|
|
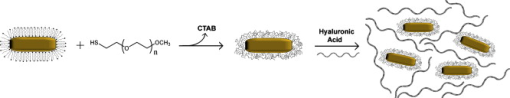
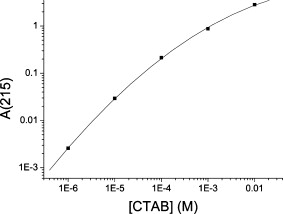
1.IntroductionPhotothermal laser welding is a technique used to seal accidental or surgical wounds. It exploits the interaction of laser light with an optical absorber, which can be endogenous as body water or exogenous as an organic dye, to transduce the laser light into heat.1, 2 When the weld is mediated by the topical interposition of a laser-activated material (solder), this technique is typically referred to as laser soldering. Over the last decade, the combination of near-infrared (NIR) radiation, which penetrates deep into the body, with an exogenous chromophore such as indocyanine green (ICG) has improved critical aspects of previous laser welding and soldering approaches, including collateral tissue overheating and lack of selectivity.3, 4, 5 However, despite the significant experimental and clinical achievements of the last few years, some limitations still hinder a standardized clinical application of this technology. The principal limitations are ascribed to the use of organic dyes, which exhibit poor photochemical stability, excessive diffusiveness in the biological environment, and inadequate stability when stored in an aqueous solution or dispersed in a physiological environment.2 This jeopardizes the sustainability of the product and the efficacy of the photothermal conversion. Substantial breakthrough in the laser welding (and soldering) of biological tissues may come from the advent of nanotechnologies. Recent advances in colloidal chemistry have driven the design of nanoparticles with excellent optical response, which hold the potential to outclass the organic dyes presently in use. One relevant example is hybrid materials as organic or inorganic nanoparticles embedded with ICG, which becomes relatively protected and stabilized in the biological environment.6 Another innovative example are metal oxide and metal nanoparticles with magnetic and plasmonic resonances, respectively, which may provide high efficiency and localization of heat release. Recent reports have described experimental tests on the use of electromagnetically excited iron oxide nanospheres7 and laser-activated gold nanoshells8 to seal arteries and skin, respectively. Among the other metal nanoparticles, gold nanorods (GNRs) appear an ideal candidate to replace the organic dyes in use.9, 10 These are cylindrical gold nanoparticles with plasmon absorption bands in the NIR window, which can be tuned by a controllable modulation of their size and shape.11 Remarkable features of these nanoparticles include exceptional optical absorption and excellent biochemical versatility and stability under physiological conditions, as well as high laser irradiances.10 In addition, their lower diffusivity through the tissue matrix with respect to that of organic dyes (e.g., for gold nanoparticles12 compared with for indocyanine green through connective tissues13) provides for better localization of the photothermal conversion, which translates into a minimization of overheating problems and optimization of efficiency and selectivity.14 The probable biocompatibility of these nanoparticles represents another attraction, which justifies the intense interest in these new materials for several biomedical applications. Recently, we reported the use of a solution of GNRs in the direct welding of sandwiches of lens capsular tissues obtained from porcine eyes ex vivo.14 These results provided the first proof of the capacity of laser-activated GNRs to mediate substantial and functional photothermal effects in a model connective tissue. In this work, we report the first application of GNRs for in vivo laser closure of a rabbit carotid artery. GNRs were functionalized with a biopolymeric shell and then embedded in a hyaluronan viscous solution to achieve a highly stabilized and handy material, suitable for manipulation by the surgeon. In addition, the choice of hyaluronan offers the advantage of well-known physiochemical and biological properties,15 which makes it a unique solution for the preparation of new biocompatible and biodegradable composites for wound repair purposes.16 2.Experimental Section2.1.MaterialsAll chemicals were purchased from Sigma-Aldrich (Saint Louis, Missouri) unless otherwise noted and used without further purification. Methoxypoly-(ethylene glycol) (mPEG-SH) with a mass was supplied by Laysan Bio Incorporated (Arab, Alabama). We used hyaluronan obtained from a bacterial source to minimize possible protein-mediated aggregation of polymer chains when storing for long times in aqueous solutions. In particular, hyaluronan sodium salt from Streptococcus equi (Esperis S.p.A., Milan, Italy) having a molecular mass of approximately and with a protein content lower than 0.025%, as previously measured,15 was used in the experiment. 2.2.Synthesis of the Gold Nanorod Hyaluronic Acid FormulationThe exogenous chromophore consisted of a gel-like paste of functionalized GNRs (Fig. 1 ). In brief, colloidal suspensions of GNRs were obtained by the seed-mediated surfactant-assisted (cetrimonium bromide or CTAB) reduction of chloroauric acid by ascorbic acid (according to a variant of the Nikoobakht method; see Ref. 14 for details). As-grown GNRs were further subjected to overgrowth by implementation of a protocol recently developed by our group.11 This allowed us to achieve a aqueous suspension of average average length GNRs (aspect ratio ) (Fig. 2 , left inset). The solution was then concentrated to by centrifugation and redispersed into water, showing enhanced absorption in the NIR (see Fig. 2, solid line), with a measured absorption coefficient at of about . The surfactant bilayer that coats the GNRs was replaced by mPEG-SH to make the nanoparticles stable in a physiological solution and ready for the following dispersion in the hyaluronan matrix. The substitution was carried out by a previously described protocol.17 In brief, of mPEG-SH and of 2 were added to GNRs suspension. The mixture was set overnight at room temperature, centrifuged, and resuspended in distilled water two times to remove the replaced CTAB and the excess mPEG-SH. Fig. 2Comparison among the optical absorbances of the colloidal GNRs suspension (solid line) of the GNRs-HA formulation at the time of the synthesis (dashed line) and after of storage at daylight and room temperature (dotted line). Common characteristics are the longitudinal band in the NIR (at ) and the transversal band in the visible (at ). Inset: (left) transmission electron micrograph of the gold nanorods used in the tests (aspect ), and (right) appearance of the GNRs-HA composite. 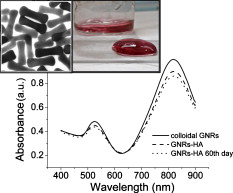 2.3.Measure of the Residual Cetrimonium BromideSince CTAB is cytotoxic, its presence in the batch used for the fabrication of the GNRs-HA solder needed to be quantified. After pegylation and washing, the residual CTAB contained in the GNRs solution was extracted into chloroform as reported in the literature.18 We carried out two extraction cycles by using a total of chloroform. The organic phases were combined and left to evaporate at room temperature, and the dry residual was dispersed again in of pure water. The absorbance of the sample at [A(215)] was considered for the quantification of the CTAB present in the sample,19 on comparison with a calibration curve of CTAB aqueous solutions in the range (Fig. 3 ). Using this procedure, we found a residual CTAB not above the detection limit of our method (i.e., ) (average of three replicates), which is below the 50% inhibitory concentration (IC50) of CTAB (9.1 ), as previously reported.18 This insight suggests a negligible toxicity of our pegylated GNRs, which are thus regarded as potentially safe for use in vivo. 2.4.Preparation of the Gold Nanorod Hyaluronic Acid FormulationThe pegylated nanoparticles were centrifuged and suspended in phosphate buffered saline (PBS, pH 7.4, phosphate buffer, NaCl). Hyaluronan was added to the suspension until a final 3% (w/v) concentration was reached. The mixture (referred to hereafter as GNRs-HA) was gently shaken for two days to obtain a homogeneous dark-red transparent paste (Fig. 2, right inset), which was stable at room temperature for at least a four-months period without significant modifications of its optical properties (Fig. 2, dotted line). 2.5.Surgical ProcedureThe surgeries were performed on four rabbits in the vivarium of the Catholic University in Rome. The animals were anesthetized with ketamine hydrochloride and medetomidine intramuscularly. During the follow-up period, the animals were treated with antibiotics ( of enrofloxacin per day). Protocols of animal tests were designed according to the recommendation and regulation of the Italian Ministry of Health. The laser used in the tests was an AlGaAs diode laser (model WELD 800 by El.En. SpA, Calenzano, Italy) emitting at and equipped with a -diam optical fiber terminating in a hand piece to enable easy operation. After the anaesthesia, a segment of the right common carotid artery was dissected and then clamped proximally and distally. The laser surgery consisted of the closure of longitudinal cuts previously executed along the artery, which represents a clinically relevant situation in neurosurgery.20, 21, 22 Prior to laser irradiation, a total quantity of of GNRs-HA formulation was first applied above and inside the cut by means of a spatula. Then excess material was immediately removed, which resulted in a residual -thick solder layer on the artery surface. This operation was performed with the aid of a surgical microscope to avoid entrance of solder material into the lumen, and consequently, to confine the photothermal effect to the vascular surface. Laser irradiation of the cuts was then performed by slowly moving the fiber tip, held at a constant distance of from the tissue. A continuous laser power (out of the fiber) of , corresponding to a power density of at the surface of the artery, and an average time exposure were found to induce an effective closure of the cut edges. Control tests performed with the same laser parameters and blank hyaluronan failed to induce any detectible photothermal effect nor wound closure. Upon clamp removal immediately after the surgery, no bleeding was observed from the laser-soldered cuts (see Fig. 4 ). At the end of the procedure, the carotid patency was verified by Doppler analysis, the artery was closed in layers, and the animal was left to recover from the anaesthesia. Fig. 4Sequence of images recorded during the laser welding procedure. (a) The artery is clamped and a longitudinal incision is performed. (b) After the application of the GNRs-HA formulation, the incision is treated with the diode laser light. (c) Appearance of the sealed artery immediately after intervention.  2.6.Histological Evaluation after Follow-UpAfter a follow-up period of , the animals were examined and reanesthetized. After animal sacrifice, artery specimens including the laser-treated segment were excised and treated following standard pathology laboratory procedures for the histological evaluation. In brief, the samples were fixed in 10% buffered formalin and embedded in paraffin. Semi-thin sections were obtained by cutting the paraffin-embedded samples with a microtome (model RM2235, Leica Microsystems GmbH, Germany). The final geometry of the sections included both the treated superficial and adventitial portion of the vessel wall in the central area, and the two hemilines of the endothelium at the periphery. To obtain this geometry, we isolated the treated area of the vessel, opened the vessel, and operated a superficial longitudinal cut along the laser-treated line to comprise the endothelial, subendothelial, muscular, and partially, adventitial stratum of the vessel wall. The so-obtained samples were then embedded in a shallow block of paraffin by laying them flat and finally sliced. The slices were laid onto electrostatically charged slides (Superfrost®, Histoline, Milan, Italy) and left overnight with a thermostat at . Then, after deparaffinization and rehydration, the sections were stained with hematoxylin and eosin, van Gieson’s stain for collagen and elastic fibres, and Masson’s trichrome stain for connective tissue. Additionally, tissue sections were immunohistochemically tested for the presence of CD31 and von Willebrand factor (vWF) by use of a monoclonal mouse antihuman CD31 and a mouse antihuman factor VIII antibodies (DAKO, Glostrup, Denmark), respectively. Immunostaining was performed with a biotinylated secondary antibody (Vectastain ABC kit, Vector Laboratories, Burlingame, California) and diaminobenzidine-HCl (Vector). Other samples were fixed in 2.5% glutaraldehyde, postfixed with 1% osmium tetroxide, and infiltrated in epoxy resin. Ultrathin sections of were contrasted with uranyl acetate and lead citrate, and inspected under a transmission electron microscope operating at . 2.7.Characterization of the Morphological and Optical Changes of Gold Nanorods upon Laser IrradiationThe effect induced by laser irradiation to the nanoparticles was studied in detail. A small amount of GNRs-HA gel was spread onto a small ( diameter) glass dish to obtain a thin layer on the order of . In an attempt to simulate the surgical environment, some drops of PBS were added in such a way that the liquid could only wet the gel borders. The laser treatment of the sample was achieved by delivering laser light of power density obtained by keeping the fiber tip fixed at a distance of from the sample surface (in a similar way to a previous experimental setup23) in accordance with a standard laser soldering procedure. The irradiation time was fixed to , which is a good approximation of the operative time needed by the surgeon to obtain an effective photothermal effect (this time is referred to a irradiated spot by considering a 0.24 fiber numerical aperture). The experiment was repeated by irradiating different areas of the sample for double and quadruple the time (10 and ) to investigate the effect of overirradiation of the sample. After irradiation, the samples were subject to spectromicroscopy by a homemade setup24 composed of a transmission microscope (model DM 2500 by Leica Microsystems GmbH, Germany), a visible-NIR spectrometer (model EPP200 by Stellarnet Incorporated, Tampa, Florida) and a set of interchangeable high-pass filters. The visible and NIR windows were examined under different conditions to optimize the signal detection. Afterward, samples (including also an untreated region used as a control) were carefully removed from the dishes, resized, placed onto copper grids, contrasted with uranyl acetate and lead citrate, and finally analyzed by transmission election microscopy (TEM). 3.Results and DiscussionAll the laser-soldered arteries were found to be patent at the end of the follow-up period. Histology on operated samples demonstrated substantial integrity of the vascular wall at the site treatment, with no damage to the tunica elastica externa, nor observable collagen homogenization [Figs. 5 and 5 ]. The absence of microgranuloma formation and/or dystrophic calcification of the vasal media and/or intima showed that no host reaction to nanoparticles interspersed throughout the treated vascular tissue occurred. Fig. 5Histochemical results demonstrating a good preservation of the carotid wall after laser soldering. Note the typical physiological pattern maintained by collagen and elastic fibers. The central laser-treated area is characterized by a bright blue or dark orange color during staining with (a) Masson’s trichrome and (b) Van Gieson, respectively. (Color online only.) 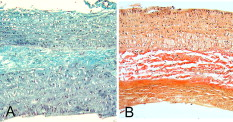 Immunohistochemical results showed the integrity and viability of the endothelium with a constant and intense expression of CD31 and vWF [Figs. 6 and 6 ]. This suggests that the introduction of the GNRs-HA formulation in laser welding surgeries may give substantial control over laser dosimetry, which is critical to avoid thrombogenic effects during the wound healing period. Interestingly, these results improve previous findings when a conventional chromophore, i.e., ICG, was used to seal rabbit vessels.3 In summary, we achieved conspicuous photothermal effects, which were able to induce local tissue fusion while preserving the endothelial viability and preventing thermally induced thrombogenicity. Fig. 6Immunohistochemistry of laser-treated vessels: (a) vWF and (b) CD31 good expression by the endothelium (arrows) over the treated area , indicating preservation of endothelial viability. 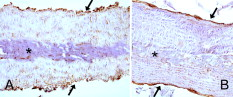 TEM micrographs of the laser-treated site showed spherical nanoparticles interdispersed among intact collagen fibers (Fig. 7 ), which indicates a morphological remodeling of the GNRs. This finding suggests the possibility of a self-termination of the laser soldering process. Indeed, the blue shift of the absorption spectrum of the GNRs associated with their thermally activated transformation into more stable shapes may inhibit further laser power absorption and photothermal conversion. The possibility that the solder may undergo a self-terminating process during the surgery was studied in some detail by carrying out focused bench experiments, as introduced in Sec 2. The optical properties of GNRs-HA films subject to different laser irradiation times at constant power density were investigated by optical spectromicroscopy and electron microscopy. The spectral analysis evidenced a consistent blue shift of the longitudinal band originally peaked at to a final [Fig. 8 ]. The wavelength position of this new band and its integrated intensity ratio to the transversal band at proved comparable for all the samples (5-, 10-, and irradiation), as summarized in Fig. 8. TEM analysis of the control sample revealed in in diameter (aspect ratio ) GNRs well spread within a regular hyaluronan matrix and never assembled in clusters [Fig. 9 ]. This result supports the spectrophotometric analysis of the GNRs-HA gel summarized in Fig. 2, which indicated a substantial preservation of the optical properties of the nanoparticles after pegylation and inclusion in the hyaluronan gel. The TEM micrographs of the irradiated sample showed in in diameter fat GNRs (aspect ratio ) dispersed in a blurred and spotted hyaluronan network. This sample was then compared to the films irradiated for double and quadruple the time (10 and ). No detectible difference in the average nanoparticle size nor evident changes in the hyaluronan matrix were observed [see Fig. 9], confirming that the remodeling of GNRs and modification of bulk HA were essentially negligible after additional 5 or of laser irradiation. Identical effects were also observed at different output powers (at least within a factor of 2) and hydration conditions, which were tested to challenge the possibility to translate our observations. The blue shift of the longitudinal band is in good agreement with the rounding of the rods, as detected by TEM analysis.25 These observations imply a drastically reduced possibility of NIR photothermal conversion, which in turn supports the occurrence of a self-terminating process during the laser soldering procedure with the GNRs-HA nanocomposite, thus corroborating the previously formulated speculations. The partial remodeling of GNRs observed in this bench experiment is apparently in partial disagreement with the TEM analysis of the treated rabbit arteries after the follow-up, as displayed in Fig. 7, which reveals in diameter nanospheres (highly monodispersed) within the connective matrix. We suggest that the complete rod-to-sphere change of the GNRs can effectively conclude during the wound healing of the artery, which is well presumable in consideration of the kinetic behavior of GNR remodeling,26 which may slowly develop at a physiological temperature (though being much more rapid on laser activation in the millisecond time scale). Fig. 7TEM micrograph of the weld site. Approximately spherical nanoparticles (as the one indicated by a circle) are visible, interdispersed among intact collagen fibers, indicating a rod-to-sphere reshaping. 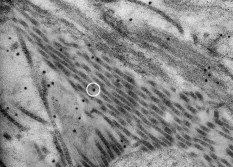 Fig. 8(a) Change of the optical properties of the untreated and laser-treated GNRs-HA films (considered irradiation times are 5, 10, and ) showing a blue shift of the longitudinal absorbance band at on NIR laser treatment (laser emission is indicated with a dashed line). (b) Variation of the position of the longitudinal band and of the integrated intensity ratio between the longitudinal and transversal bands with irradiation time. 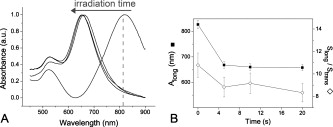 4.ConclusionsIn our previous work, we had reported the ability of a colloidal GNR suspension to induce conspicuous thermal effects, which led to local tissue fusion in porcine lens capsules excised ex vivo.14 Here we confirm in vivo the potential of the laser technique performed by laser activation of a semisolid and biocompatible formulation of GNRs. First, the dispersion of the GNRs into the hyaluronan matrix provides better manipulation and a highly stabilized product, which can be easily stored with minimal optical and structural modifications over time. Second, the low diffusivity of gold nanoparticles allows us to minimize collateral overheating and to increase the selectivity of the treatment, as revealed by the substantial preservation of the endothelium and inner wall structures of arteries. Third, the complete absence of a host reaction after a follow-up is encouraging toward a good biocompatibility of the GNR formulation, which is further supported by the negligible residual CTAB detected in the GNR solution used to fabricate the gel. Fourth, interestingly, GNRs undergo conspicuous reshaping on laser irradiation, which suggests the possibility of a self-terminating process and thus of additional safety of the minimally invasive laser procedure. Overall, this work highlights the feasibility of in vivo laser soldering operations by means of NIR-absorbing GNRs. This outcome represents an advancement toward the introduction of GNRs in clinical practice, which will require further investigation to solve critical questions raised by the use of metal nanoparticles, such as their sustainability and metabolism dynamics. ReferencesK. M. McNally,
““Laser tissue welding,” Chap. 39,”
Biomedical Photonics Handbook, 1
–45 CRC Press, Boca Raton, FL
(2003). Google Scholar
R. Pini, F. Rossi, P. Matteini, and F. Ratto,
“Laser tissue welding in minimally invasive surgery and microsurgery,”
Biophotonics. Series: Biological and Medical Physics, Biomedical Engineering, 275
–299 Springer, Berlin
(2008). Google Scholar
A. Puca, A. Albanese, G. Esposito, G. Maira, B. Tirpakova, G. Rossi, A. Mannocci, and R. Pini,
“Diode laser-assisted carotid bypass surgery: an experimental study with morphological and immunohistochemical evaluations,”
Neurosurgery, 59
(6), 1286
–1295
(2006). https://doi.org/10.1227/01.NEU.0000249217.27214.EC 0148-396X Google Scholar
S. D. DeCoste, W. Farinelli, T. Flotte, and R. R. Anderson,
“Dye-enhanced laser welding for skin closure,”
Lasers Surg. Med., 12
(1), 25
–32
(1992). https://doi.org/10.1002/lsm.1900120107 0196-8092 Google Scholar
F. Rossi, R. Pini, L. Menabuoni, R. Mencucci, U. Menchini, S. Ambrosini, and G. Vannelli,
“Experimental study on the healing process following laser welding of the cornea,”
J. Biomed. Opt., 10
(2), 024004
(2005). https://doi.org/10.1117/1.1900703 1083-3668 Google Scholar
V. Saxena, M. Sadoqi, and J. Shao,
“Indocyanine green-loaded biodegradable nanoparticles: preparation, physicochemical characterization and in vitro release,”
Int. J. Pharm., 278 293
–301
(2004). https://doi.org/10.1016/j.ijpharm.2004.03.032 0378-5173 Google Scholar
M. Reinert, A. Bregy, A. Kohler, B. Steitz, A. Petri-Finkb, S. Bogni, A. Alfieri, M. Munker, I. Vajtai, M. Frenz, and H. Hofmann,
“Electromagnetic tissue fusion using superparamagnetic iron oxide nanoparticles: first experience with rabbit aorta,”
Open Surg. J., 2 3
–9
(2008). https://doi.org/10.2174/1874300500802010003 Google Scholar
A. M. Gobin, D. P. O’Neal, D. M. Watkins, N. J. Halas, R. A. Drezek, and J. L. West,
“Near infrared laser-tissue welding using nanoshells as an exogenous absorber,”
Lasers Surg. Med., 37
(2), 123
–129
(2005). https://doi.org/10.1002/lsm.20206 0196-8092 Google Scholar
M. Hu, J. Chen, Z. Y. Li, L. Au, G. V. Hartland, X. Li, M. Marqueze, and Y. Xia,
“Gold nanostructures: engineering their plasmonic properties for biomedical applications,”
Chem. Soc. Rev., 35 1084
–1094
(2006). https://doi.org/10.1039/b517615h 0306-0012 Google Scholar
P. K. Jain, K. S. Lee, I. H. El-Sayed, and M. A. El-Sayed,
“Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine,”
J. Phys. Chem. B, 110 7238
–7248
(2006). https://doi.org/10.1021/jp057170o 1089-5647 Google Scholar
F. Ratto, P. Matteini, F. Rossi, and R. Pini,
“Size and shape control in the overgrowth of gold nanorods,”
J. Nanopart. Res.,
(2009). https://doi.org/10.1007/s11051-009-9712-0 1388-0764 Google Scholar
G. Sonavane, K. Tomoda, A. Sano, H. Ohshima, H. Terada, and K. Makino,
“In vitro permeation of gold nanoparticles through rat skin and rat intestine: effect of particle size,”
Colloids Surf. B Biointerfaces, 65 1
–10
(2008). https://doi.org/10.1016/j.colsurfb.2008.02.013 Google Scholar
E. A. Genina, M. Y. Kuzmina, S. S. Pankov, A. N. Bashkatov, and V. V. Tuchin,
“In vitro study of indocyanine green solution interaction with skin,”
Proc. SPIE, 6535 65351H
(2007). https://doi.org/10.1117/12.740992 0277-786X Google Scholar
F. Ratto, P. Matteini, F. Rossi, L. Menabuoni, N. Tiwari, S. K. Kulkarni, and R. Pini,
“Photothermal effects in connective tissues mediated by laser-activated gold nanorods,”
Nanomed. Nanotechnol. Biol. Med., 5 143
–151
(2009). https://doi.org/10.1016/j.nano.2008.10.002 Google Scholar
P. Matteini, L. Dei, E. Carretti, N. Volpi, A. Goti, and R. Pini,
“Structural behavior of hyaluronan under high concentration conditions,”
Biomacromolecules, 10 1516
–1522
(2009). https://doi.org/10.1021/bm900108z 1525-7797 Google Scholar
W. Y. J. Chen and G. Abatangelo,
“Function of hyaluronan in wound repair,”
Wound Repair Regen, 7 79
–89
(1999). https://doi.org/10.1046/j.1524-475X.1999.00079.x 1067-1927 Google Scholar
T. Niidome, M. Yamagata, Y. Okamoto, Y. Akiyama, H. Takahashi, T. Kawano, Y. Katayama, and Y. Niidome,
“PEG-modified gold nanorods with a stealth character for in vivo applications,”
J. Controlled Release, 114 343
–347
(2006). https://doi.org/10.1016/j.jconrel.2006.06.017 0168-3659 Google Scholar
H. Takahashi, Y. Niidome, T. Niidome, K. Kaneko, H. Kawasaki, and S. Yamada,
“Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity,”
Langmuir, 22 2
–5
(2006). https://doi.org/10.1021/la0520029 0743-7463 Google Scholar
M. Medenica, D. Ivanovic, and A. Malenovic,
“Simultaneous determination of compounds in Septalen pellets by derivative spectrophotometry,”
J. Serb. Chem. Soc., 65 339
–344
(2000). 0352-5139 Google Scholar
N. Sanai, Z. Zador, and M. T. Lawton,
“Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass,”
Neurosurgery, 65 670
–683
(2009). https://doi.org/10.1227/01.NEU.0000348557.11968.F1 0148-396X Google Scholar
R. A. Hanel and R. F. Spetzler,
“Surgical treatment of complex intracranial aneurysms,”
Neurosurgery, 62
(6), 1289
–1297
(2008). https://doi.org/10.1227/01.neu.0000333794.13844.d9 0148-396X Google Scholar
M. T. Lawton, A. Quinones-Hinojosa, N. Sanai, J. Y. Malek, and C. F. Dowd,
“Combined microsurgical and endovascular management of complex intracranial aneurysms,”
Neurosurgery, 52 263
–274
(2003). https://doi.org/10.1227/01.NEU.0000043642.46308.D1 0148-396X Google Scholar
P. Matteini, F. Ratto, F. Rossi, R. Cicchi, C. Stringari, D. Kapsokalyvas, F. S. Pavone, and R. Pini,
“Photothermally-induced disordered patterns of corneal collagen revealed by SHG imaging,”
Opt. Express, 17 4868
–4878
(2009). https://doi.org/10.1364/OE.17.004868 1094-4087 Google Scholar
F. Ratto, P. Matteini, S. Centi, F. Rossi, and R. Pini,
“Stability of cetrimonium and silica modified gold nanorods/polyvinyl alcohol nano-composites upon near infrared laser excitation,”
Proc. SPIE, 7577 757716
(2010). https://doi.org/10.1117/12.840971 0277-786X Google Scholar
J. Pérez-Juste, I. Pastoriza-Santos, L. M. Liz-Marzán, and P. Mulvaney,
“Gold nanorods: synthesis, characterization and applications,”
Coord. Chem. Rev., 249 1870
–1901
(2005). https://doi.org/10.1016/j.ccr.2005.01.030 0010-8545 Google Scholar
H. Petrova, J. Perez Juste, I. Pastoriza-Santos, G. V. Hartland, L. M. Liz-Marzán, and P. Mulvaney,
“On the temperature stability of gold nanorods: comparison between thermal and ultrafast laser-induced heating,”
Phys. Chem. Chem. Phys., 8 814
–821
(2006). https://doi.org/10.1039/b514644e 1463-9076 Google Scholar
|