|
|
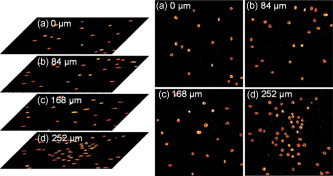
1.IntroductionTargeted cancer-cell microsurgery is critical to realizing zero damage to adjacent healthy cells or tissue, which is especially important for damage-free ablation of tumor cells while they are deeply embedded in complex tissue structures. Chemotherapy, radiation therapy, and surgery have been widely adopted as cancer management options. However, the effectiveness of chemotherapy is often limited by toxicity to other tissues in the body. Radiation can also cause damage to normal tissue. Surgery often requires the removal of a wide surgical margin or a free margin. The propensity of cancers to invade adjacent tissue or to spread to distant sites by microscopic metastasis often limits the effectiveness of those traditional methods. Alternative methods have been sought to reach the goal of cancer treatment. One of the promising treatment methods is microsurgery of cancer cells using pulsed near-infrared (NIR) laser beams and optical microscopy,1 which has allowed for ablation of cancer cells.2 In addition, this method also provides a tool for 3-D imaging through thick tissue media. However, pulsed near-infrared laser-based microsurgery has not been adopted medically for cancer treatment due to the following hurdles. First, the utilization of bulky optics and multilaser systems limits the microscopes to be potentially useful in in vivo inner organs and noninvasive environments. Second, the weak light absorption of both malignant and normal tissues to near-infrared light makes selective and low energy (medically safe) treatment infeasible. To address these hurdles, it is necessary to adopt an effective tool for delivering a near-infrared laser beam to a malignant tissue in a minimally invasive and observable manner without damaging surrounding healthy tissues, and to enhance the light absorption and heat conversion in malignant tissues. Two-photon endomicroscopy is a useful tool for 3-D in vivo imaging. A two-photon endomicroscope utilizes a femtosecond near-infrared laser as an energy source and delivers a laser beam through an optical fiber to a miniaturized probe. This design makes it possible to deliver a laser beam directly to the targeted tissue in a minimally invasive manner. Recently, photothermal therapy using surface plasmonic gold nanoparticles has shown to be a promising method for a localized treatment of cancer cells.3, 4, 5 Among the various gold nanoparticles investigated, the gold nanorod is one of special interest due to the easiness of synthesis and their near-infrared absorption. The strong two-photon luminescence of gold nanorods also makes them suitable to 3-D imaging.6 Therefore, a combination of two-photon endomicroscopy with gold nanorods should lead to a breakthrough in near-infrared pulsed laser-based microsurgery. In this work, we use a portable nonlinear optical endomicroscope with a small probe connected by a long flexible fiber to deliver a NIR pulsed beam.7 The use of a double-clad fiber (DCF) allows for the transmission of the laser beam to a sample through a miniature probe.7 At the sample, the NIR pulses were measured as by an autocorrelator (FR-103XL, Femtochrome, Berkeley, California). The electromagnetically driven resonant tuning fork in the probe facilitates a fast scanning mechanism in the horizontal axis,7 and an axial movement through a cantilever. This 3-D scanning unit leads to a maximum field of view with and a fast imaging speed of . 2.Endoscopic Imaging of Gold Nanorods and Cancer Cells Labeled by Gold NanorodsGold nanorods were prepared according to a method previously reported.5, 8 The as-synthesized gold nanorods have a maximal plasmonic absorption at . Detailed characterization is available in our previous publication.5 The human cervical cancer-cell line HeLa was used as a cell model. To achieve efficient targeting of gold nanorods to the cancer cells, transferrin molecules were conjugated to the surface of the nanorods.5 Transferrin has been proven to be efficient in enhancing cancer targeting by nanoparticles, including gold nanoparticles.5, 9 To stabilize the nanorods in cell culture buffer, polyethylene glycol (PEG) molecules were also conjugated on the gold nanorods. The molecular ratios of transferrin and PEG to gold nanorods are 3000:1 and 650:1, respectively. The concentration of gold nanorods in the culture medium is . The cytotoxicity of the transferrin-conjugated gold nanorods was examined using trypan blue. After incubating for , the viability of the cells is (cells not incubated with gold nanorods were used as controls). This means that the conjugated gold nanorods at this concentration are not toxic to cells. To realize cancer-cell microsurgery in a precisely observable manner, we first identify the cancer cells using the nonlinear endomicroscope through its small probe. 3-D two-photon-excited photoluminescence images of the HeLa cells labeled with gold nanorods within a field of view of were obtained by gently pressuring the tip of the probe onto the surface of the collagen matrix tissue phantom. The unique automatic axial scanning function of the nonlinear endomicroscope allows for 3-D imaging of the cancer cells by obtaining a set of slice images with a slice spacing of and a penetration depth of . Figure 1 shows some selected two-photon-excited photoluminescence images of the cancer cells from a set of the slice images. The images clearly reveal individual cancer cells at different depths. The HeLa cells without gold nanorod labeling cannot be detected by the endomicroscope. 3.Localized Three-Dimensional Cancer-Cell Treatment by EndomicroscopyWith the help of this visualization method of cancer cells, the gold-nanorod-enhanced cancer-cell necrosis process can be investigated. Necrosis is a process of cell death by the compromising of membrane integrity.10 To this end, live HeLa cells labeled by transferrin-conjugated gold nanorods were placed in the collagen tissue phantom mixed with propidium iodide (PI). PI can be used to examine the death of cells after being irradiated by an excitation laser beam. The nuclei of dead cells could be stained with PI, while that of live cells could not. The cancer-cell microsurgery was achieved by imaging the cancer cells first, then selecting a single cancer cell and focusing the laser beam to the selected cell for a period of time (Fig. 2 ). We first obtained the two-photon-excited photoluminescence image (Fig. 2 part A1) of the live cancer cells at the surface of the collagen using a laser beam of wavelength , which is the longitudinal surface plasmon resonance wavelength of the transferrin-conjugated gold nanorods. The power of the excitation laser beam at the sample was . This visualization facilitates that particular cells within the field of view (here the cells in the “L” shape) can be selected. The excitation laser beam was focused on each of those cells with a dwelling time of . Six minutes after the entire selected cells were illuminated, the cells were imaged by the nonlinear endomicroscope again, while the excitation wavelength was changed to (Fig. 2 part A2), which is the peak two-photon absorption wavelength of PI. Figure 2 part A2 shows a clear “L” shape in the field of view, and confirms that the selected cells irradiated with a dwelling time of were dead while the rest of cells were not. Fig. 2Video1 showing 3-D imaging and localized necrosis of cancer cells in the collagen matrix tissue phantom mixed with propidium iodide (PI). Parts A1, B1, and C1 show two-photon photoluminescence images of HeLa cells labeled with biofunctional gold nanorods at the collagen surface, , and below the surface, respectively. Parts A2, B2, and C2 show HeLa cells selectively killed by the focused excitation laser beam at the collagen surface, , and below the surface, respectively. (QuickTime, 3.2 MB) 1 . 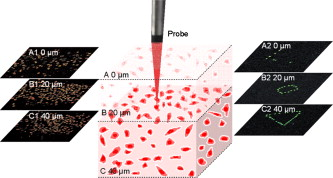 Figures 2 part B1, B2, C1, and C2 show the same process of the localized necrosis effect at 20 and below the surface of the collagen with cells at a square and a triangular shape being killed. These images display that the cells outside the laser irradiated area were not affected at all, indicating that the endomicroscope can be used for the highly localized 3-D necrosis treatment of the selected cancer cells. Such a 3-D treatment is significant to cancer therapy, especially at early stages before it metastasises. In contrast, when cancer cells are not labeled with gold nanorods, the experiment shows that the cells cannot be killed by a laser power level as high as . This indicates that the presence of gold nanorods can significantly reduce the power and energy threshold for cancer-cell necrosis. Since cancer cells can take more transferrin-conjugated gold nanorods, the energy threshed for injuring a healthy cell is much higher than that for a cancer cell. 4.Cancer Cell Necrosis and Apoptosis by EndoscopyIn the prior experiments, the high power illumination can lead to fragmentation of the gold nanorods11 and the associated localized mechanical shock to the cell membrane, causing membrane perforation and thus inducing cell necrosis. The intense heat produced at high power/energy fluence can also destroy the cell membrane, leading to cell necrosis. In fact, cell proliferation can be stopped either by the necrosis or apoptosis processes. The photothermal effect of gold nanorods can potentially lead to the dysfunction of subcellular structures that govern the apoptosis of cells at a laser power level lower than that for necrosis. To examine whether apoptosis can be induced with the nonlinear optical endomicroscopic system, a combination of two dyes, namely annexinV-Cy3.18 and PI was used for this purpose. Annexin V is a kind of protein that binds phospholipids in the presence of calcium. During the early stages of apoptosis, cell membrane phosphatidyserine is transported from the inner plasma membrane leaflet to the outer leaflet, where it binds with annexin-V molecules. Therefore, apoptotic cells are only membrane stained; dead cells are both membrane and nuclei stained, while live cells cannot be stained with either of the two dyes. To differentiate the apoptosis and necrosis processes by a single laser beam, cells were illuminated at laser power levels of 2 and , respectively. Interestingly, we observed that although was not high enough to kill all the cells (necrosis), apoptosis could be induced. As shown in Fig. 3 , only the membrane of the cells treated at this laser power level was stained. As a comparison, both the membrane and nuclei of the cells treated at the laser power of were labeled [Fig. 3]. The calculated energy fluence levels at 10 and are 1.74 and , which are far above the energy used in identifying a cell from the photoluminescence of gold nanorods. This means that during in vivo treatment, cells surrounding a tumor will not be harmed during the imaging (identifying or diagnosis) process. Fig. 3Apoptosis and necrosis induction of HeLa cells at power levels of (a) and (b) , respectively, and (c) average irradiation time for a single cell through necrosis and apoptosis processes as well as (d) the probability of a cell through necrosis and apoptosis processes caused by the nanorod-induced photothermal effect. The scale bars in (a) and (b) are . 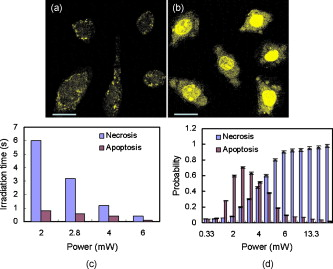 To induce necrosis and apoptosis of single cells, the required irradiation time of a laser beam depends on the excitation laser power, as shown in Fig. 3. For a given laser irradiation time of at a single cancer cell, the statistic probability of a Hela cell to be necrotic and apoptotic as a function of the laser power is displayed in Fig. 3. At a laser power below , the probabilities of a cell to be necrotic and apoptotic are close to almost zero; thus a cancer cell is not affected by laser illumination. For laser power higher than , the probability of a cell to be dead is close to 100%. Therefore, a cancer cell is definitely damaged with laser power over . As the laser power decreases from , while the probability of a cell through a necrosis process declines from 100 to 30%, the probability for a cell through the apoptosis process increases up to 70%. In other words, a cancer cell can be killed with a 100% death rate through necrosis or apoptosis processes when the laser power is larger than , corresponding to an energy fluence of . 5.ConclusionIn summary, we develop a novel technique for cancer-cell microsurgery by combining our recently developed nonlinear endomicroscope with the photothermal effects of gold nanorods. With the strong photoluminescence of gold nanorods, cancer cells can be imaged and selected for apoptosis and necrosis induction by controlling the laser energy. This bifunctionality (imaging and treatment) opens an immediately bright future for this technique to be used in minimal-invasive cancer treatment with localized and selective therapeutic effects. ReferencesW. Denk,
J. H. Strickler, and
W. W. Webb,
“Two-photon laser scanning fluorescence microscopy,”
Science, 248 73
–76
(1990). https://doi.org/10.1126/science.2321027 0036-8075 Google Scholar
R. L. Amy and
R. Storb,
“Selective mitochondrial damage by a ruby laser microbeam: an electron microscope study,”
Science, 150 756
–758
(1965). https://doi.org/10.1126/science.150.3697.756 0036-8075 Google Scholar
V. P. Zharvo and
V. Galitovsky,
“Photothermal detection of local thermal effects during selective nanophotothermolysis,”
Appl. Phys. Lett., 83 4897
–4899
(2003). https://doi.org/10.1063/1.1632546 0003-6951 Google Scholar
X. H. Huang,
I. H. El-Sayed,
W. Qian, and
M. A. El-Sayed,
“Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods,”
J. Am. Chem. Soc., 128 2115
–2120
(2006). https://doi.org/10.1021/ja057254a 0002-7863 Google Scholar
J. L. Li,
D. Day, and
M. Gu,
“Ultra-low energy threshold for cancer photothermal therapy using transferrin-conjugated gold nanorods,”
Adv. Mater. (Weinheim, Ger.), 20 3866
–3871
(2008). https://doi.org/10.1002/adma.200800941 0935-9648 Google Scholar
H. F. Wang,
T. B. Huff,
D. A. Zweifel,
W. He,
P. S. Low,
A. Wei, and
J. X. Cheng,
“In vitro and in vivo two-photon luminescence imaging of single gold nanorods,”
Proc. Natl. Acad. Sci. U.S.A., 102 15752
–15756
(2005). https://doi.org/10.1073/pnas.0504892102 0027-8424 Google Scholar
H. C. Bao,
J. Allen,
R. Pattie,
R. Vance, and
M. Gu,
“A fast handheld two-photon fluorescence micro-endoscope with a field of view for in vivo imaging,”
Opt. Lett., 33 1333
–1335
(2008). https://doi.org/10.1364/OL.33.001333 0146-9592 Google Scholar
T. K. Sau and
C. J. Murphy,
“Seeded high yield synthesis of short Au nanorods in aqueous solution,”
Langmuir, 20 6414
–6420
(2004). https://doi.org/10.1021/la049463z 0743-7463 Google Scholar
B. D. Chithrani and
W. C. W. Chan,
“Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes,”
Nano Lett., 7 1542
–1550
(2007). https://doi.org/10.1021/nl070363y 1530-6984 Google Scholar
L. Tong,
Y. Zhao,
T. B. Huff,
M. N. Hansen,
A. Wei, and
J. X. Cheng,
“Gold nanorods mediated tumor cell death by compromising membrane integrity,”
Adv. Mater. (Weinheim, Ger.), 19 3136
–3141
(2007). https://doi.org/10.1002/adma.200701974 0935-9648 Google Scholar
S. Link,
C. Burda,
B. Nikoobakht, and
M. A. El-Sayed,
“Laser-induced shape changes of colloidal gold nanorods using femtosecond and nanosecond laser pulses,”
J. Phys. Chem. B, 104 6152
–6163
(2000). https://doi.org/10.1021/jp000679t 1089-5647 Google Scholar
|