|
|
|
Molecular imaging combines molecular biology and medical imaging, allowing the visualization of cellular processes in living subjects at the molecular level. Reporter gene/reporter probe technology is a powerful molecular imaging technique that provides a generalizable method for noninvasive imaging of protein expression, protein function, and protein-protein interaction.1 Reporter gene imaging has been widely used in biomedical research to address many fundamental biological problems, including monitoring the progress of cancers, screening drugs, monitoring gene therapy, and tracking the fate of cells.2 Many reporter gene/reporter probe systems have been coupled with different imaging modalities over the past two decades. For reporter gene optical bioluminescence imaging (BLI), luciferase as a reporter gene with D-luciferin or coelenterazine as the substrate has been widely used.2 Because of the extremely low background, this imaging system can monitor luminescence light with high sensitivity. However, the light produced from the enzyme/substrate reaction usually has a peak less than 600 nm and thus displays poor tissue penetration.3 For nuclear imaging, a well-established radionuclide-based imaging reporter gene/reporter probe system is the herpes simplex virus type-1 thymidine kinase (HSV1-tk) enzyme, and radio-labeled uracil nucleoside or acycloguanosine derivatives such as 9-(4-18F-fluoro-3-[hydroxymethyl] butyl)guanine ([18F]FHBG), or 2′-[18F]fluoro-5-ethyl-1-β-D-arabinofuranosyluracil ([18F]FEAU) as the reporter probe.4, 5 Another is the sodium iodide symporter and the reporter probe radioiodine or 99mTc-pertechnetate.6, 7 In using these approaches, several disadvantages arise, namely that nuclear reporter gene systems require positron emission tomography (PET) or single photon emission computed tomography (SPECT), which are expensive and may not be easily accessible to many researchers. Recently, we and others have found that a variety of radioactive materials (β+ and β− emitters) could be detected by OI techniques. It has been successfully demonstrated that radioactive molecular probes such as 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG), Na18F, Na131I, 90YCl3, and 90Y labeled tumor targeting peptides could be noninvasively imaged in small animals by optical imaging instruments. This is mainly attributed to the ability of radioactive materials to produce low energy visible photons (1.2 to 3.1 eV, 400 to 1000 nm) associated with Cerenkov or Bremsstrahlung radiation.8, 9, 10, 11 In this research study, we further evaluated the feasibility of using OI to monitor the nuclear reporter gene/reporter probe systems. HSV1-tk and [18F]FHBG was thus chosen as a model system in this study. The C6 rat glioma cell line stably transfected with HSV-sr39tk plasmids (C6-tk) (12) was used, and the in-vitro [18F]FHBG uptakes in C6-tk cells were measured by both an IVIS optical imaging system (Caliper, Hopkinton, Massachusetts) and a gamma counter (Perkin Elmer, Waltham, Massachusetts). Finally, we performed in-vivo imaging studies of the reporter gene/reporter probe using both OI and PET, followed by a biodistribution study. The in-vitro [18F]FHBG cell uptake study was performed as previously described with minor modifications.12 Briefly, C6-tk cells were seeded into 12-well plates at a density of 5×105 cells per well 12 h prior to the experiment. The C6 cell line without transfection was used as a negative control. The cells were then incubated with [18F]FHBG (∼107 kBq/well, 3 μCi/well) at 37°C for 15, 30, 60, and 120 min. Tumor cells were washed three times with chilled phosphate buffered saline (PBS) and harvested with 0.25% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA) (Invitrogen, Carlsbad, California). The cell suspensions were transferred to a 96-well flat bottom black plate (Nunc) and imaged by an IVIS 200 system (Caliper). Luminescent images were taken without a filter [see Fig. 1]. The exposure time was 5 min. Then, the radioactivity of the cell suspension was measured in a gamma counter (Packard, Meriden, Connecticut). The in-vitro cell uptake results were expressed as the percentage of the applied activity with decay correction [see Fig. 1]. Experiments were performed twice with triplicate samples for each time point. Both OI and gamma counting show a rapid and high uptake of [18F]FHBG in C6-tk cells, whereas nontransfected C6 cells show very low cell uptake [see Figs. 1 and 1]. More importantly, an excellent correlation has been obtained between OI and gamma counting results [see Fig. 1, r 2 > 0.95]. These findings lay the foundation for further in vivo studies to validate the feasibility of using OI for monitoring the nuclear reporter gene system. Fig. 1In-vitro cell imaging (a), (b), and (c) and sensitivity comparison studies of OI and PET (d). Shown are (a) C6 cell uptakes monitored by OI; (b) quantification and (c) correlation of C6 cell uptakes from both OI and gamma counting (GC) results; and (d) different radioactivity monitored by OI and PET [OI: 0.96, 0.60, 0.24 and 0.12 μCi (35.48, 22.18, 8.88 and 4.44 kBq); PET: 0.10, 0.06, 0.02 and 0.01 μCi (3.55, 2.22, 0.89 and 0.44 kBq)] (n = 4). 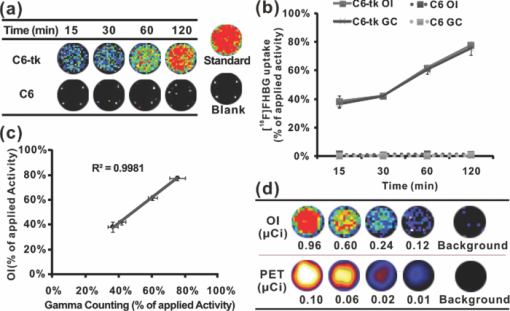 Prior to in-vivo imaging, comparison studies were performed to evaluate the detection sensitivity of the IVIS 200 OI system (Caliper) and the small animal PET (microPET R4 rodent model scanner, Siemens Medical Solutions USA, Washington, D.C.). Serial samples containing different amounts of radioactivity (Na18F) were prepared in 300 μl of water (ranged from 0.01 to 1 μCi) and imaged by OI and PET [see Fig. 1]. A 5-min imaging time was applied to both modalities. For PET, signal-to-noise ratio (SNR) of 32.1 is obtained from 0.01 μCi (0.44 kBq) of 18F, while OI can detect 0.1 μCi (4.4 kBq) of 18F with a SNR of 1.7. It was found that 18F samples lower than 0.1 μCi could hardly be differentiated from background in OI images. PET excels OI in both sensitivity and imaging contrast. The difference in sensitivity between the two modalities could be attributed to the weak photon production of radionuclides and thus low signal intensities from 18F in OI. Although less sensitive than PET, OI demonstrates promising detection capability and warrants further in-vivo experiments. All animal studies were carried out in compliance with federal and local institutional rules for the conduct of animal experimentation. Mice bearing two tumors (C6-tk and C6) were then used for in-vivo studies. The C6-tk (left) and C6 (right) cells were injected contralaterally in the dorsal shoulder region of each mouse (2×106 cells/mouse; athymic nude mice were purchased from Charles River Laboratories, Wilmington, Massachusetts, n = 6). Approximately 10 days later, when tumor size reached about 1 to 1.5 cm in diameter, small animal PET and OI were performed using the mice and the PET probe [18F]FHBG. Images at 1 and 2 h were obtained by both OI and PET. Results are shown by average radiance (photon/second/cm2/str) for OI and percentage of injected dose per gram (%ID/g) for PET based on the method previously described.13 At 1 h after tail vein injection of [18F]FHBG (10 to 11 MBq, 270 to 300 μCi), C6-tk tumors can be clearly delineated using OI, while there is minimum tracer uptakes in the control C6 tumors (see Fig. 2). The strong signals in the lower part of the mouse body [Fig. 2] are due to the presence of the radioactivity in the bladder. PET imaging shows a similar pattern of tracer distribution [Fig. 2]. At 2-h postinjection, with the elimination of [18F]FHBG, only C6-tk tumors can be clearly visualized in both imaging modalities (see Fig. 2). Figure 2 shows the statistical analysis of tumor to normal tissue (T/N) ratio for both imaging modalities at 1- and 2-h postinjection. Compared to OI, PET displays higher T/N ratios (OI: 2.6 ± 0.5 at 1 h, 5.1 ± 1.1 at 2 h; PET: 43.2 ± 8.4 at 1 h, 143.0 ± 14.8 at 2 h). Fig. 2In-vivo imaging using OI and PET. Mice bearing C6-tk and C6 tumors can be imaged by both (a) OI and (b) PET. (c) Statistical analysis was performed for both modalities (n = 6). 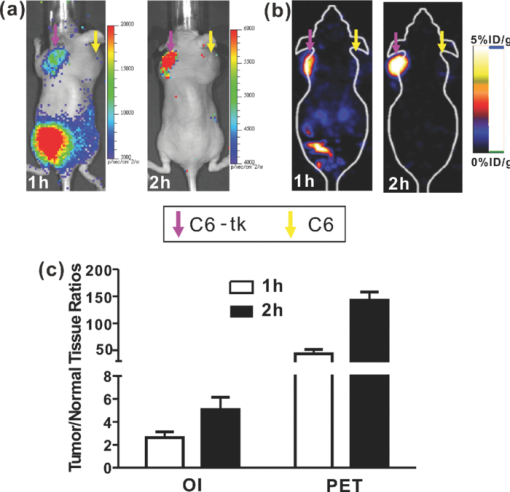 To further compare the imaging results obtained from OI and PET, biodistribution studies were conducted. Mice were sacrificed and different organs were collected at 1-h postinjection of [18F]FHBG. OI images were obtained for these radioactive organs, and quantification analysis of optical images was performed using Living Image software version 3.1 [see Fig. 3]. Meanwhile, the radioactivity of the organs was also measured using a gamma counter after weighing, and the radioactivity uptake in the tumor and normal tissues was calculated and expressed as a percentage of the injected radioactive dose per gram of tissue (%ID/g) [see Fig. 3]. For optical imaging of the organs, the C6-tk tumor, the kidney, and the intestine were clearly visible at 1-h postinjection. Quantification analysis also revealed that they were the organs with the highest optical signals, while all the other organs displayed minimum light intensities [see Fig. 3]. It was also found that biodistribution patterns obtained through these two approaches were consistent [see Figs. 3 and 3]. Compared with the intestines, much higher signals from the kidney are observed, which indicates that the clearance route of [18F]FHBG is mainly through urinary excretion. Fig. 3Biodistribution studies measured by (a) OI or (b) gamma counting at 1-h postinjection of [18F]FHBG (n=6). 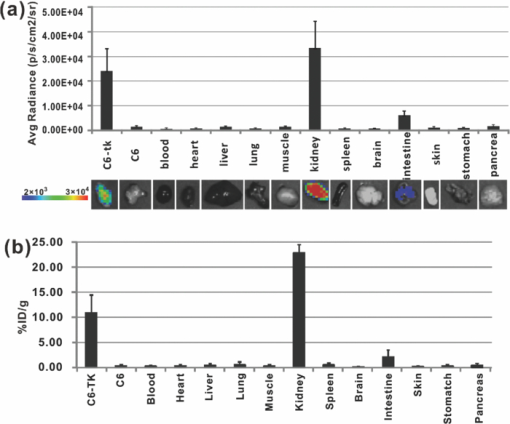 The ratio of kidney to tumor uptake of [18F]FHBG at 1 h was also calculated to be 2.3 versus 1.4, for gamma counting versus OI quantification, respectively. This discrepancy could mainly originate from the diverse optical properties of different tissues, which ultimately affects light imaging and quantification. Compared with the C6-tk tumor, the kidney contains more hemoglobin that absorbs more optical photons. Moreover, unlike high energy gamma rays, the low energy photons have very weak tissue penetrability. Light sources deeper in the tissue contribute less for the total optical signals than those closer to the surface. Therefore, the kidney/tumor ratio measured by OI is lower than that obtained by gamma counting. Overall, PET has a better quantitative capability than OI. However, radioactive OI can still serve as a qualitative or semiquantitative research tool for radioactive reporter probe studies. The radioactive OI signals also have a uniquely wide wavelength of emission spectrum.9 The emissions in the near-infrared range are especially favorable for OI in living subjects. In comparison with current reporter gene imaging using BLI probes, radioactive reporter probes may offer a better option for 3-D OI reconstruction. In conclusion, the low energy photons produced by [18F]FHBG radiation can be easily imaged by OI instruments both in vitro and in living small animals. This work for the first time demonstrates the feasibility of using OI as an alternative tool for monitoring reporter gene expression with radioactive probes. Considering the wide availability of OI instruments and many radioactive reporter probes such as [18F]FHBG and radioiodine, OI with radioactive reporter probes will facilitate and broaden the applications of reporter gene/reporter probe techniques in medical research. AcknowledgmentWe acknowledge support from the National Cancer Institute (NCI) R21 CA121842 (to Cheng) and a China Scholarship Council fellowship (to Liu). ReferencesJ. J. Min and
S. S. Gambhir,
“Molecular imaging of PET reporter gene expression,”
Hand. Exp. Pharmacol., 185 277
–303
(2008). https://doi.org/10.1007/978-3-540-77496-9_12 Google Scholar
T. F. Massoud and
S. S. Gambhir,
“Molecular imaging in living subjects: seeing fundamental biological processes in a new light,”
Genes Dev., 17
(5), 545
–580
(2003). https://doi.org/10.1101/gad.1047403 Google Scholar
J. W. Hastings,
“Chemistries and colors of bioluminescent reactions: a review,”
Gene, 173
(1), 5
–11
(1996). https://doi.org/10.1016/0378-1119(95)00676-1 Google Scholar
J. G. Tjuvajev,
A. Joshi,
J. Callegari,
L. Lindsley,
R. Joshi,
J. Balatoni,
R. Finn,
S. M. Larson,
M. Sadelain, and
R. G. Blasberg,
“A general approach to the non-invasive imaging of transgenes using cis-linked herpes simplex virus thymidine kinase,”
Neoplasia, 1
(4), 315
–320
(1999). https://doi.org/10.1038/sj.neo.7900053 Google Scholar
S. S. Gambhir,
J. R. Barrio,
M. E. Phelps,
M. Iyer,
M. Namavari,
N. Satyamurthy,
L. Wu,
L. A. Green,
E. Bauer,
D. C. MacLaren,
K. Nguyen,
A. J. Berk,
S. R. Cherry, and
H. R. Herschman,
“Imaging adenoviral-directed reporter gene expression in living animals with positron emission tomography,”
Proc. Natl. Acad. Sci. USA, 96
(5), 2333
–2338
(1999). https://doi.org/10.1073/pnas.96.5.2333 Google Scholar
T. Groot-Wassink,
E. O. Aboagye,
M. Glaser,
N. R. Lemoine, and
G. Vassaux,
“Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene,”
Hum. Gene Ther., 13
(14), 1723
–1735
(2002). https://doi.org/10.1089/104303402760293565 Google Scholar
A. Merron,
I. Peerlinck,
P. Martin-Duque,
J. Burnet,
M. Quintanilla,
S. Mather,
M. Hingorani,
K. Harrington,
R. Iggo, and
G. Vassaux,
“SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene,”
Gene Ther., 14
(24), 1731
–1738
(2007). https://doi.org/10.1038/sj.gt.3303043 Google Scholar
R. Robertson,
M. S. Germanos,
C. Li,
G. S. Mitchell,
S. R. Cherry, and
M. D. Silva,
“Optical imaging of Cerenkov light generation from positron-emitting radiotracers,”
Phys. Med. Biol., 54
(16), N355
–365
(2009). https://doi.org/10.1088/0031-9155/54/16/N01 Google Scholar
H. Liu,
G. Ren,
Z. Miao,
X. Zhang,
X. Tang,
P. Han,
S. S. Gambhir, and
Z. Cheng,
“Molecular optical imaging with radioactive probes,”
PLoS ONE, 5
(3), e9470
(2010). https://doi.org/10.1371/journal.pone.0009470 Google Scholar
A. E. Spinelli,
D. D'Ambrosio,
L. Calderan,
M. Marengo,
A. Sbarbati, and
F. Boschi,
“Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers,”
Phys. Med. Biol., 55
(2), 483
–495
(2010). https://doi.org/10.1088/0031-9155/55/2/010 Google Scholar
A. Ruggiero,
J. P. Holland,
J. S. Lewis, and
J. Grimm,
“Cerenkov luminescence imaging of medical isotopes,”
J. Nucl. Med., 51
(7), 1123
–1130
(2010). https://doi.org/10.2967/jnumed.110.076521 Google Scholar
S. S. Gambhir,
E. Bauer,
M. E. Black,
Q. Liang,
M. S. Kokoris,
J. R. Barrio,
M. Iyer,
M. Namavari,
M. E. Phelps, and
H. R. Herschman,
“A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography,”
Proc. Natl. Acad. Sci. U S A, 97
(6), 2785
–2790
(2000). https://doi.org/10.1073/pnas.97.6.2785 Google Scholar
Z. Cheng,
O. P. De, M. Namavari,
A. De,
J. Levi,
J. M. Webster,
R. Zhang,
B. Lee,
F. A. Syud, and
S. S. Gambhir,
“Small-animal PET imaging of human epidermal growth factor receptor type 2 expression with site-specific 18F-labeled protein scaffold molecules,”
J. Nucl. Med., 49
(5), 804
–813
(2008). https://doi.org/10.2967/jnumed.107.047381 Google Scholar
|

