removing the culture medium, a single cell was measured in the petric dish to ensure that the signal obtained by FCBS spectroscopy was obtained from a single cell every time. The lateral resolution of the FCBS spectrometer was calibrated with a standard resolution board of linewidth (Shanghai Institute of Optical Instruments). An area in the standard resolution board was scanned using the electronic control stage with steps, and the light intensity was recorded at each step. The reconstructed image of the standard resolution board subsequently demonstrated that the system’s lateral resolution is . The axial resolution of the FCBS was measured by moving a mirror axially using the same stage. Light intensity was recorded at each step, so that the axial resolution can be calculated with of the peak light intensity as criteria. Before measurement, maximum intensity was obtained by adjusting the mirror manually, and then moving the mirror axially. When moving eight steps from the maximal intensity location, the light intensity changed by of the maximum intensity, corresponding to a distance that can be resolved of . A silicon slice with reflectivity of 30% in both visual light and NIR was used as a standard reflector in the cell backscattering spectrum analysis. The cell backscattering spectra are illustrated in Figs. 3 and 3 . Figures 3 and 3 show the backscattering spectra of GES-1 and NCI-N87, respectively. The vertical ordinate represents the backscattering intensity ratio of the cell backscattering spectrum to the reference silicon slice backscattering light. The wavelength coverage of the curve is from . Figure 3 indicates that the NCI-N87’s spectra show greater scattering light intensity than the GES-1’s, and from the GES-1’s spectral curve has regular intensity changes, but these are not present in NCI-N87. Fig. 3Cell backscattering spectra obtained by FCBS spectrometry with wavelength range from . (a) Microbackscattering spectrum of normal gastric cell GES-1. (b) Cancerous gastric cell NCI-N87. 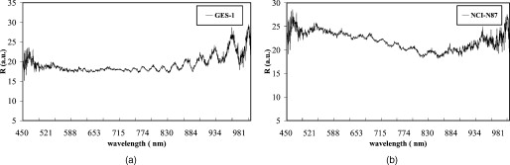 Statistical analysis (ANOVA in Excel, Microsoft) was performed to evaluate differences between the two different cell lines. 50 samples of GES-1 and NCI-N87 were studied, respectively; the results showed that there is a significant difference between the spectra of GES-1 and NCI-N87 . A light beam emerges from the objective as a converging beam and focuses down onto a single cell located at the front focal plane of the objective: the scattering signal of the cell thus comes from an effective illumination volume with diameters of laterally and axially. The scattering spectral difference is based on the spatial variation of the concentration of intracellular solids, and that the variation results give rise to spatial fluctuations in the refractive index of the cell within the effective illumination volume.17 From , the NCI-N87’s spectrum shows greater scattering light intensity than the GES-1’s. This is just due to alterations in chromatin structure, resulting in increased heterogeneity of refractive index variations: the system collects more backscattering signals, which lead to increased light scattering intensity. Figure 3 shows that the GES-1’s spectral curve has regular intensity changes from wavelength range due to ordinary, regular cell internal structures. Normal nuclei have a characteristic spheroid Mie scatterer of a diameter . In the visible range, the spectral wavelength is smaller than the cellular nuclei diameter, so the Van de Hulst approximation can be used to describe optical scattering, that the scattering cross section of the nuclei exhibits a periodicity along that wavelength.18 Although heterogeneities are present in the cellular structure, they are not significant enough to disrupt the peaks resulting from the cytoplasm and nuclear boundaries. Comparing cancer cells with normal gastric cells (Fig. 2), it is obvious that cancer cells have larger nuclear-cytoplasmic ratios, nuclear enlargement, polycaryon, asymmetrical nuclear shape, increased DNA content, and so on. Accordingly, the dysplastic cells have larger mean nuclear index and broader variations in the range of nuclear index and broader spatial frequency range. The distinct fluctuating light intensity of dysplastic cells is not present. Figure 3 shows that there is no regular intensity change in cancerous spectral curves. The changes during the development of cancer cells will correspond to characteristic alterations in light and cell interaction. To show how the cell morphological changes affect cell backscattering, Drezed, Dunn, and Richards-Kortum19 analyzed normal and cancer cells with the mentioned light scattering characteristic property using a finite difference time domain (FDTD) method. Their theoretic prediction is that abnormal cells have higher backscattering light intensity than normal cells. In addition, there are no distinct interference peaks distributed in backscattering spectra from abnormal cells, although much fewer irregular structures in normal cells cause no breaks in the interference peaks between cytoplasm and cell borders. Therefore, our experiments by FCBS agree well with previous theoretical predictions in the spectral trend. A cellular microspectrometer has been developed combining fiber-confocal microscopy with elastic backscattering spectroscopy. Experimentally, normal human gastric epithelial cells and human gastric carcinoma cells at logarithmic phase have been researched, and specific spectrum curves have been identified. The FCBS is a cofocal spectroscopic system, and it can achieve 3-D spectroscopic characteristics of tissue. Especially when comparing with the CLASS microscopy13, 14 and partial wave spectroscopy (PWS),17, 20 the FCBS is able to enter internal body cavities via the work channel of an endoscopy, because we substitute fiber couplers for beamsplitters to separate illumination and signal light and interface of fibers for pinholes to realize cofocal construction, the FCBS system will be realized miniaturization. Although signals obtained by the FCBS and PWS were all recorded from microscopic volumes, the PWS utilized a slit to scan the amplified backscattering imaging of an object in each scanning step and results in a data cube to obtain the nanoscale morphology of a cell by statistical analysis based on the mesoscopic light transport theory. It cannot obtain spectroscopic characteristics of tissue or cells in 3-D. If further work will not only distinguish the cancerous cell from the normal cell, but also detect histological unapparent cells, minimizing the size of the optical probe of FCBS spectroscopy, and studying the inversion algorithm of backscattered cell spectra will facilitate the acquisition of information about microscopic alterations of the cell architecture and subcellular components in vivo, then the FCBS spectrometer will realize high-resolution, in vivo, early stage medical diagnosis. AcknowledgmentsThe project was financially supported by the Shanghai Education Development Foundation (2007CG62) and the Eastern Scholar Project of the Shanghai Education Committee. Guoyan Zhou has kindly helped with statistical analysis. ReferencesV. Backman, M. B. Wallace, L. T. Pereleman, J. T. Arendt, and M. S. Feld,
“Detection of preinvasive cancer cells,”
Nature, 406
(6), 35
–36
(2000). https://doi.org/10.1038/35017638 0028-0836 Google Scholar
J. R. Mourant, M. Canpolat, C. Brocker, and J. P. Freyer,
“Light scattering from cells: the contribution of the nucleus and the effects of proliferative status,”
J. Biomed. Opt., 5
(2), 131
–137
(2000). https://doi.org/10.1117/1.429979 1083-3668 Google Scholar
J. R. Mourant, J. P. Freyer, A. H. Heilscher, A. A. Eick, D. Shen, and T. M. Johnson,
“Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics,”
Appl. Opt., 37
(16), 3586
–3593
(1998). https://doi.org/10.1364/AO.37.003586 0003-6935 Google Scholar
R. Drezek, M. Guillaud, C. Thomas, I. Boiko, and R. R. Kortum,
“Light scattering from cervical cells throughout neoplastic progression: influence of nucleus morphology, DNA content, and chromatin texture,”
J. Biomed. Opt., 8
(1), 7
–16
(2003). https://doi.org/10.1117/1.1528950 1083-3668 Google Scholar
J. G. Fujimoto,
“Optical coherence tomography for ultrahigh resolution in vivo imaging,”
Nat. Biotechnol., 21 1361
–1367
(2003). https://doi.org/10.1038/nbt892 1087-0156 Google Scholar
L. Lovat and S. Bown,
“Elastic scattering spectroscopy for detection of dysplasia in Barrett’s esophagus,”
Gastrointest Endosc Clin. N. Am., 14
(3), 507
–517
(2004). https://doi.org/10.1016/j.giec.2004.03.006 1052-5157 Google Scholar
P. J. Dwyer, C. A. Dimarzio, J. M. Zavislan, W. J. Fox, and M. Rajadhyaksha,
“Confocal reflectance theta line scanning microscope for imaging human skin in vivo,”
Opt. Lett., 31 942
–944
(2006). https://doi.org/10.1364/OL.31.000942 0146-9592 Google Scholar
T. Perelman Lev, Z. George, V. Backman, R. Gurjar, and M. S. Feld,
“Quantitative analysis of mucosal tissues in patients using light scattering spectroscopy,”
Proc. SPIE, 3597 474
–479
(1999). https://doi.org/10.1117/12.356844 0277-786X Google Scholar
R. S. Gurjar, V. Backman, L. T. Perelman, I. Georgakoudi, and M. S. Feld,
“Imaging human epithelial properties with polarized light scattering spectroscopy,”
Nat. Med., 7
(11), 1245
–1248
(2001). https://doi.org/10.1038/nm1101-1245 1078-8956 Google Scholar
I. Georgakoudi, B. C. Jacobson, J. V. Dam, V. Backman, and M. S. Feld,
“Reflectance and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus,”
Gastroenterology, 120 1620
–1629
(2001). https://doi.org/10.1053/gast.2001.24842 0016-5085 Google Scholar
T. T. Wu and J. Y. Qu,
“Assessment of the relative contribution of cellular components to the acetowhitening effect in cell culture and suspensions using elastic light-scattering spectroscopy,”
Appl. Opt., 46
(21/20), 4834
–4842
(2007). https://doi.org/10.1364/AO.46.004834 0003-6935 Google Scholar
Y. Liu, X. Li, Y. L. Kim, and V. Backman,
“Elastic backscattering spectroscopic microscopy,”
Opt. Lett., 30
(18), 2445
–2447
(2005). https://doi.org/10.1364/OL.30.002445 0146-9592 Google Scholar
H. Fang, L. Qiu, E. Vitkin, M. M. Zaman, L. T. Perelman,
“Confocal light absorption and scattering spectroscopic microscopy,”
Appl. Opt., 46
(10), 1760
–1769
(2007). https://doi.org/10.1364/AO.46.001760 0003-6935 Google Scholar
I. Itzkan, L. Qiu, H. Fang, M. M. Zaman, E. Vitkin, and L. T. Perelman,
“Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels,”
PNAS, 103
(44), 17255
–17260
(2007). https://doi.org/10.1073/pnas.0708669104 Google Scholar
M. Xu, T. T. Wu, and J. Y. Qu,
“Unified Mie and fractal scattering by cells and experimental study on application in optical characterization of cellular and subcellular structures,”
J. Biomed. Opt., 13
(3), 024015
(2008). https://doi.org/10.1117/1.2907790 1083-3668 Google Scholar
K. Sung,
“Fiber optic confocal reflectance microscopy: in vivo detection of pre-cancerous lesions in epithelial tissue,”
(2003). Google Scholar
H. Subramanian, P. Pradhan, Y. Liu, I. R. Capoglu, and V. Backman,
“Optical methodology for detecting histologically unapparent nanoscale consequences of genetic alterations in biological cells,”
PNAS, 105
(51), 20124
–20129
(2008). https://doi.org/10.1073/pnas.0806563106 Google Scholar
L. T. Perelman,
“Observation of periodic fine structure in reflectance from biological tissue: a new technique for measuring nuclear size distribution,”
Phys. Rev. Lett., 80
(3), 627
–630
(1998). https://doi.org/10.1103/PhysRevLett.80.627 0031-9007 Google Scholar
R. Drezek, A. Dunn, and R. Richards-Kortum,
“A pulsed finited-difference time-domain (FDTD) method for calculating light scattering from biological cells over broad wavelength ranges,”
Opt. Express, 6
(7), 147
–156
(2000). https://doi.org/10.1364/OE.6.000147 1094-4087 Google Scholar
H. Subramanian, P. Pradhan, Y. Liu, I. R. Capoglu, J. D. Rogers, V. Backman,
“Partial-wave microscopic spectroscopy detects subwavelength refractive index fluctuations: an application to cancer diagnosis,”
Opt. Lett., 34
(4), 518
–520
(2009). https://doi.org/10.1364/OL.34.000518 0146-9592 Google Scholar
|