|
|
1.IntroductionHuman ex vivo skin has been used as a model of in vivo epithelia for transdermal delivery,1 for topical penetration studies,2, 3 and in cosmeceutical applications.4 In addition, ex vivo skin is often used as a source of transplantable allografts4 to treat burns and scalds, providing immediate barrier protection and pain relief while stimulating reepithelialization.5, 6 An issue in using ex vivo skin is maintaining viability and metabolic activity, as these are required to optimally simulate in vivo conditions for percutaneous penetration and to ensure the best clinical outcome for allograft recipients.4, 7 The most appropriate control for assessing the viability of ex vivo skin is in vivo skin, recognizing that ex vivo skin viability decreases over time as a consequence of ischemic necrosis. This is caused by the loss of blood supply after surgical excision, hypoxia, and the accumulation of toxic metabolites.8 Methods commonly used to assess the viability of ex vivo skin include the morphology of the tissue,9 metabolic activity by MTT,10, 11, 12 lactate dehydrogenase (LDH) activity assays,13 skin pH,14 and oxygen consumption.11, 15, 16 Several studies have used the MTT assay to measure the decreasing viability of ex vivo skin.7, 8, 11, 17, 18 The MTT assay, which measures tetrazolium reduction by metabolic enzymes, is a technique widely used by researchers and skin banks for estimating the metabolic viability of ex vivo skin.2, 18, 19 However, the measurement of metabolic viability by tetrazolium-based assays is limited, as it does not yield direct and real-time cellular information. This assay is also limited by the necessity to destroy the tissue, which restricts clinical applicability. Several other methodologies have been used to assess skin viability including trypan blue dye exclusion, oxygen consumption, histopathological examination, and pH change.11, 15, 16 Oxygen consumption and pH measurements have both been used to monitor skin metabolism noninvasively. Stratum corneum pH is considered to be critical for maintaining outer barrier function at pH 4.2 to 5.6. Therefore, alterations in skin surface pH indicate a potential disruption in that barrier. Messager 15 showed that both excised and frozen skin had a much higher pH ( 8) and that over time the pH of the fresh skin decreased ( 7.5), while the pH of the frozen skin did not change. Oxygen consumption is a hallmark of aerobic respiration and activity relates to the activity of mitochondria. Metabolic activity is especially critical for epidermal function. This important index of skin viability is closely related to NAD(P)H lifetime imaging, also tightly coupled to metabolic and mitochondrial activity. Overall, both pH and oxygen consumption yield different but complementary information about skin viability to NAD(P)H imaging by multiphoton tomography and fluorescence lifetime imaging (MPT-FLIM). Oxygen consumption measurements have the advantage of low cost and ease of use, but the limitations include a lack of resolution in terms of which cells or layers of the skin are the most active or impaired. On the other hand, MPT-FLIM is a relatively expensive technique in terms of equipment, technical expertise, and data processing time required to obtain useful information, but has the advantage of subcellular resolution and multiple types of data output (i.e., microenvironment changes, semiquantitative concentration, physical distribution of metabolic species, and morphological information). Reduced nicotinamide adenine dinucleotide (NADH) and reduced nicotinamide adenine dinucleotide phosphate (NADPH) have also been used as metabolic indicators for the viability of cells and tissues.20, 21, 22, 23 Recently, fluorescence lifetime imaging microscopy (FLIM) analysis of intracellular NAD(P)H has been used to monitor metabolic activity in healthy and diseased tissue.24, 25, 26, 27 NAD(P)H refers to the summation of the molecules NADH and NADPH. The fluorescence lifetime of a molecule is the average time the molecule stays in the excited state.28 FLIM can distinguish between compounds with similar fluorescence spectral properties and is concentration independent, only changing with temperature, pH, and molecular interactions.29 Combined with multiphoton tomography, MPT-FLIM is a powerful means of analyzing fluorophore populations and interactions in tissues. Traditional confocal microscopy is a linear optical process that uses a single-photon to excite a fluorophore and measure the subsequent fluorescent emission. The benefits of this method are low cost, compared to MPT systems, and widespread use. Single-photon confocal microscopy suffers from some drawbacks including scattering, photobleaching, limited depth penetration, and photodamage.30 Multiphoton tomography, a much more expensive imaging technology, significantly reduces the effect of photobleaching and photodamage while increasing the depth at which tissues can be imaged.30, 31 While cost and complexity of running MPT systems has hindered widespread use, there are significant technological advantages. The MPT-FLIM system can measure fluorescence intensity, separate components of the skin, and quantify the fluorescence lifetime of NAD(P)H without significant variation within or between samples (Fig. 1 ). Figure 1 also includes photon-normalized spectra redrawn from previous work.32, 33 Fig. 1Fluorescence intensity and NAD(P)H lifetime intra and inter sample variation in study subjects. The images were acquired with a scan speed of at in size. (a) The average fluorescence intensity and standard deviation (SD) were calculated for the six subjects. (b) The photon contribution variability of NAD(P)H, keratin and FAD; the inset illustrates the photon normalized spectra redrawn from Zvyagin 33 (c) and (d) The mean and SD of the average fast and slow NAD(P)H fluorescence lifetime decays, respectively. 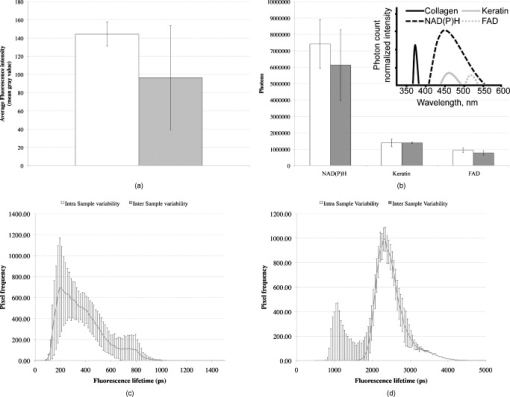 The fluorescence lifetimes for free and protein-bound NAD(P)H differ and are approximately 400 and , respectively,26, 29, 34, 35 with the ratio of free to protein-bound NAD(P)H being used as an indicator of the cellular ratio state.36 These data have been used to characterize NADH in breast cancer in vitro cell lines36 as well as to differentiate between normal, precancerous, and cancerous epithelia of various grades37, 38, 39, 40 and to define metabolic changes during apoptosis and necrosis.21 Both NADH and NADPH share the same absorption and fluorescence spectra,41 though neither nor are fluorescent.41, 42 Additionally, these molecules possess the same fluorescence lifetime and are often used for live metabolic and free:bound NAD(P)H ratio imaging.26, 41 NADH is a central coenzyme molecule in several energy metabolic pathways, including anaerobic glycolysis, the electron transport chain, and the citric acid cycle.43 Intracellular NADH concentration and distribution are useful and natural metabolic indicators because they are sensitive to pathological and physiological changes.36, 40, 44 NADPH is a reducing agent that can counter the accumulation of reactive oxygen species20 (ROS). Studies have concluded that the relative fractions of free and protein-bound NAD(P)H, which can be inferred from the measured fluorescence lifetime,36 can influence the cell redox state, defined by the ratio36, 41 . Different rates of electron transfer are likely to prevail in the free and bound states. In this study, we used the autofluorescence and fluorescence lifetime properties of NAD(P)H to monitor the metabolic deterioration of ex vivo skin stored at different temperatures in the presence and absence of nutrient media over a time course. Two controls were used: images of the ex vivo skin taken at day 0 and in vivo images of the ventral forearm from three volunteers. We showed that MPT-FLIM of ex vivo skin yields characteristic and reproducible imaging data that can be used to monitor ischemic necrosis. These observations may contribute to the development of an optical assay for clinicians and researchers to assess the viability of skin flaps, preserved skin for transplants as well as ex vivo skin for experimentation that is metabolically comparable to in vivo skin. 2.Results2.1.Autofluorescence Live ImagesFigure 2 shows representative autofluorescence images of in vivo and ex vivo skin, at day 0, and ex vivo skin stored in the presence and absence of media at the indicated temperatures over the time course. Ex vivo skin stored at in the absence of media maintained the most consistent autofluorescence with time (Fig. 2). Skin stored at in media and at either or room temperature without media showed less consistency. Autofluorescence was lost rapidly under the other conditions studied. Fig. 2Autofluorescence of ex vivo skin kept at , room temperature (RT), and in the presence and absence of nutrient media. The scale bar represents a distance of . The skin depths of the images range between 30 and . The absence of native cellular autofluorescence is indicated by N.D. (no detection). 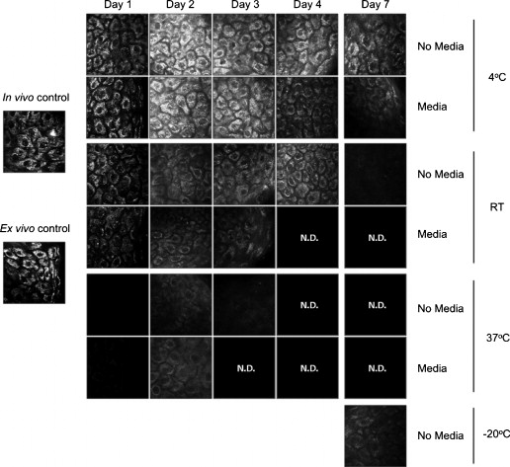 2.2.Autofluorescence Intensity and Photon Counts of NAD(P)H, Keratin, and FADFluorescence intensity and NAD(P)H photon counts (total and normalized to either FAD or keratin photon counts) for each of the samples are shown in Fig. 3 . Autofluorescence intensity and NAD(P)H photon counts (total and normalized) were relatively constant over time at and at room temperature except at day 7. In contrast, tissue incubated at exhibited a significant decrease in autofluorescence intensity at day 1, which progressively decreased to zero over time (Fig. 3). A similar pattern was seen for the FAD-normalized NAD(P)H photon count with temperature and over time. Fig. 3Autofluorescence intensity and photon counts of NAD(P)H, keratin and FAD from ex vivo skin stored at , room temperature, and . The mean fluorescence intensities of the autofluorescence images from are shown in the first row. The second row plots the mean photon counts of NAD(P)H, as obtained from FLIM channel 1 . The third row shows normalized NAD(P)H photon counts to keratin photon counts, determined from FLIM channel 2 . The final row shows normalized NAD(P)H photon counts to FAD photon counts, calculated from FLIM channel 3 . The error bars represent the SD of the means from three independent ex vivo skin samples. 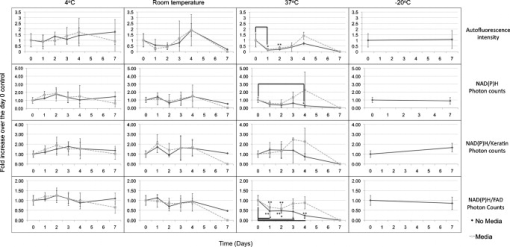 2.3.Fluorescence Lifetime Distribution HistogramsFigure 4 shows the histograms of the lifetimes of the fast [ , free NAD(P)H] and slow decay components [ , protein-bound NAD(P)H] for both the in vivo and ex vivo controls. There was a major peak at for both in vivo and ex vivo lifetime histograms for free NAD(P)H. A secondary peak at was seen in ex vivo but not in vivo skin. A peak was also seen at with an additional peak at . In general, ex vivo skin samples kept at for without media showed two histogram peaks at and , comparable to the in vivo and ex vivo controls. Other samples showed much less consistency and other multiple peaks. Fig. 4Free and protein-bound NAD(P)H fluorescence lifetime histograms of in vivo and ex vivo skin stored at , room temperature, and in the presence and absence of media. The fluorescence lifetime histograms of free and protein-bound NAD(P)H from FLIM imaged ex vivo skin are shown from day 7 of the time course. The separate histograms in each box represent ex vivo skin samples from up to three individuals. 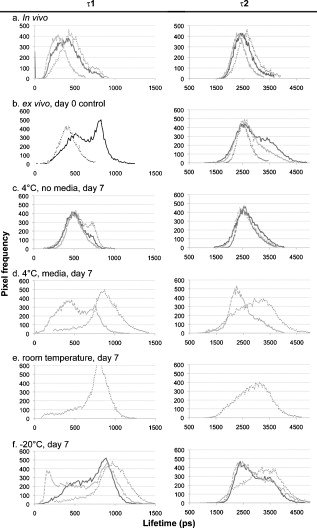 2.4.Free:Bound NAD(P)H Lifetime Ratio ImagesFigure 5 shows representative pseudocolored images, between the range of , of in vivo control and ex vivo skin samples stored over . The in vivo and ex vivo controls had a mean fluorescence lifetime of . Ex vivo skin stored at , without media, maintained this mean lifetime up to . With media, mean lifetime of skin kept at was comparable to the controls only up to . For the remaining conditions, the mean lifetime progressively approached until tissue expiration. Fig. 5Fluorescence lifetime images of ex vivo skin kept in the presence and absence of media, at various temperatures, across a time course. The images have been pseudocolored according to , which is calculated by weighting the mean fluorescence lifetime of free and protein-bound NAD(P)H ( and , respectively) by their respective contributions: and . These are representative images taken from three independent experiments. 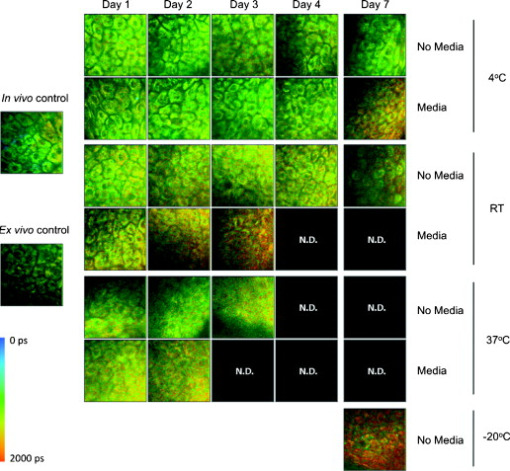 2.5.Image QualityFigure 6 displays the average image quality measurement (IQM) values over time of the imaged skin for each storage condition. Image quality for all the storage conditions initially increased after relative to the day 0 control. For skin stored at , with and without media, this value fell to the control level and persisted up until day 7. Figure 6 also shows that mean peak signal-to-noise ratio (PSNR) values from the ex vivo skin images stored at different conditions did not significantly change with time. However, the mean PSNR was higher for skin stored at , without media than at and room temperature without media, after . The noise frequency spectrum profiles are displayed in Fig. 7 . Figure 7 aligns the autofluorescence images adjacent to their respective noise frequency spectra. Autofluorescence images representing viable epidermal cells (i.e., the controls and ex vivo skin at ) all displayed an L-shaped noise frequency spectrum. In contrast, tissues with high background/noise typical of cells damaged due to ischemic necrosis, such as ex vivo skin kept at and , showed a flat noise frequency spectrum. Fig. 6Mean PSNRs of the autofluorescence images , relative to the day 0 control (upper) and “image quality” mean from ex vivo skin samples , from the three study subjects, across the time course (lower). PSNRs were determined by ImageJ using a custom script. Autofluorescence images (Fig. 2) were processed with the image processing software Image Quality from a Natural Scene (MITRE, Bedford, Massachusetts) to obtain a quantitative IQM value. 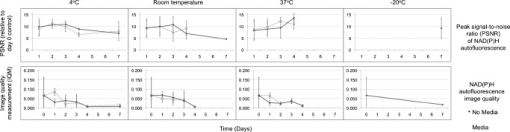 Fig. 7Noise frequency spectra of in vivo skin and ex vivo skin at day 0, at (no media), and at (no media), shown next to the corresponding representative autofluorescence image. The spectra were obtained by calculating the Fourier transform of the autofluorescence images using the software package Imatest (Imatest LCC, Boulder, Colorado). Data were collected from three independent experiments. 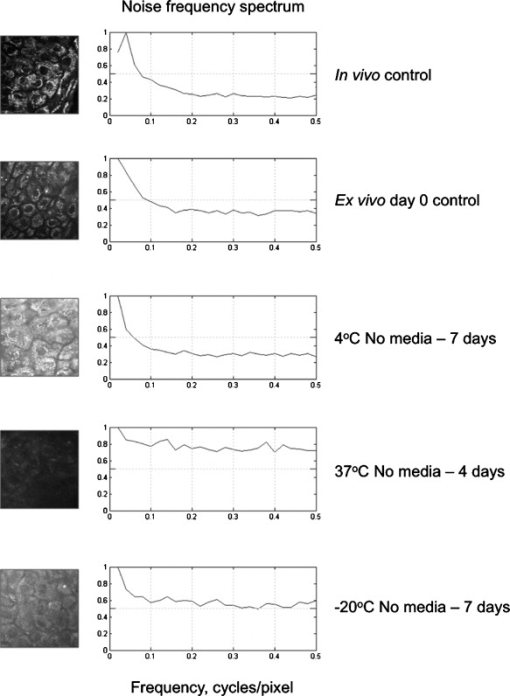 3.DiscussionPrevious studies demonstrate that cell death can alter NAD(P)H autofluorescence intensity in human cells. For example, autofluorescence increases during the early stages of apoptosis in primary human epithelial cells treated with cisplatin.22 Ischemia has been well described to cause a physical reduction in NAD(P)H and a corresponding decrease in autofluorescence in a variety of cells and tissues.23, 45, 46 The correlation with NADH autofluorescence intensity is related47 to the physical concentration of the NADH. NADH autofluorescence has been used to assess the onset of skin flap necrosis in rats and showed a significant decrease in autofluorescence with time and necrosis.48 In this study, a progressive loss of tissue autofluorescence intensity and FAD-normalized NAD(P)H photon counts occurred for ex vivo skin kept at and to a lesser extent at room temperature. Ex vivo skin kept at maintained autofluorescence across the time course, which agrees with previous findings4, 11, 18, 49 based on the MTT assay that the metabolic viability of ex vivo skin was prolonged at . Our results also appear to parallel the decrease in NAD(P)H autofluorescence in yeast cells after reversible injury by the addition of or to stimulate oxidative stress.23 As the epidermal cells in our experiment were progressing through ischemic necrosis, the reduction in autofluorescence may represent an initial depletion of NAD(P)H while the cells respond to metabolic demands and antioxidant responses, which are also associated with cell death.23, 50, 51 In the ex vivo skin we examined, the recovery of autofluorescence and thus NAD(P)H levels may be due to a temporary burst of metabolic activity stimulated by ischemic-injury-induced damage and response systems. In contrast, hepatocytes undergo an initial increase in autofluorescence from NADH during the early stages of hypoxia following reversible hypoxic injury.46 When hepatocytes were exposed to longer hypoxic conditions, the autofluorescence gradually decreased from a peak level below the control until irreversible damage and cell death due to ischemia had occurred.46 These changes occurred within after hypoxia. A similar trend was seen in this study, with the autofluorescence of the ex vivo skin initially increasing after incubation at and room temperature (Fig. 2). Speculatively, the increase in autofluorescence may be due to hypoxic injury or alterations in metabolic pathways. By the end of the time course, all autofluorescence had been lost for tissue kept at room temperature, indicating cell death. In skin, this peak occurred at in both the room temperature and groups (Fig. 3). Although the trend in autofluorescence is similar in hepatocytes and skin, the time scale and temperature aspects do not clearly correlate. More investigation is required to fully understand the mechanisms that contribute to this phenomenon. The absence of oxygen delivery to the viable epidermis in ex vivo skin may cause a significant dependence on anaerobic respiration for ATP production. The by-product of increased glycolysis is the production of lactate, which has been demonstrated to stimulate the production of reactive oxygen species.52 Studies that have found lactate-stimulated ROS generation noted that this occurred while some mitochondrial respiration was active. A similar effect may be occurring in viable epidermis cells as the remaining oxygen levels within the skin, postexcision, would be depleted over time with further metabolic activity. Mitochondrial respiration would eventually be superseded by glycolysis in the viable epidermis as oxygen levels decrease and lactate production increases. This transition period may be a source of ROS generation and subsequent oxidative injury that takes place in the skin. The loss of NAD(P)H autofluorescence was not seen in skin kept at (Fig. 2). This may be due to the low temperature that significantly suppressed any metabolic activity,8 irrespective of the presence of media. Generally, cellular autofluorescence for skin kept at room temperature and lasted longer in the absence of media (Fig. 2). This may be explained by the supply of metabolic nutrients in media to promote higher metabolic activity, and thus the accumulation of metabolic waste. If this were the case, the presence of media would accelerate the path toward ischemic necrosis. This hypothesis has been suggested in a similar study conducted by others.8 The mean lifetimes of free and protein-bound NAD(P)H ( and , respectively) closely matched other in vitro measurements.27, 29 Additionally, the values of the in vivo and ex vivo stratum spinosum fluorescence lifetimes agreed with another study.53 Throughout the time course we observed the emergence of an additional peak lifetime between 800 and in addition to the control peak at in some skin samples (Fig. 4). The appearance of this peak was a common pattern for skin that had lost autofluorescence prior to tissue expiration. Similarly, for protein-bound NAD(P)H, a broad right-tailed histogram peak at was also observed. The formation of these additional lifetime profiles may correlate with the interaction of NAD(P)H with an intracellular enzyme. This may be similar to the different lifetimes seen for NAD(P)H when bound to lactose dehydrogenase or mitochondrial malate dehydrogenase25 (mMDH). Interestingly, the recorded fluorescence lifetime of a solution of NADH:mMDH is25 . Thus, the formation of the peak in Fig. 4 may be indicative of NADH binding to mMDH. There are also suggestions36, 40, 44 that similar peaks can be attributed to extended and folded populations of free NADH. However, the relationships between these observations and the death of skin are not known. Similarly, the appearance of a peak in the histogram may be indicative of NADPH binding to NADPH oxidase (Nox2), which displays a comparable lifetime for this interaction.54 Interestingly, Nox2 is involved in the production of ROS in endothelial cells in response to various agonists and stimulants.55 One study also found that Nox2 −/− mice displayed impaired neovascularization in response to ischemia, as Nox2 is involved in angiogenesis within endothelial cells via ROS production.55 Further studies are necessary to determine whether these hypothetical interactions occur under these conditions. The increase in the fluorescence lifetimes of NAD(P)H for skin undergoing ischemic necrosis contrasts with the decrease in lifetime recorded for in vitro cells treated with potassium cyanide (KCN), a metabolic inhibitor.36 The authors concluded36 that the decrease of fluorescence lifetime correlated with an increase in the ratio, potentially due to the underutilization of NADH resulting from the inhibition of the electron transport chain by KCN. In this study however, the ex vivo skin suffering from ischemic necrosis does not have a fresh source of nutrients and a means of extracting waste. Thus, the lack of nutrients during continued metabolic activity may inhibit the replenishing of fresh NADH/NADPH and lead to the accumulation of . FLIM images of cultured cells pseudocolored by the mean fluorescence lifetime and weighted by relative amplitudes of components have been used as a visual indicator of the cellular free:bound NAD(P)H ratio state and related to cancer progression.36, 38 In our study, the progressive increase of the mean lifetime from appears to be an indicator of ischemic necrosis. Interestingly, these results contrast with other studies that have described the average lifetime decreasing with hypoxia in cancerous tissue.38, 39 While a marked decrease in the supply of oxygen during metabolic activity is common to these physiological states, the contrast in fluorescence lifetimes may be explained by the finding that human epidermal skin metabolism is already predominantly anaerobic.56 Thus, the lifetime increases throughout the onset of ischemic necrosis may be more related to removal of metabolic waste from the tissue via the vasculature rather than the lack of oxygen supply. Further studies are required to examine this hypothesis. Noise frequency spectra have been largely used to assess noise compensation or filter software for digital cameras, with flat spectra indicating uncompensated white noise.57 Cameras that use digital software to compensate for white noise display a roll-off in noise frequency spectra, characteristic of spectral noise.57 We found that tissue with advanced ischemic necrosis gave a cloudy image, presumably due to leaking intracellular material.58 In addition, there was a flat noise frequency spectrum rather than the L-shaped spectrum seen for the control images and the best-preserved tissue stored at up to (Fig. 7). In summary, this study has examined changes in NAD(P)H autofluorescence following the progressive onset of ischemic necrosis in ex vivo skin. We have found that ischemic necrosis of the tissue is marked by several characteristics: (1) a loss in NAD(P)H autofluorescence, possibly due to a physical depletion of the molecule; (2) a reduction in the FAD normalized NAD(P)H photon count; (3) a significant deviation in the fluorescence lifetime histogram of free and protein-bound NAD(P)H away from a normal “control” state (i.e., freshly excised ex vivo skin); (4) an increase in the average fluorescence lifetime above ; and (5) a qualitative loss is cellular integrity, revealed by noise frequency spectra analysis. These patterns may serve as the basis for MPT-FLIM to be used as a visual noninvasive biopsy for assessing the onset of ischemic necrosis of ex vivo skin. 4.Materials and Methods4.1.DermaInspect/MPT-FLIMMultiphoton tomography was carried out using the DermaInspect system (JenLab GmbH, Jena, Germany). Excitation light for multiphoton imaging was provided by an ultra-short-pulsed ( pulse width) mode-locked titanium sapphire laser (MaiTai, Spectraphysics, Mountain View, California). The tuning range of the excitation wavelength was . To enable FLIM measurements, a time-correlated single-photon counting SPC 830 FLIM system (Becker & Hickl, Berlin, Germany) was integrated into the tomography system. FLIM analyses were performed by an SPC 830 2.9 software module installed on a dual core computer with a Windows XP operating system. The SPC module builds59 a photon distribution over the and scan coordinates along with the time within the fluorescence decay, . The signals from four photon-counting detectors operated at different wavelength intervals and were processed simultaneously. To analyze the FLIM data, the software data analysis package SPCImage (Becker & Hickl GmbH, Berlin, Germany) was used. This package uses iterative convolution with either a single-, double-, or triple-exponential model to calculate the decay parameters for each individual pixel within the scan. The amplitude weighted average of the double-exponential fit was used analyze the photon decay profiles from the scanned images of the epidermis unless otherwise noted. The resulting two components are and . When a tissue sample was excited with (two-photon), the and fluorescence lifetime decay histograms have been shown to correlate with free and protein-bound NAD(P)H respectively within the cells.60 Deviations away from these native fluorescence lifetimes indicate changes in the state of NAD(P)H within the cell. Changes in the fluorescence lifetime of NAD(P)H can be caused by alterations in temperature, pH and protein binding.61 The objectives lens used in this study was the Plan-Neofluar oil-immersion (Carl Zeiss, Germany) with the distance between the objective lens and the in vivo adaptor filled with index-matching oil. The MPT imaging system (JenLab GmbH, Jena, Germany) uses ultra-short-pulse excitation laser light that is narrowly focused onto a sample via a high-numerical-aperture (NA) oil-immersion microscope objective ( , NA, 1.3). Two IR photons emitted by the ultrashort-pulsed-laser were simultaneously absorbed by a single fluorophore molecule, raising it to an excited state in a process known as two-photon excited fluorescence (TPEF). Through the objective, the focused laser light within the femtoliter focal volume in the sample resulted in a large and instantaneous intensity that caused the sample’s nonlinear optical response. The fluorescence emission occurred exclusively within the focal volume. This resulted essentially in an “optical section,” with the thickness of each section calculated by the axial transfer function of the imaging system, or with our instrument. To image ex vivo and in vivo skin, the excitation wavelength was set to (two-photon) with a laser power of at the sample to excite NAD(P)H, which is responsible for cellular autofluorescence. The focal point within the sample was raster-scanned using two galvanometer mirrors. The same objective lens was used to collect all emitted fluorescence, which was transmitted through a dichroic mirror and optical filter for detection by photon-multiplier tubes (PMTs). The dichroic mirror simultaneously reflects any IR laser excitation photons to prevent feedback. A BG39 filter ( , Schott glass color filter) was used to optically filter the fluorescence light to either the live image or FLIM PMT. In front of the FLIM detectors, a bandpass filter was used to isolate the NAD(P)H signal and the FAD signal was isolated with a bandpass filter. All live and FLIM images were acquired with a scan speed of at a size. The skin was imaged at a depth range between 30 and , which encompassed the stratum spinosum. This range was necessary due to the undulating profile of the viable epidermis and subsequent variable depth of the stratum spinosum. The imaging depth was determined by keratinocyte morphology, with an emphasis on maintaining uniform cell diameter between samples. 4.2.Skin SamplesExcised human abdomen skin samples from three individuals were obtained from volunteers immediately following elective surgery. The donors gave informed written consent, and approval for the collection of the skin samples was given by the Princess Alexandra Hospital Research Committee (Approval no. 097/090, administered by the University of Queensland Human Ethics Committee). The skin samples were processed on delivery to remove the underlying fat and connective tissue. The resulting full-thickness skin was then cut into -diam circles and placed into yellow-capped sample containers, either in the presence or absence of of nutrient serum-free Dulbecco modified Eagle medium (DMEM). The media was not supplemented with antibiotics due to their metabolic effects on the ex vivo skin, as determined by FLIM (data not shown). For in vivo control images, a drop of water was placed on the ventral forearm of three individual volunteers, followed by a -thick microscope glass coverslip. Immersion oil was placed between the coverslip and the objective lens prior to imaging. Scan settings and depths were identical to those used for ex vivo skin samples. 4.3.Time Course IncubationsThe skin samples were stored at , room temperature, or , with a partially unscrewed lid to permit gas exchange, in the presence or absence of nutrient media. Samples kept at were placed within a tissue culture incubator with 5% . Skin was also stored at without media for the incubation period. The control for this experiment was a sample of the skin imaged by MPT-FLIM prior to incubation at room temperature. Tissue stored at , room temperature, and was imaged every by MPT-FLIM over . After from the day of incubation (i.e., the day 0 control), the skin stored at was thawed and imaged along with the remaining tissue samples. 4.4.Autofluorescence Intensity and Photon CountsThe autofluorescence images were analyzed for fluorescence intensity using the Java-based image-processing program ImageJ (National Institutes of Health). The autofluorescence intensity from the images taken from three independent ex vivo skin samples, stored under the conditions already indicated, were averaged and plotted against the time course in days. Photon counts were obtained using the SPCImage software. FLIM data were separated by optical filters into three emission channels: channel 1 , channel 2 , and channel 3 . An excitation wavelength of (two-photon) was used to observe autofluorescence. A bandpass filter was used to isolate NAD(P)H. Keratin and FAD were imaged through 450 to 515- and bandpass filters, respectively. Using SPCImage, these channels were used to obtain and normalize NAD(P)H photon counts to keratin and FAD as a quantitative approach to measure the levels of this metabolic molecule within the image field of view. 4.5.Free:Bound NAD(P)H Ratio ImagingBoth in vivo and the stored ex vivo skin imaged by FLIM was pseudocolored to display the mean lifetime weighted by their relative contributions of free and protein-bound NAD(P)H . This provided a qualitative representation of the free:bound NAD(P)H ratio of the tissue while providing an effective means of comparison to the control across the time course. 4.6.Image Quality AnalysisTo obtain a quantitative value for the level of image quality, in relation to noise distortion, we used the image processing software Image Quality from a Natural Scene (IQM; MITRE, Bedford, Massachusetts). Autofluorescence images (represented in Fig. 2) were processed to obtain an IQM value using an objective image quality system, which has been described.62 The autofluorescence images were analyzed by IQM to obtain an arbitrary value for image quality, based on the level of noise and spatial image power spectrum. The second approach used to ascertain image quality was to measure the noise frequency spectra of the autofluorescence images using the software package Imatest (Imatest LCC, Boulder, Colorado). The noise frequency spectrum was obtained by calculating the Fourier transform of the spatial image.57 Noise frequency spectrum analysis has been used to discern between white noise and spectral (nonwhite) noise.63 White noise demonstrates a flat noise frequency spectrum profile while spectral noise possesses an L-shaped curve.63 In this study, we attempted to use noise frequency spectrum analysis to differentiate between images where cellular integrity was high (i.e., in vivo and ex vivo day 0 controls) and low, due to ischemic necrosis (i.e., cell images prior to tissue expiration). The final approach used the program ImageJ again to measure the PSNR, using a custom script. The ex vivo day 0 control served as the reference image for autofluorescence images across the time course, for each respective individual. AcknowledgmentsWe acknowledge the support of the Australian National Health & Medical Research Council (NHMRC) and the Asian Office of Research and Development (AORD) in undertaking this work. The authors state no conflict of interest. ReferencesG. K. Menon,
“New insights into skin structure: scratching the surface,”
Adv. Drug Delivery Rev., 54
(Suppl. 1), S3
–17
(2002). https://doi.org/10.1016/S0169-409X(02)00121-7 0169-409X Google Scholar
B. Godin and E. Touitou,
“Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models,”
Adv. Drug Delivery Rev., 59
(11), 1152
–1161
(2007). https://doi.org/10.1016/j.addr.2007.07.004 0169-409X Google Scholar
R. C. Wester, and H. I. Maibach,
“Percutaneous absorption of drugs,”
Clin. Pharm., 23
(4), 253
–266
(1992). https://doi.org/10.2165/00003088-199223040-00002 0278-2677 Google Scholar
H. Ben-Bassat,
“Performance and safety of skin allografts,”
Clin. Dermatol., 23
(4), 365
–375
(2005). https://doi.org/10.1016/j.clindermatol.2004.07.021 0738-081X Google Scholar
M. H. Hermans,
“Clinical experience with glycerol-preserved donor skin treatment in partial thickness burns,”
Burns Incl Therm Inj, 15
(1), 57
–59
(1989). 0305-4179 Google Scholar
E. S. Kalter and T. M. de By,
“Tissue banking programmes in Europe,”
Br. Med. Bull., 53
(4), 798
–816
(1997). 0007-1420 Google Scholar
D. Alotto, S. Ariotti, S. Graziano, R. Verrua, M. Stella, G. Magliacani, and C. Castagnoli,
“The role of quality control in a skin bank: tissue viability determination,”
Cell Tissue Bank, 3
(1), 3
–10
(2002). https://doi.org/10.1023/A:1021846703301 Google Scholar
J. N. Kearney,
“Guidelines on processing and clinical use of skin allografts,”
Clin. Dermatol., 23
(4), 357
–364
(2005). https://doi.org/10.1016/j.clindermatol.2004.07.018 0738-081X Google Scholar
A. R. Companjen, L. I. van der Wel, L. Wei, J. D. Laman, and E. P. Prens,
“A modified ex vivo skin organ culture system for functional studies,”
Arch. Dermatol. Res., 293
(4), 184
–90
(2001). https://doi.org/10.1007/s004030100219 0340-3696 Google Scholar
C. Gelis, A. Mavon, M. Delverdier, N. Paillous, and P. Vicendo,
“Modifications of in vitro skin penetration under solar irradiation: evaluation on flow-through diffusion cells,”
Photochem. Photobiol., 75
(6), 598
–604
(2002). https://doi.org/10.1562/0031-8655(2002)075<0598:MOIVSP>2.0.CO;2 0031-8655 Google Scholar
D. Bravo, T. H. Rigley, N. Gibran, D. M. Strong, and H. Newman-Gage,
“Effect of storage and preservation methods on viability in transplantable human skin allografts,”
Burns, 26
(4), 367
–378
(2000). https://doi.org/10.1016/S0305-4179(99)00169-2 0305-4179 Google Scholar
C. Antille, C. Tran, O. Sorg, and J. H. Saurat,
“Penetration and metabolism of topical retinoids in ex vivo organ-cultured full-thickness human skin explants,”
Skin Pharmacol. Physiol., 17
(3), 124
–128
(2004). https://doi.org/10.1159/000077238 Google Scholar
N. A. Monteiro-Riviere, A. O. Inman, T. H. Snider, J. A. Blank, and D. W. Hobson,
“Comparison of an in vitro skin model to normal human skin for dermatological research,”
Microsc. Res. Tech., 37
(3), 172
–179
(1997). https://doi.org/10.1002/(SICI)1097-0029(19970501)37:3<172::AID-JEMT2>3.0.CO;2-Q 1059-910X Google Scholar
N. Issachar, Y. Gall, M. T. Borell, and M. C. Poelman,
“pH measurements during lactic acid stinging test in normal and sensitive skin,”
Contact Dermatitis, 36
(3), 152
–155
(1997). https://doi.org/10.1111/j.1600-0536.1997.tb00399.x 0105-1873 Google Scholar
S. Messager, A. C. Hann, P. A. Goddard, P. W. Dettmar, and J. Y. Maillard,
“Assessment of skin viability: is it necessary to use different methodologies?,”
Skin Res. Technol., 9
(4), 321
–330
(2003). https://doi.org/10.1034/j.1600-0846.2003.00039.x 0909-752X Google Scholar
M. A. Zieger, E. E. Tredget, and L. E. McGann,
“A simple, effective system for assessing viability in split-thickness skin with the use of oxygen consumption,”
J. Burn Care Rehabil., 14
(3), 310
–318
(1993). https://doi.org/10.1097/00004630-199305000-00002 0273-8481 Google Scholar
H. L. Bank and M. K. Schmehl,
“Parameters for evaluation of viability assays: accuracy, precision, specificity, sensitivity, and standardization,”
Cryobiology, 26
(3), 203
–211
(1989). https://doi.org/10.1016/0011-2240(89)90015-1 0011-2240 Google Scholar
C. Castagnoli, D. Alotto, I. Cambieri, R. Casimiri, M. Aluffi, M. Stella, S. T. Alasia, and G. Magliacani,
“Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolioum salt assay,”
Burns, 29
(8), 759
–767
(2003). https://doi.org/10.1016/j.burns.2003.01.001 0305-4179 Google Scholar
M. B. Klein, D. Shaw, S. Barese, G. A. Chapo, and C. B. Cuono,
“A reliable and cost-effective in vitro assay of skin viability for skin banks and burn centers,”
J. Burn Care Rehabil., 17
(6), 565
–570
(1996). https://doi.org/10.1097/00004630-199611000-00016 0273-8481 Google Scholar
W. Ying,
“ and in cellular functions and cell death: regulation and biological consequences,”
Antioxid. Redox. Signal., 10
(2), 179
–206
(2008). https://doi.org/10.1089/ars.2007.1672 Google Scholar
H. W. Wang, V. Gukassyan, C. T. Chen, Y. H. Wei, H. W. Guo, J. S. Yu, and F. J. Kao,
“Differentiation of apoptosis from necrosis by dynamic changes of reduced nicotinamide adenine dinucleotide fluorescence lifetime in live cells,”
J. Biomed. Opt., 13
(5), 054011
(2008). https://doi.org/10.1117/1.2975831 1083-3668 Google Scholar
J. M. Levitt, A. Baldwin, A. Papadakis, S. Puri, J. Xylas, K. Munger, and I. Georgakoudi,
“Intrinsic fluorescence and redox changes associated with apoptosis of primary human epithelial cells,”
J. Biomed. Opt., 11
(6), 064012
(2006). https://doi.org/10.1117/1.2401149 1083-3668 Google Scholar
J. Liang, W. L. Wu, Z. H. Liu, Y. J. Mei, R. X. Cai, and P. Chen,
“Study the oxidative injury of yeast cells by NADH autofluorescence,”
Spectrochim. Acta A Mol. Biomol. Spectrosc., 67
(2), 355
–359
(2007). https://doi.org/10.1016/j.saa.2006.07.035 Google Scholar
Q. Yu, M. Proia, and A. A. Heikal,
“Integrated biophotonics approach for noninvasive and multiscale studies of biomolecular and cellular biophysics,”
J. Biomed. Opt., 13
(4), 041315
(2008). https://doi.org/10.1117/1.2952297 1083-3668 Google Scholar
Q. Yu and A. A. Heikal,
“Two-photon autofluorescence dynamics imaging reveals sensitivity of intracellular NADH concentration and conformation to cell physiology at the single-cell level,”
J. Photochem. Photobiol., B, 95
(1), 46
–57
(2009). https://doi.org/10.1016/j.jphotobiol.2008.12.010 1011-1344 Google Scholar
H. Schneckenburger and K. Konig,
“Fluorescence decay inetics and imaging of NAD(P)H and flavins as metabolic indicators,”
Opt. Eng., 31
(7), 1447
–1451
(1992). https://doi.org/10.1117/12.57704 0091-3286 Google Scholar
V. K. Ramanujan, J. H. Zhang, E. Biener, and B. Herman,
“Multiphoton fluorescence lifetime contrast in deep tissue imaging: prospects in redox imaging and disease diagnosis,”
J. Biomed. Opt., 10
(5), 051407
(2005). https://doi.org/10.1117/1.2098753 1083-3668 Google Scholar
P. I. Bastiaens and A. Squire,
“Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell,”
Trends Cell Biol., 9
(2), 48
–52
(1999). https://doi.org/10.1016/S0962-8924(98)01410-X 0962-8924 Google Scholar
J. R. Lakowicz, H. Szmacinski, K. Nowaczyk, and M. L. Johnson,
“Fluorescence lifetime imaging of free and protein-bound NADH,”
Proc. Natl. Acad. Sci. U.S.A., 89
(4), 1271
–1275
(1992). https://doi.org/10.1073/pnas.89.4.1271 0027-8424 Google Scholar
R. M. Williams, D. W. Piston, and W. W. Webb,
“Two-photon molecular excitation provides intrinsic 3-dimensional resolution for laser-based microscopy and microphotochemistry,”
FASEB J., 8
(11), 804
–813
(1994). 0892-6638 Google Scholar
J. Paoli, M. Smedh, and M. B. Ericson,
“Multiphoton laser scanning microscopy—a novel diagnostic method for superficial skin cancers,”
Semin Cutan Med. Surg., 28
(3), 190
–195
(2009). https://doi.org/10.1016/j.sder.2009.06.007 1085-5629 Google Scholar
M. S. Roberts, M. J. Roberts, T. A. Robertson, W. Sanchez, C. Thorling, Y. Zou, X. Zhao, W. Becker, and A. V. Zvyagin,
“In vitro and in vivo imaging of xenobiotic transport in human skin and in the rat liver,”
J. Biophoton., 1
(6), 478
–493
(2008). https://doi.org/10.1002/jbio.200810058 Google Scholar
A. V. Zvyagin, X. Zhao, A. Gierden, W. Sanchez, J. A. Ross, and M. S. Roberts,
“Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo,”
J. Biomed. Opt., 13
(6), 064031
(2008). https://doi.org/10.1117/1.3041492 1083-3668 Google Scholar
H. Schneckenburger, M. H. Gschwend, W. S. Strauss, R. Sailer, M. Kron, U. Steeb, and R. Steiner,
“Energy transfer spectroscopy for measuring mitochondrial metabolism in living cells,”
Photochem. Photobiol., 66
(1), 34
–41
(1997). https://doi.org/10.1111/j.1751-1097.1997.tb03135.x 0031-8655 Google Scholar
K. Konig,
“Clinical multiphoton tomography,”
J. Biophoton., 1
(1), 13
–23
(2008). https://doi.org/10.1002/jbio.200710022 Google Scholar
D. K. Bird, L. Yan, K. M. Vrotsos, K. W. Eliceiri, E. M. Vaughan, P. J. Keely, J. G. White, and N. Ramanujam,
“Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH,”
Cancer Res., 65
(19), 8766
–8773
(2005). https://doi.org/10.1158/0008-5472.CAN-04-3922 0008-5472 Google Scholar
M. W. Conklin, P. P. Provenzano, K. W. Eliceiri, R. Sullivan, and P. J. Keely,
“Fluorescence lifetime imaging of endogenous fluorophores in histopathology sections reveals differences between normal and tumor epithelium in carcinoma in situ of the breast,”
Cell Biochem. Biophys., 53
(3), 145
–157
(2009). https://doi.org/10.1007/s12013-009-9046-7 1085-9195 Google Scholar
M. C. Skala, K. M. Riching, A. Gendron-Fitzpatrick, J. Eickhoff, K. W. Eliceiri, J. G. White, and N. Ramanujam,
“In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,”
Proc. Natl. Acad. Sci. U.S.A., 104
(49), 19494
–19499
(2007). https://doi.org/10.1073/pnas.0708425104 0027-8424 Google Scholar
M. C. Skala, K. M. Riching, D. K. Bird, A. Gendron-Fitzpatrick, J. Eickhoff, K. W. Eliceiri, P. J. Keely, and N. Ramanujam,
“In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia,”
J. Biomed. Opt., 12
(2), 024014
(2007). https://doi.org/10.1117/1.2717503 1083-3668 Google Scholar
I. Georgakoudi,
“NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes,”
Cancer Res., 62
(3), 682
–687
(2002). 0008-5472 Google Scholar
D. W. Piston, and S. M. Knobel,
“Real-time analysis of glucose metabolism by microscopy,”
Trends Endocrinol. Metab., 10
(10), 413
–417
(1999). https://doi.org/10.1016/S1043-2760(99)00204-0 Google Scholar
B. Chance, V. Legallais, and B. Schoener,
“Metabolically linked changes in fluorescence emission spectra of cortex of rat brain, kidney and adrenal gland,”
Nature, 195 1073
–1075
(1962). https://doi.org/10.1038/1951073a0 0028-0836 Google Scholar
S. J. Lin and L. Guarente,
“Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease,”
Curr. Opin. Cell Biol., 15
(2), 241
–246
(2003). https://doi.org/10.1016/S0955-0674(03)00006-1 0955-0674 Google Scholar
H. D. Vishwasrao, A. A. Heikal, K. A. Kasischke, and W. W. Webb,
“Conformational dependence of intracellular NADH on metabolic state revealed by associated fluorescence anisotropy,”
J. Biol. Chem., 280
(26), 25119
–25126
(2005). https://doi.org/10.1074/jbc.M502475200 0021-9258 Google Scholar
J. T. Fitzgerald, S. Demos, A. Michalopoulou, J. L. Pierce, and C. Troppmann,
“Assessment of renal ischemia by optical spectroscopy,”
J. Surg. Res., 122
(1), 21
–28
(2004). https://doi.org/10.1016/j.jss.2004.05.025 0022-4804 Google Scholar
E. T. Obi-Tabot, L. M. Hanrahan, R. Cachecho, E. R. Beer, S. R. Hopkins, J. C. Chan, J. M. Shapiro, and W. W. LaMorte,
“Changes in hepatocyte NADH fluorescence during prolonged hypoxia,”
J. Surg. Res., 55
(6), 575
–580
(1993). https://doi.org/10.1006/jsre.1993.1187 0022-4804 Google Scholar
A. Mayevsky and G. G. Rogatsky,
“Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies,”
Am. J. Physiol.: Cell Physiol., 292
(2), C615
–640
(2007). https://doi.org/10.1152/ajpcell.00249.2006 0363-6143 Google Scholar
M. Mokry, P. Gal, M. Harakalova, Z. Hutnanova, J. Kusnir, S. Mozes, and J. Sabo,
“Experimental study on predicting skin flap necrosis by fluorescence in the FAD and NADH bands during surgery,”
Photochem. Photobiol., 83
(5), 1193
–1196
(2007). https://doi.org/10.1111/j.1751-1097.2007.00132.x 0031-8655 Google Scholar
J. N. Kearney,
“Quality issues in skin banking: a review,”
Burns, 24
(4), 299
–305
(1998). https://doi.org/10.1016/S0305-4179(98)00039-4 0305-4179 Google Scholar
M. C. Gendron, N. Schrantz, D. Metivier, G. Kroemer, Z. Maciorowska, F. Sureau, S. Koester, and P. X. Petit,
“Oxidation of pyridine nucleotides during Fas- and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation,”
Biochem. J., 353
(Pt. 2), 357
–367
(2001). https://doi.org/10.1042/0264-6021:3530357 0264-6021 Google Scholar
G. Kroemer, P. Petit, N. Zamzami, J. L. Vayssiere, and B. Mignotte,
“The biochemistry of programmed cell death,”
FASEB J., 9
(13), 1277
–1287
(1995). 0892-6638 Google Scholar
T. Hashimoto, R. Hussien, S. Oommen, K. Gohil, and G. A. Brooks,
“Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis,”
FASEB J., 21
(10), 2602
–2612
(2007). https://doi.org/10.1096/fj.07-8174com 0892-6638 Google Scholar
K. Konig, A. Ehlers, F. Stracke, and I. Riemann,
“In vivo drug screening in human skin using femtosecond laser multiphoton tomography,”
Skin Pharmacol. Physiol., 19
(2), 78
–88
(2006). https://doi.org/10.1159/000091974 Google Scholar
R. Niesner, P. Narang, H. Spiecker, V. Andreson, K. H. Gericke, and M. Gunzer,
“Selective detection of NADPH oxidase in polymorphonuclear cells by means of NAD(P)H-based fluorescence lifetime imaging,”
J. Biophys., 2008 602
–639
(2008). Google Scholar
M. Ushio-Fukai and Y. Nakamura,
“Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy,”
Cancer Lett., 266
(1), 37
–52
(2008). https://doi.org/10.1016/j.canlet.2008.02.044 0304-3835 Google Scholar
G. Ronquist, A. Andersson, N. Bendsoe, and B. Falck,
“Human epidermal energy metabolism is functionally anaerobic,”
Exp. Dermatol., 12
(5), 572
–579
(2003). https://doi.org/10.1034/j.1600-0625.2003.00018.x 0906-6705 Google Scholar
, “Imatest stepchart,”
Digital Image Quality Testing 2009, http://www.imatest.com/guides/modules/stepchart Google Scholar
G. Kroemer, B. Dallaporta, and M. Resche-Rigon,
“The mitochondrial death/life regulator in apoptosis and necrosis,”
Annu. Rev. Physiol., 60 619
–642
(1998). https://doi.org/10.1146/annurev.physiol.60.1.619 0066-4278 Google Scholar
D. C. Dai, S. J. Xu, S. L. Shi, M. H. Xie, and C. M. Che,
“Efficient multiphoton-absorption-induced luminescence in single-crystalline ZnO at room temperature,”
Opt. Lett., 30
(24), 3377
–3379
(2005). https://doi.org/10.1364/OL.30.003377 0146-9592 Google Scholar
T. G. Scott, R. D. Spencer, N. J. Leonard, and G. Weber,
“Emission properties of NaDH. studies of fluorescence lifetimes and quantum efficiencies of NADH, AcPyADH, and simplified synthetic models,”
J. Am. Chem. Soc., 92
(3), 687
–695
(1970). https://doi.org/10.1021/ja00706a043 0002-7863 Google Scholar
A. Gafni and L. Brand,
“Fluorescence decay studies of reduced nicotinamide adenine dinucleotide in solution and bound to liver alcohol dehydrogenase,”
Biochemistry, 15
(15), 3165
–3171
(1976). https://doi.org/10.1021/bi00660a001 0006-2960 Google Scholar
N. B. Nill and B. H. Bouzas,
“Objective image quality measure derived from digital image power spectra,”
Opt. Eng., 31
(4), 813
–825
(1992). https://doi.org/10.1117/12.56114 0091-3286 Google Scholar
, “Noise in photgraphic images,”
Digital Image Quality Testing 2009, http://www.imatest.com/docs/noise.html Google Scholar
|

