|
|
|
Head and neck cancer is one of the most common malignancies worldwide due to its high incidence and high mortality rates. In the United States, more than 35,000 new cases of head and neck malignancies were reported in 2009, accounting for approximately 3% of all newly diagnosed cancers.1 In Singapore, over 2000 patients between 35 and old have been diagnosed with head and neck cancer from 1998 to 2002.2 Early diagnosis and localization of head and neck cancer with effective treatment is critical to decreasing the mortality rates. However, identification of early cancer can be difficult using conventional white-light reflectance (WLR) endoscopy, which relies heavily on visualization of tissue morphological changes associated with neoplastic transformation. Subtle tissue changes may not be apparent, limiting its diagnostic accuracy. Positive endoscopic biopsy is the standard means for head and neck cancer diagnosis, but it is invasive and impractical for screening high-risk patients who may have multiple suspicious lesions. Hence, it is highly desirable to develop advanced optical techniques to complement WLR endoscopy for improving early cancer diagnosis and characterization during clinical examinations. In the past two decades, the autofluorescence (AF) imaging technique, which is capable of detecting changes in the endogenous fluorophores and morphological architectures of tissue, has been developed to significantly improve the diagnostic sensitivity of early neoplastic lesions at endoscopy, but AF imaging still suffers from moderate diagnostic specificities.3 Optical spectroscopic techniques, such as AF spectroscopy and diffuse reflectance (DR) spectroscopy, which provide information about tissue optical properties (e.g., absorption and scattering coefficients), morphologic structures, endogenous fluorophore distribution, blood content (e.g., hemoglobin), and oxygenation associated with neoplastic transformation, have been comprehensively investigated for in vitro or in vivo precancer and cancer diagnosis in various organs with high diagnostic specificity.4, 5, 6, 7 The combination of AF imaging with optical spectroscopy offers a potential of providing both high diagnostic sensitivity and specificity for cancer tissue diagnosis and detection.6, 7, 8, 9 In this letter, we report on the development of an integrated point-wise spectroscopy (AF/DR) and AF endoscopic imaging technique for real-time in vivo tissue measurements at endoscopy. Different from previous work that combined imaging with spectroscopy limited to spectral measurements at the centroid of the endoscopic field of view,8, 9 in our configuration, the in vivo point-wise AF/DR spectra can be quickly acquired from any specific areas of the imaged tissue of interest under the AF/WLR imaging guidance during endoscopic examination. Figure 1 shows the schematic of the integrated point-wise spectroscopy and AF endoscopic imaging technique developed for in vivo tissue measurements at endoscopy. This system consists mainly of a dedicated xenon short arc lamp coupled with two customized bandpass (BP) filters (BP1: for AF excitation; BP2: for WL illumination) for AF/DR spectroscopy and imaging; a medical endoscope (Hopkins II 7230BP, Karl Storz, Germany); a sensitive three-chip charge-coupled device (CCD) camera [red (R) channel ; green (G) channel ; and blue (B) channel ; ; Tricam SL II, Karl Storz, Germany]; a spectrograph equipped with a CCD detector (FWHM of with a holographic grating; USB2000, Ocean Optics, Inc., Dunedin, FL); and a specially designed point spectrum optical adapter (inset in Fig. 1) for realizing simultaneous in vivo endoscopic imaging and point-wise AF/DR spectral measurements on the specific areas of the imaged tissue of interest. The customized optical adaptor comprises three lenses ; a thin quartz glass plate coated with a gold mirror (diameter of ; reflection of in ); and a 3-D motorized translational stage (travel range: ; 8MT184-13, Standa, Inc., Lithuania) for controlling the rapid movement of the gold mirror to realize the spectral measurements on the points of interest of the imaged tissue. For simultaneous AF imaging and spectroscopy measurements, the filtered blue excitation light is conducted into the endoscope via a flexible fiber optic light guide and shines onto the tissue with an incident power of on the fiber tip of the endoscope. AF emitted from the tissue is collected by the same fiber tip of the endoscope, then is coupled into the customized optical adapter by passing through a long-pass (LP) filter (cut off at ) for removing the interference of the excitation light scattered from tissue, and then is focused onto the quartz glass plate, which is positioned at the interim imaging plane of lens 1 with an orientation of with respect to the incident light direction. The tissue fluorescence light passes through the oriented glass plate and is focused onto the three-chip CCD camera through lens 2 for fluorescence imaging measurements. Meanwhile, a very small portion of tissue fluorescence is reflected from the gold mirror coated on the quartz plate and focused onto a fiber via lens 3, which is connected to the spectrograph for fluorescence spectroscopic measurements. Further, an automatic motorization of the small gold mirror coated on the quartz plate together with the point-wise spectral measurement module enables a rapid movement of the dark spot (of in diameter due to the refection of gold mirror in the point spectrum optical adapter) on the image to any spot of the imaged tissue of interest (see Video 1 ). Hence, the AF imaging and point-wise AF spectroscopy can be simultaneously acquired from the same imaged tissue without introducing an optical fiber catheter into the instrument channel of an endoscope, as in conventional endoscopic spectral measurements, which prolong the endoscopic operation procedures. Similarly, the simultaneous WLR imaging and point-wise DR spectroscopy on the same tissue can also be realized simply by switching the excitation light filter to the white-light illumination mode (BP2: ) and removing the LP filter in the customized optical adaptor. In this work, we have also developed a LabView-based software for controlling real-time endoscopic image (WLR/AF) acquisition and point-wise spectral measurements and processing (e.g., wavelength calibration, system spectral response calibration, CCD dark-noise subtraction, signal saturation detection, etc.). Both the live AF/WLR image and the AF/DR spectrum can be simultaneously displayed on the computer monitor for real-time review and stored in the computer for further analysis. Fig. 1Schematic of the integrated point-wise spectroscopy and autofluorescence (AF) imaging system for in vivo tissue measurements at endoscopy. , , and stand for translational stages. 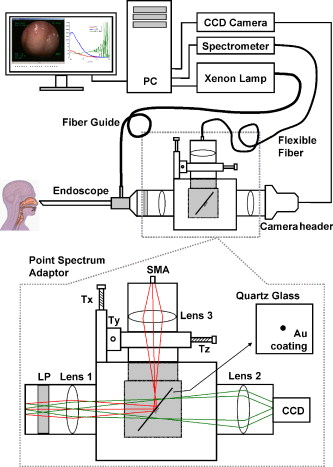 Video 1Video illustrating simultaneous AF imaging and point-wise AF spectral measurements of the cheek in vivo in real-time during AF endoscopic imaging (QuickTime, ). . 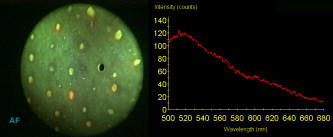 We have applied the integrated point-wise spectroscopy and endoscopic imaging technique developed for in vivo tissue measurements in the head and neck. Figure 2 shows an example of in vivo WLR images and the corresponding DR spectra of different tissue sites (i.e., chin, buccal mucosa, dorsal of the tongue, and lower lip) simultaneously acquired from a healthy volunteer under the white-light illumination mode. Point-wise DR spectra from different anatomical locations [dark spots in the WLR images in Fig. 2] in the oral cavity can be acquired within , and the absorption peaks (e.g., 420, 540, and ) attributed to hemoglobin absorptions in the vessels can be clearly identified, but with large absorption variations among different tissue locations. Fig. 2(a) In vivo white-light images and the corresponding diffuse reflectance (DR) spectra from different anatomical locations (chin, buccal mucosa, dorsal of the tongue, and lower lip) simultaneously acquired from a healthy volunteer. (b) Comparison of in vivo AF images and the corresponding point-wise AF spectra from different anatomical locations (chin, buccal mucosa, dorsal of the tongue, and lower lip) simultaneously acquired from a healthy volunteer. Note that each DR spectrum is acquired within , whereas the AF spectrum is acquired within . 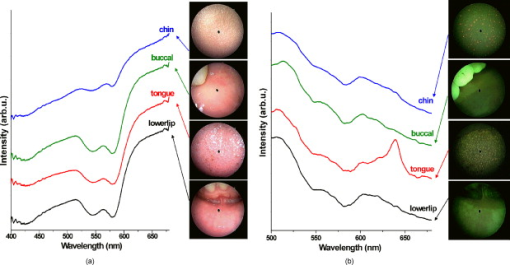 Swapping the excitation filter to the blue BP filter in the xenon lamp for tissue fluorescence excitation, in vivo tissue AF images and point-wise AF spectra can also be simultaneously acquired from the head and neck. Figure 2 shows the representative in vivo AF images and AF spectra of different locations in the oral cavity from a healthy volunteer. Obviously, AF images [Fig. 2] that contain information about endogenous fluorophore distributions in tissue provide a higher image contrast as compared to WLR images [Fig. 2]. High-quality in vivo tissue AF spectra can be acquired within from the dark spot areas on the AF images [Fig. 2]. Again, AF spectra from different anatomical tissue locations also vary, revealing the differences in concentrations of endogenous fluorophores among different tissue locations. For instance, the prominent fluorescence peak at for flavins is observed in all different tissues, but a much stronger fluorescence at for protoporphyrins is found, particularly in the chin and the tongue. By rapidly moving the gold reflection mirror in the point spectrum optical adapter, we also demonstrate the ability of the integrated endoscopic imaging and spectroscopy technique developed for pinpointing the spectral properties of specific areas of interest on the imaged tissue. Video 1 illustrates the in vivo AF image of the cheek acquired together with simultaneous AF spectral measurements on different spots of the tissue imaged in real-time during rapid scanning of the gold reflection mirror (shown as a dark spot in the AF image). The point-wise spectral measurements across the entire image size of can be quickly completed within , making the in vivo AF measurements feasible in clinical settings. In vivo AF spectral differences of different spots on the same imaged tissue can also be clearly identified (Video 1), indicating the capability of our developed technique for revealing the inhomogeneity of endogenous fluorophore distributions in tissue. Our point spectrum adapter design associated with rapid scanning and optics allows for a more convenient spectral measurement on the point of interest of the entire imaged tissue for spectral analysis and may have a significant impact on practical clinical applications. In conclusion, an integrated autofluorescence endoscopic imaging and point-wise spectroscopy system has been built and evaluated in the head and neck. The simultaneous acquisition of an endoscopic AF image and an AF/DR spectrum from a specific area of imaged tissue in vivo can be realized within , which may facilitate rapid, noninvasive, in vivo tissue diagnosis and characterization in clinical settings. One notes that the unique AF image-guided point-wise spectroscopy technique developed in this work can also be readily adapted to study other internal organs in vivo by using different flexible medical endoscopes (e.g., bronchoscope, colonoscope, gastroscope, etc.). AcknowledgmentsThis research was supported by the Biomedical Research Council, the National Medical Research Council, and the Faculty Research Fund of the National University of Singapore. ReferencesA. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu, and A. M. J. Thun,
“Cancer statistics, 2009,”
Ca-Cancer J. Clin., 59 225
–249
(2009). https://doi.org/10.3322/caac.20006 0007-9235 Google Scholar
A. Seow, W. P. Koh, K. S. Chia, L. M. Shi, H. P. Lee, and K. Shanmugaratnam,
“Trends in cancer incidence in Singapore, 1968–2002,”
(2004). Google Scholar
B. Palcic, S. Lam, J. Hung, and C. MacAulay,
“Detection and localization of early lung cancer by imaging techniques,”
Chest, 99
(3), 742
–743
(1991). https://doi.org/10.1378/chest.99.3.742 0012-3692 Google Scholar
D. C. DeVeld, M. J. H. Witjes, H. J. Sterenborg, and J. L. Roodenburg,
“The status of in vivo autofluorescence spectroscopy and imaging for oral oncology,”
Oral Oncol., 41
(2), 117
–131
(2005). https://doi.org/10.1016/j.oraloncology.2004.07.007 0964-1955 Google Scholar
R. Mallia, S. S. Thomas, A. Mathews, R. Kumar, P. Sebastian, J. Madhavan, and N. Subhash,
“Oxygenated hemoglobin diffuse reflectance ratio for in vivo detection of oral pre-cancer,”
J. Biomed. Opt., 13
(4), 041306
(2008). https://doi.org/10.1117/1.2952007 1083-3668 Google Scholar
Y. S. Fawzy, M. Petek, M. Tercelj, and H. Zeng,
“In vivo assessment and evaluation of lung tissue morphologic and physiological changes from noncontact endoscopic reflectance spectroscopy for improving lung cancer detection,”
J. Biomed. Opt., 11
(4), 044003
(2006). https://doi.org/10.1117/1.2337529 1083-3668 Google Scholar
M. P. L. Bard, A. Amelink, M. Skurichina, V. Noordhoek Hegt, R. P. W. Duin, H. J. C. M. Sterenborg, H. C. Hoogsteden, and J. G. J. V. Aerts,
“Optical spectroscopy for the classification of malignant lesions of the bronchial tree,”
Chest, 129
(4), 995
–1001
(2006). https://doi.org/10.1378/chest.129.4.995 0012-3692 Google Scholar
H. Zeng, M. Petek, M. T. Zorman, A. McWilliams, B. Palcic, and S. Lam,
“Integrated endoscopy system for simultaneous imaging and spectroscopy for early lung cancer detection,”
Opt. Lett., 29
(6), 587
–589
(2004). https://doi.org/10.1364/OL.29.000587 0146-9592 Google Scholar
C. M. Lee, C. J. Engelbrecht, T. D. Soper, F. Helmchen, and E. J. Seibel,
“Scanning fiber endoscopy with highly flexible, catheterscopes for wide-field, full-color imaging,”
J. Biophoton., 3
(5–6), 385
–407
(2010). https://doi.org/10.1002/jbio.200900087 Google Scholar
|

