|
|
|
Calcium is a very important messenger in all cells and tissues, relaying information within cells to regulate their activities,1 especially those related to apoptosis signaling.2 It plays different roles within the cytoplasm, nucleus, and organelles. Free calcium in the nucleus affects gene transcription and cell growth significantly. The endoplasmic reticulum (ER) is recognized as the principal intracellular calcium store,3 while mitochondria function as a modulator of calcium by their ability to release and uptake free calcium.4 Calcium can be discharged from the nuclear envelope directly into the nucleoplasm,5 and it can also diffuse into the nucleus from the cytoplasm through nuclear pores6 or nuclear tubular structures by some special mechanisms. Yet despite these discoveries, the exact location of calcium stores remains uncertain. Although several possible stores have been proposed,7 the traditional method of stimulating by chemicals such as InsP3 produces a global effect, and thus cannot identify the store at the subcellular level and pinpoint the pathway of diffusion into the nucleus. With the recent advances of lasers, the femtosecond (fs) laser has become a powerful and precise tool in biological research. A report in 20018 described the induction of a wave in a cell when it was irradiated by a finely focused fs laser beam, which activated both the intracellular store and the stretch-activated channel or other channels by light-induced pressure on the membrane, or by shock waves generated from the focal spot to cause an extracellualr influx.9 The mechanisms were further investigated by another work in 2009,10 which utilized the fs laser as a controllable stimulation in highly localized space and time. Inspired by these results, we also attempted to irradiate HeLa cells by a fs laser with the aim of pinpointing the exact location of the store within a cell. This becomes possible because the fs laser can provide a controllable and precise trigger of slow release at the subcellular level, thereby enabling a much easier observation of intracellular dynamics that cannot be achieved by traditional chemical stimulations. In this study, HeLa cells obtained from American Type Culture Collection (Manassas, Virginia) were cultured in RPMI 1640 medium (Sigma Aldrich, Saint Louis, Missouri) supplemented with 10% (v/v) fetal calf serum (FCS) (Gibco, Invitrogen, Carlsbad, California) at and 5% . They were seeded on a petri dish with a glass slide ( thick), stained with Fluo-4/AM [Invitrogen (Carlsbad, California), final concentration of ) for and washed two times by phosphate buffered saline (PBS) for study. The fluorescence was excited by a laser at . The central wavelength of the fs laser [Calmar (Sunnyvale, California) FPL-04] employed in this study was , and the average power at the fiber output was about at a pulse width of and a repetition rate of . The fs laser was coupled into the microscope by a fiber collimator and a dichroic mirror, and then focused by a objective . The coupling efficiency of the system is around 60% and the pulse dispersion is around . The full width at half maximum diameter of the focus was around , and the focal plane was controlled by confocal scanning (Nikon C1 confocal microscope). The optical design is shown in Fig. 1 . For clarity, we use , , and to represent the intracellular concentration in the whole cell, the nucleus, and the cytoplasm, respectively. Fig. 1(a) Optical setup of the experiment. L1: the fs laser at . L2: the laser at for fluorescence excitation. scans versus time after the laser exposure (b) at cytoplasm and (c) at nucleus. In (c), the object appearing in the lower-left-hand corner is a neighbor cell. Red cross: exposure spot. Bar is . (Color online only.) 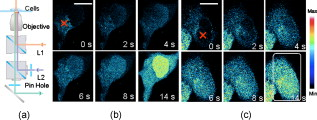 The first group of HeLa cells (20 cells) was irradiated at the cytoplasm for , which elicited a slow release of whose diffusion pattern into the cell nucleus is shown in Fig. 1. The observed increase in fluorescence intensity subsequent to the laser excitation is consistent with the theory that any initial release can trigger more release from the store, forming loop amplification11 or -induced release (CICR).12 Since the diffusion of requires a concentration gradient, should be less than . This is examined in the next two figures. The reason for the apparently strong Fluo-4 fluorescence in the nuclear region despite its lower concentration is that Fluo-4 excited at gives a stronger fluorescence in the cell nucleus than in the cytoplasm.13 Next, a second group of cells (20 cells) was irradiated by the fs laser for at the nucleus (guided by the white light image), and the corresponding Fluo-4 fluorescence is shown in Fig. 1. It is seen that the fs pulses also locally triggered a slow release of in the nucleus, which gradually diffused out into the cytoplasm. The diffused , on reaching the store in the cytoplasm, could cause it to release more . These results thus confirm that is stored in both the cytoplasm and the nucleus, which can diffuse into the nucleus and the cytoplasm, respectively, once its host store is released, and can influence the local store at the migrated site. A third group of cells was incubated in -free HEPES buffer [concentration in mM: 1 EGTA, 140 NaCl, 5 KCl, 1 , 10 glucose, 10 -2-hydroxyethylpiperazine- -2-ethanesulphonic acid (HEPES), final pH 7.2]. Femtosecond laser treatment was performed on the cells, after which the cell medium was replaced by PBS, and then ionomycin was added to work as ionophores by which can diffuse through cell membranes. Since the free concentration in PBS is around , while it is only inside cells, an extracellular influx into cells would occur. The maximum fluorescence intensity of (Fmax) can thereby be obtained for data normalization, where all fluorescence intensity is normalized as . As shown in Fig. 2 , the cells that were first exposed for at the cytoplasm, labeled cell 1 (5 cells), would release slowly which then started to decay after approximately . After , the cells were exposed again for , but at the nucleus. A slow rise in level was also observed, but the increase was much smaller. The measured fluorescence intensity versus time is shown in Fig. 2 cell 1, standard deviation less than 17% of the value at each point). To ensure that the low-intensity second peak in the curve is not caused by the depletion of store or fluorescence bleaching after the first laser exposure, another batch of cells from the same group, labeled cell 2, was irradiated by the fs laser first at the nucleus and then at the cytoplasm following the same protocol. The fluorescence scans of a typical cell 2 are shown in Fig. 2. Its intensity versus time plot is shown in Fig. 2 cell 2, standard deviation less than 20% of the value at each point). The rise due to the exposure at the cytoplasm is observed to be much higher than that caused by the exposure at the nucleus in both cells 1 and 2. Since there was no external source of , it can be concluded that the release of is much higher in the cytoplasm than in the nucleus. Consequently, one can infer that the store in the cytoplasm is larger than that in the nucleus. Fig. 2Typical fluorescence intensity of cells after exposure by the fs beam for at at the sample in -free buffer. (a) Cells were exposed at cytoplasm first and then at nucleus [cell 1 in (c)]. (b) Cells were exposed at nucleus first and then at cytoplasm [cell 2 in (c)]. Fmax is the maximum fluorescence resulting from ionomycin treatment (Fmax). Red cross: fs laser focusing spot. Bar is . Fluorescence intensity versus time from the whole cell (c), from cytoplasm and nucleus separately in (d) cell 1 and (e) cell 2 . Black arrow: irradiation instant. Blue arrow: irradiation at cytoplasm. Purple arrow: irradiation at nucleus. (Color online only.) 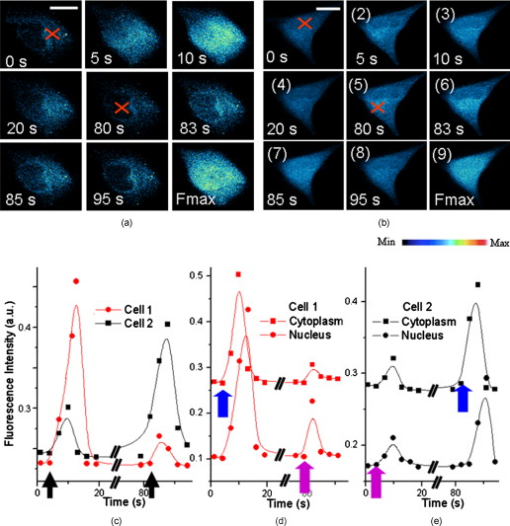 The fluorescence intensities from the nucleus and cytoplasm regions were separately extracted from the scans and plotted as shown in Figs. 2 and 2 for cell 1 and 2, respectively. It is interesting to note that when the exposure was at the cytoplasm, there existed a delay of between the peak from the cytoplasm and that from the nucleus. On the contrary, there was no delay between the two peaks when the exposure was at the nucleus. Naturally, the observed delay is caused by the diffusion of from the cytoplasm to the nucleus, while the nonexistence of delay in the reverse direction may be due to a much faster diffusion mechanism from the nucleus to the cytoplasm. The asymmetric diffusion rates can arise from the regulation of proteins in the nuclear membrane acting as nuclear pore complexes.14 Next, we investigated the contribution of extracellular in the response elicited by the fs laser irradiation with the help of a channel blocker, nifedipine, which can block all channels in the membrane. At first, two groups of cells, labeled cells 3 and 4 (five cells in each group), were incubated in PBS and exposed by the fs beam for at the cytoplasm and the nucleus, respectively. According to the reported mechanism, the fs laser would release from the intracellular store and open up the channels in the cell membrane to let the extracellular diffuse into cells. This rise subsequently generated more by CICR, which would be taken up by mitochondria slowly. Then, nifedipine was added to the medium after , and the cells were incubated for another to block all channels in the cell membrane. The cells were subsequently irradiated by the fs laser for a second time at the same location, but this time the extracellular could not diffuse into the cells. In the corresponding control groups, the cells were just exposed twice in PBS without any nifedipine addition as a reference. As in previous experiments, ionomycin was added at the end of the measurements to all cells to get the maximum fluorescence intensity of , Fmax, for data normalization. The scans of a typical cell 3 (irradiated at the cytoplasm) and cell 4 (irradiated at the cell nucleus) are respectively shown in Figs. 3 and 3 . Their corresponding normalized fluorescence intensities are plotted in Figs. 3 and 3, respectively, where two peaks corresponding to the double exposures in each group are clearly seen (standard deviation less than 19% of the value at each point). By comparing the second peaks with the first ones in the control, there was obviously no bleaching effect on the fluorescence after the second laser exposure. Cells 3 and 4 behaved nearly the same and just like their control in the first irradiation event. It should be noted that the fluorescence peaks of cells 3 and 4 after the first irradiation event are around 70% of Fmax from Figs. 3 and 3. This is probably due to the balance achieved between the release triggered by the laser and amplified by CICR, and the uptake by mitochondria. The laser irradiation has been proven to be safe to the mitochondria,15 so that they can still take up the extra to protect the cells. On the other hand, ionomycin treatment is quite different, as the high concentration of in the media freely diffusing into cells will depolarize all mitochondria and cause their dysfunction irreversibly. Consequently, even though the stores in the cytoplasm and nucleus are different, the intensities of the first fluorescence peaks from both groups of cells are almost the same at around 70% of Fmax. Fig. 3Typical fluorescence scans of Fluo-4 by confocal microscope: (a) cell 3 and (b) cell 4. Fmax is the maximum fluorescence obtained after ionomycin treatment. Red cross: fs laser focusing spot. Bar is . Normalized fluorescence intensity versus time is shown in (c) for cell 3 and (d) for cell 4 . Blue arrow in (c) and (d): irradiation instant of cells 3 and 4. (Color online only.) 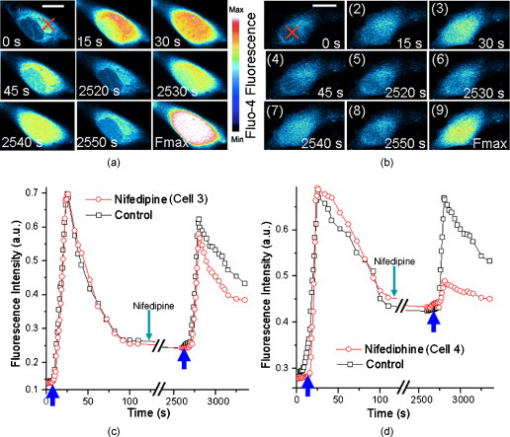 After the addition of nifedipine, the fluorescence peak of in cell 3 is lower than that of the first peak by about 0.1-fold of Fmax. This reduction should correlate with the absence of influx of extracellular . On the other hand, cell 4 exhibits a second peak lower than that of the first peak by about 0.2-fold of Fmax, as shown in Fig. 3. This amount of reduction should also reflect the contribution due to extracellular . By comparing these two peaks, it can be inferred that the store in the nucleus is notably smaller than that in the cytoplasm. The fluorescence signals coming from the cytoplasm or nucleus were then separately extracted from cells 3 and 4, and they are very similar to the corresponding signals from the whole cells (data now shown). The results also exhibit the delay between the peak signals from the cytoplasm and from the nucleus when the diffusion is from the cytoplasm to the nucleus. It should be noted that apart from the nucleus and cytoplasm, the nucleoplasmic reticulum developed from the nuclear membrane in the nucleus can also be a store of .16 However, this structure is developed mainly during apoptosis, and the recorded there was found to be fluctuating in our previous work,17 because the high concentration could induce mitochondria migration to uptake the . Therefore, our present study for live cells only focused on the generation of in the nucleus and the cytoplasm. In conclusion, it was found that a controlled dose of fs laser irradiation at can induce a slow wave in HeLa cells. The increase arose from stores in both the nucleus and cytoplasm, with the cytoplasm being the major store. Extracellular was also verified to contribute to the rise, whose contribution was more significant when the irradiation was focused on the nucleus. The diffusion patterns were experimentally recorded for excitation locations in both the cytoplasm and nucleus. A time delay of was observed when the excitation was on the cytoplasm between the peak signals measured at the cytoplasm, and at the nucleus due to the finite diffusion time from cytoplasm to nucleus. By contrast, no such delay is observed when diffused from the nucleus to the cytoplasm, presumably at a much faster rate. Hence, our technique provides a means for the slow and precise release of at the subcellular level. The subfemtoliter focus spot, laser power, duration of irradiation, and instant of irradiation can all be finely controlled, resulting in triggers of high spatial and temporal resolution. Last but not least, the technique is noncontact, noninvasive, free of any chemical compounds, and safe to cells, so that the dynamics of cells in live condition can be obtained. AcknowledgmentThis work was supported in part by The Research Grants Council of HKSAR Government under Grant Nos. CUHK410708 and 410809. ReferencesM. J. Berridge,
M. D. Bootman, and
P. Lipp,
“Calcium-a life and death signal,”
Nature, 395 645
–648
(1998). https://doi.org/10.1038/27094 0028-0836 Google Scholar
K. F. Ferri and
G. Kroemer,
“Organelle-specific initiation of cell death pathways,”
Nat. Cell Biol., 3 E255
–E265
(2001). https://doi.org/10.1038/ncb1101-e255 1465-7392 Google Scholar
M. J. Berridge,
P. Lipp, and
M. D. Bootman,
“The versatility and universality of calcium signaling,”
Nat. Rev. Mol. Cell Biol., 1 11
–21
(2000). https://doi.org/10.1038/35036035 1471-0072 Google Scholar
L. Scorrano,
S. A. Oakes,
J. T. Opferman,
E. H. Cheng,
M. D. Sorcinelli,
T. Pozzan, and
S. J. Korsmeyer,
“BAX and BAK regulation of endoplasmic reticulum : a control point for apoptosis,”
Science, 300 135
–139
(2003). https://doi.org/10.1126/science.1081208 0036-8075 Google Scholar
A. N. Malviya,
P. Rogue, and
G. Vincendon,
“Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus,”
Proc. Natl. Acad. Sci. U.S.A., 87 9270
–9274
(1990). https://doi.org/10.1073/pnas.87.23.9270 0027-8424 Google Scholar
N. L. Allbritton,
E. Oancea,
M. A. Kuhn, and
T. Meyer,
“Source of nuclear calcium signals,”
Proc. Natl. Acad. Sci. U.S.A., 91 12458
–12462
(1994). https://doi.org/10.1073/pnas.91.26.12458 0027-8424 Google Scholar
M. T. Alonso,
C. Villalobos,
P. Chamero,
J. Alvarez, and
J. García-Sancho,
“Calcium microdomains in mitochondria and nucleus,”
Cell Calcium, 40 513
–525
(2006). https://doi.org/10.1016/j.ceca.2006.08.013 0143-4160 Google Scholar
N. I. Smith,
K. Fujita,
T. Kaneko,
K. Katoh,
O. Nakamura,
S. Kawata, and
T. Takamatsu,
“Generation of calcium waves in living cells by pulsed-laser-induced photodisruption,”
Appl. Phys. Lett., 79 1208
–1210
(2001). https://doi.org/10.1063/1.1397255 0003-6951 Google Scholar
S. Iwanaga,
T. Kaneko,
K. Fujita,
N. Smith,
O. Nakamura,
T. Takamatsu, and
S. Kawata,
“Location-dependent photogeneration of calcium waves in HeLa cells,”
Cell Biochem. Biophys., 45 167
–176
(2006). https://doi.org/10.1385/CBB:45:2:167 1085-9195 Google Scholar
Y. Zhao,
X. Liu,
X. Lv,
W. Zhou,
Q. Luo, and
S. Zeng,
“Photostimulation of astrocytes with femtosecond laser pulses,”
Opt. Express, 17 1291
–1298
(2009). https://doi.org/10.1364/OE.17.001291 1094-4087 Google Scholar
M. C. Wei,
W. X. Zong,
E. H. Y. Cheng,
T. Lindsten,
V. Panoutsakopoulou,
A. J. Ross,
K. A. Roth,
G. R. MacGregor,
C. B. Thompson, and
S. J. Korsmeyer,
“Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death,”
Science, 292 727
–730
(2001). https://doi.org/10.1126/science.1059108 0036-8075 Google Scholar
J. W. Deitmer,
A. Verkhratsky, and
C. Lohr,
“Calcium signalling in glial cells,”
Cell Calcium, 24 405
–416
(1998). https://doi.org/10.1016/S0143-4160(98)90063-X 0143-4160 Google Scholar
K. R. Gee,
K. A. Brown,
W. N. Chen,
S. J. Bishop,
D. Gray, and
I. Johnson,
“Chemical and physiological characterization of fluo-4 Ca -indicator dyes,”
Cell Calcium, 27 97
–106
(2000). https://doi.org/10.1054/ceca.1999.0095 0143-4160 Google Scholar
M. D. Bootman,
C. Fearnley,
I. Smyrnias,
F. MacDonald, and
H. Llewelyn Roderick,
“An update on nuclear calcium signaling,”
J. Cell. Sci., 122 2337
–2350
(2009). https://doi.org/10.1242/jcs.028100 0021-9533 Google Scholar
H. He,
K. T. Chan,
S. K. Kong, and
R. K. Y. Lee,
“Mechanism of oxidative stress generation in cells by localized near-infrared femtosecond laser excitation,”
Appl. Phys. Lett., 95 233702
(2009). https://doi.org/10.1063/1.3273373 0003-6951 Google Scholar
W. Echevarria,
M. F. Leite,
M. T. Guerra,
W. R. Zipfel, and
M. H. Nathanson,
“Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum,”
Nat. Cell Biol., 5 440
–446
(2003). https://doi.org/10.1038/ncb980 1465-7392 Google Scholar
H. He,
S. K. Kong, and
K. T. Chan,
“Role of nuclear tubule on the apoptosis of HeLa cells induced by femtosecond laser,”
Appl. Phys. Lett., 96 223701
(2010). https://doi.org/10.1063/1.3447365 0003-6951 Google Scholar
|

