|
|
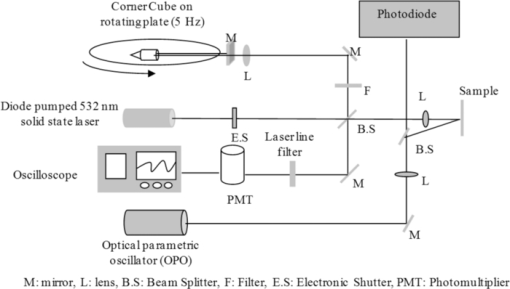
1.IntroductionAblative thermal therapy involves heating a target tissue to temperatures between 50 and 90°C to create in-situ coagulation necrosis and cause cell death. This method is considered an effective minimally invasive method to treat solid tumors. Different techniques such as radio-frequency radiation,1 high intensity focused ultrasound (HIFU),2 or interstitial laser sources3 are developed for delivering the thermal energy needed for ablative thermal treatment to a targeted region. An essential part of any successful thermal therapy is a monitoring system, allowing a more accurate assessment of the heated area and to minimize damage to the healthy tissues surrounding the target. Modalities including magnetic resonance temperature imaging,4 optical radiance/fluence probes,5 and ultrasound imaging6 have been investigated for providing such monitoring systems. These methods face challenges such as their cost and patient selectiveness (MRI), imaging depth (IR and optical thermal probes), and lack of reliable contrast between lesion and normal tissues (ultrasound imaging). Optoacoustic imaging (OA) is a new promising modality that recently has been developed and suggested for use in many areas of biomedicine from imaging of breast cancers,7 functional and structural imaging of brain,8 monitoring of oxygenation to imaging of neovasculation,9 and determining blood vessel diameters.10 It relies on ultrasonic detection of thermoelastic pressure transients11 induced in a target after its irradiation by short laser pulses. Optoacoustic imaging combines the high resolution of ultrasonic imaging with the high tissue contrast of optical imaging techniques. Several studies have shown that the shape and amplitude of optoacoustic signals depend on the optical and thermomechanical properties of the illuminated target. Since thermally induced tissue damage can lead to both changes in optical12, 13 and mechanical14 properties, optoacoustic techniques are suggested for monitoring thermal therapies. Optoacoustic techniques have been successfully applied to measure tissue temperature during heating and cooling of canine liver,15 for monitoring tissue coagulation during thermal therapies,16, 17 and to assess retinal temperature during its treatment by laser irradiation.18 The observed changes in the optoacoustic signals from thermally treated tissues are so far mainly attributed to the changes in their optical properties.16, 19 While the changes in the optical properties of thermally treated tissues have been previously studied, there is a paucity of studies that examine the role of changes in the thermal and mechanical properties of tissues and their effect on the optoacoustic signals. In this work, the dynamics of thermoelastic expansion for native and coagulated ex-vivo bovine liver after short pulse laser illumination were studied using an interferometric technique. In the technique proposed, thermomechanical and optical properties of samples were simultaneously assessed at the same sample location. 2.Theoretical DescriptionA short laser pulse that is absorbed by a target creates a small temperature rise. For the short time scales involved in a typical optoacoustic pulsing protocol (typically a few nanoseconds), one can assume that the resulting temperature distribution and the absorbed light distribution are spatially coincident. For the case of a turbid medium like biological tissues or tissue mimicking phantoms, this distribution can be approximated by an axial symmetric function with an exponentially decaying axial profile and a Gaussian radial profile: Eq. 1[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} T(r,z) = T_0 \exp (- r^2/ w^2) \exp(-z/ D)\enspace\,{\rm and}\enspace\, T_0=\frac {\bm{\Phi}\mu_a}{\rho C_V}, \end{equation}\end{document}Eq. 2[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} D = [3\mu _a (\mu _a + \mu '_ s)]^{-1/2}, \end{equation}\end{document}Eq. 3[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{eqnarray} \rho \frac{{\partial ^2 {\bf u}}}{{\partial t}} &-& \frac{E}{{2(1 + \sigma)}}\nabla ^2 {\bf u} - \frac{E}{{2(1 + \sigma)(1 + 2\sigma)}}\nabla (\nabla.{\bf u}) \nonumber\\ &&= \frac{{ - E\beta }}{{3(1 - 2\sigma)}}\nabla T(r,z), \end{eqnarray}\end{document}Assuming an initial temperature distribution in the form of Eq. 1, it is possible to find an analytical expression for the equilibrium surface displacement on the z axis during the quasi-steady-state time regime20: Eq. 4[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} u_z = \frac{{2(1 + \sigma)}}{3}\frac{\Gamma}{{\rho c^2_L }}g_0 (R){\bm \Phi}, \end{equation}\end{document}Eq. 5[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} c_L^2 = \frac{{E(1 - \sigma)}}{{\rho (1 + \sigma)(1 - 2\sigma)}}\,. \end{equation}\end{document}3.Materials and Methods3.1.Experimental SetupThe interferometric setup used to measure the thermoelastic expansion of tissue phantom samples has been described previously.22, 23 Laser pulses of 6.5-ns duration, 10-Hz repetition rate, and 750-nm wavelength from an optical parametric oscillator (OPO) system (Vibrant B-532, Opotek Incorporated, Carlsbad, California) were directed onto the front surface of tissue phantom samples. The final beam size on the target location was measured using a photographic sheet and had an elliptical cross section with major and minor axes of 5 and 3 mm, respectively. A part of the pump beam was directed onto a fast silicon photodiode (DET10A, 200 to 1100 nm, 1-ns rise time, Thorlabs, Newton, New Jersey) to measure the pulse energy and also to synchronize the data acquisition and the incident pump pulse. The incident angle of the pump beam onto the sample was approximately 36 deg. According to Snell's law, this yields an angle of refraction of 26 deg into the tissue based on indices of refraction of 1.00 for air and 1.40 for tissue. The thermoelastic movements of the sample's surface were measured using a modified Michelson interferometer. It is built around a cw solid-state laser (CrystaLaser GCL-025-S, CrystaLaser, Reno, Nevada) operating at a wavelength of 532 nm. The laser beam was split into two arms with one arm directed toward a corner cube mounted on a rotating table, permitting a constant modulation on the interference pattern when the target surface is not moving. This allowed the improvement of spatial and temporal resolution of the interferometry setup beyond those of a conventional Michelson interferometer, and also provided the capability of detecting the direction of the target surface movement. During and after OPO illumination, the resulting interference signals were detected by a photomultiplier (Hamamatsu H7732P-11, Hamamatsu City, Japan), amplified, and then digitized on an oscilloscope (Agilent DSO6054A, 500 MHz, Agilent, Santa Clara, California) for analysis. A schematic diagram of the experimental interferometry setup is shown in Fig. 1. 3.2.Sample PreparationSeven samples of bovine liver (each on a different day) were purchased from a local market. The samples remained sealed in their packaging and kept at 4°C to avoid gassing and slow tissue degradation. From each piece of liver, two samples of about 5 × 5 × 1 cm were prepared. One of the samples was placed in a 70°C water bath for 30 min. The heated samples were then removed and cut in smaller rectangular pieces with dimensions of approximately 1 to 2 cm. The untreated liver sample was also cut in smaller pieces with dimensions similar to the heated one. The accuracy of our interferometry technique is improved for tissue samples with a smooth, flat surface. As these two surface qualities can be affected by the heating process, smaller pieces were cut from the heated samples, yielding better surface uniformity. One piece from each sample was placed in the center of two identical aluminum cylindrical containers. Gelatin (type A, Sigma Life Science, Saint Louis, Missouri) was dissolved in hot water and cooled down to around 40°C before it was poured around the samples in the containers. The containers then were placed in a refrigerator at a temperature of 6°C to let the gelatin harden and fix the samples in place. After solidification of the gelatin, the front face of the tissue sample, which was exposed to air, was used for the interferometric experimental measurements. 3.3.Data AnalysisThe interference pattern detected by the photomultiplier was collected for a period of 10 μs, with the pump laser pulses firing 2 μs after the beginning of each acquisition frame. The acquired data were exported to Matlab (The Mathworks, Natick, Massachusetts) for analysis. Displacement of the target surface was calculated using the Hilbert transform on the interferogram.24 Signals from a silicon photodiode detecting a portion of the pump laser pulse were used to estimate energy of each laser pulse hitting the target surface. The measurements were calibrated prior to the experiments by placing an accurate laser power meter (Ophir Nova II meter and PE25-SH measuring head, Ophir Optronics Limited, Jerusalem, Israel) at the target location, and comparing the readings from it with data obtained from the silicon photodiode. The signal from the photodiode follows the laser pulse, and so its duration is much faster than the rate of change in the interference fringe pattern. Both signals were digitized and recorded on the same oscilloscope and within the same acquisition time frame of 10 μs. A low sampling rate for digitizing the two signals can lead to inaccuracy in the measurement of laser pulse energy, while a high sampling rate increases the size of data files recorded by the oscilloscope. Therefore, a moderate digitization sample rate of 500 MS/s was selected to reduce discretization of the readings for the laser pulse energies and at the same time keep the size of data files reasonable. 3.4.Numerical CalculationThe thermoelastic wave in Eq. 3 was numerically solved by using the finite-difference time domain (FDTD)25 method in a cylindrical coordinate system. To further simplify the problem, it is assumed that temperature field induced by laser pulse in the sample is axially symmetric. A staggered mesh was employed to discretize the space for the two remaining dimensions of the coordinate system (z and r), with the z axis set to be along the irradiation direction. Only half of the space along the r axis is discretized to reduce the required computational memory. The grid size is chosen to be smaller than one-tenth of the optical attenuation depth in the sample (grid size is 8.5×8.5 μm). Then, the temporal step size is determined to satisfy the Courant condition for solution stability.26 For the interface between air and the sample surface, a free boundary condition (i.e., pressure at z = 0 is zero) is applied. The axial symmetry of the problem requires that the radial component of displacement at the center of the cylinder (r = 0) also be zero. An absorbing boundary layer on the two outer edges with rigid constraints (displacement at the edges is equal to zero) is used to eliminate artificial reflections of the deformation wave from these edges.27 4.Results and Discussion4.1.Dynamics of Thermoelastic Deformation4.1.1.Optical attenuation depthTypical sample surface displacement profiles for native and coagulated ex-vivo bovine livers after their irradiation by a short laser pulse at 750 nm are shown in Fig. 2. Thermoelastic stresses are generated within the sample. In response to these stresses, the sample surface expands and reaches its maximum; the time it takes to reach this maximum is characterized by the optical attenuation depth and speed of sound in the sample. An approximation for slope of initial rise in the surface displacement curve is given by28: Eq. 6[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} u{(t)} = u_0 [1 - \exp (- c_L t/D) ], \end{equation}\end{document}Fig. 2Sample surface displacement traces obtained for coagulated and native ex-vivo liver tissue samples after their irradiation by short laser pulses at 750 nm (pulse fired at time t = 0 ns). 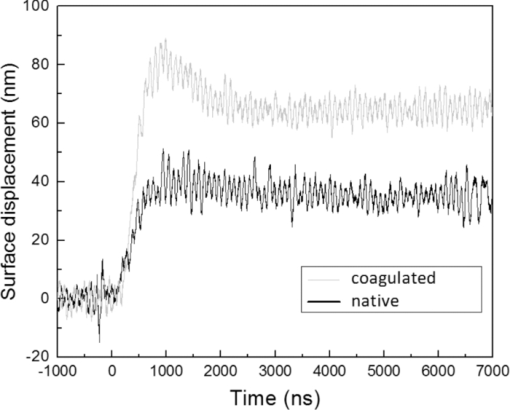 Fig. 3Measured optical attenuation depth of native and coagulated ex-vivo bovine liver samples irradiated at 750 nm (mean value ± standard error of the mean). 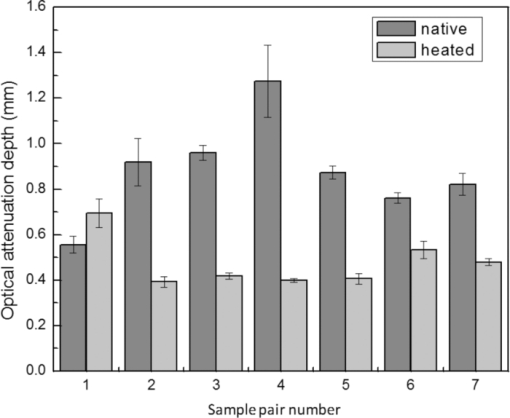 Table 1Optical attenuation depth of ex-vivo liver tissue in different species as reported in the literature.
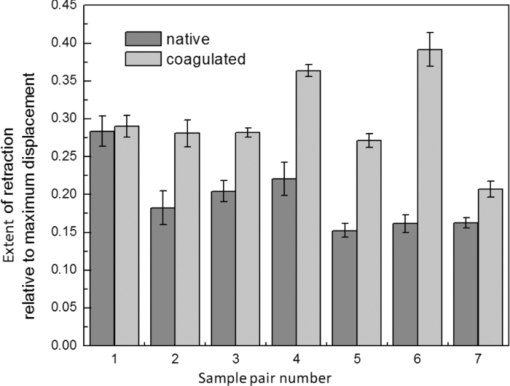
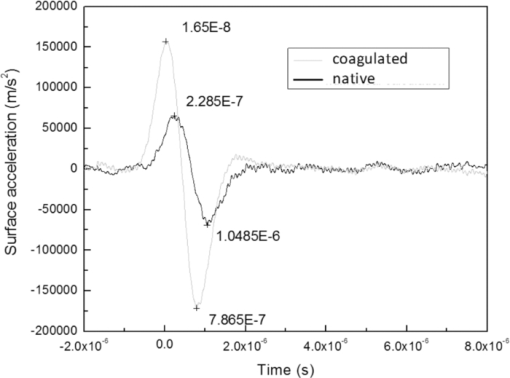
4.1.2.Grüneisen coefficientAfter an initial expansion, the sample surface may contract before reaching a new equilibrium displacement. In our experiments, prominent contractions were generally observed in the case of coagulated samples, while in the case of uncoagulated samples such contractions were considerably smaller or nonexistent (Fig. 2). In the latter case, one can assume that equilibrium and maximum displacement of the sample surface are equivalent. For assessment of the changes occurring in thermal and mechanical properties of tissue samples after coagulation, we compared the equilibrium displacement of the sample surface after its initial expansion. For each signal, the time-resolved displacement of the sample surface was smoothed by averaging over neighboring points. The resulting displacements between 4000 and 5500 ns after the firing of the laser pulse were averaged and their standard deviation was calculated. These values were considered equilibrium displacements of the sample surface. The equilibrium displacement of the sample surface was plotted against the energy of incident laser pulses to measure the Grüneisen coefficient. We considered a linear fit passing through the origin (no displacement for a laser pulse with energy of zero, see Eq. 4). An example of fitted data for a pair of native and coagulated samples is shown in Fig. 4. The correlation coefficients of the linear regressions range from 0.78 to 0.92. The results show an average of 19.6% increase in slope of the fitted lines for the coagulated samples compared to the native tissues. However, a two sample paired t-test reveals that the difference between values of slope for the native and coagulate liver tissues is significant only at P = 0.24 (t = −1.31) level. Both a decrease and increase in slope of the linear fit for the coagulated liver tissues compared to native samples are observed. A bar chart of slopes for the linear regressions is presented in Fig. 5. Since sound speeds in the native and coagulated liver samples at room temperature are similar,29 the changes of the slope cannot be explained by difference of the sound speed in the samples. The geometrical correction factor g0(R) for each sample is calculated using the mean values of the optical attenuation depth (measured as described in the previous section) and assuming a laser beam radius of 2.5 mm on the targeted tissues. These values of g 0(R) are then inserted into Eq. 4 to estimate values of the Grünesien coefficient for the native and coagulated liver samples. The result is presented as a bar chart in Fig. 6. The Grünesien coefficient for the seven native samples ranges from 0.071 to 0.16, while this parameter for the coagulated samples varies between 0.088 and 0.2. A similar variation is previously reported for values of the Grünesien coefficient for knee meniscus.23 The average value of the Grünesien coefficient for both groups of the native and coagulated liver samples is 0.12. Considering the relation of the Grüneisen coefficient with the physical properties of tissue (since [TeX:] $\Gamma = {{c_L^2 \beta } \mathord{\left/ {\vphantom {{c_L^2 \beta } {C_V }}} \right. \kern-\nulldelimiterspace} {C_V }}$ ), and using reported values of these properties for liver tissues [β = 3×10−4 K−1, C V = 3600 J.kg–1.K–1and c L = 1500 m/s],32, 33 one calculates Γ = 0.19, which is consistent with our results. However, the sensitivity of the current measurements to noise, in addition to natural variability of the biological samples (e.g., variations in fat and connective tissue content in the small liver areas irradiated) prevents detection of any significant difference between Grüneisen coefficients in the two groups of samples (t = –0.088, P = 0.93). Fig. 4Equilibrium displacement of native (empty circles) and coagulated (filled squares) liver samples versus laser pulse energy for two liver samples. Linear regressions and 95% confidence limits are given for both samples. 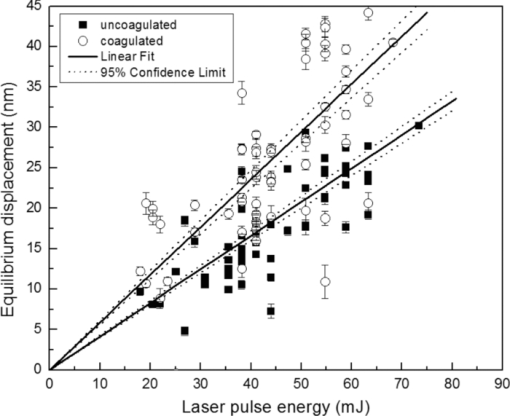 Fig. 5Slopes of linear fits for the equilibrium displacement of native and coagulated liver samples versus laser pulse energies. Error bars represent the standard deviation of the measured surface displacement. 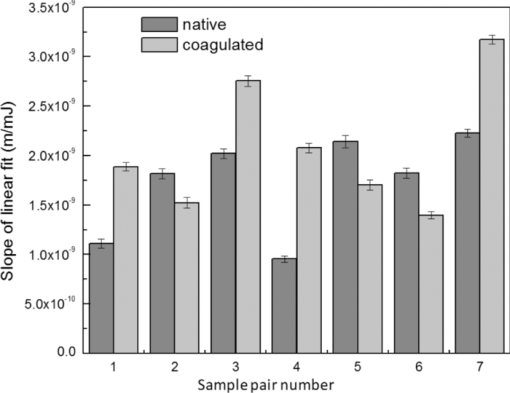 Fig. 6Estimated values of the Grüneisen coefficient for native and coagulated liver samples, using the average values of the optical attenuation depth and the slope of the linear regression to the equilibrium surface displacements, versus laser pulse energies for each sample. Error bars are propagations of the uncertainties related to the optical attenuation depths and the slope of the linear fit. 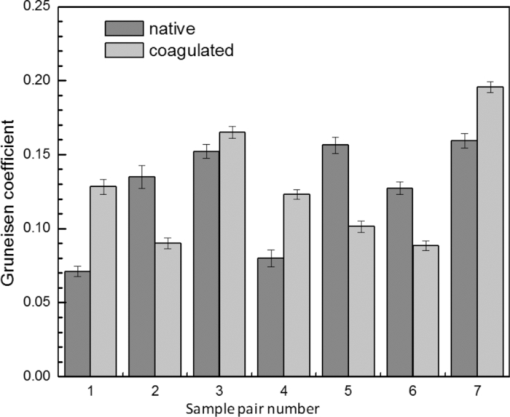 Surface displacements of tissue samples are measured along the optical axis of the sample arm in the interferometry setup. It is known that an important source of uncertainty in measuring the surface displacement is alignment of the target surface relative to this axis.44 Since soft tissues and especially liver samples are usually prone to large deformations under relatively small forces,35 environmental mechanical noise causes changes in the alignment of the tissue surface along the optical axis of the interferometer. There is also a considerable fluctuation in spatial profiles of the laser pulses, and the tissue sample in the irradiated area (47 mm2) is heterogeneous. This leads to variation of the induced heat profile in the sample when it moves because of mechanical noise. As an example, for laser pulses with the same energy, a typical maximum standard deviation of 36% for scattered measurements of the quasi-steady-state displacement was obtained. 4.1.3.Mechanical properties and elastic modulusThe dynamics of the thermoelastic expansion of the sample's surface for the two groups of native and coagulated liver tissue samples are significantly different: a more prominent retraction after the initial expansion of the tissue surface was observed in the case of coagulated liver samples. Even for the signals in which displacement in the quasi-steady-state was the same value, the coagulated sample showed a more pronounced retraction after its initial expansion compared to the native sample. Such behavior is shown in Fig. 7. To quantify this effect, a single exponential decay function in the form of: Eq. 7[TeX:] \documentclass[12pt]{minimal}\begin{document}\begin{equation} d(t) = d_0 + a \cdot\exp (- t/\tau), \end{equation}\end{document}Fig. 7Sample surface displacement traces obtained for coagulated and native ex-vivo liver tissue samples after their irradiation by short laser pulses at 750 nm. The surfaces attain a same displacement in quasi-steady-state, but the coagulated sample shows more pronounced retraction after its initial expansion. 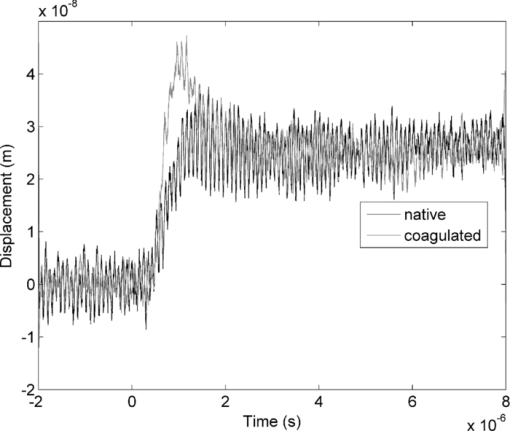 Fig. 8Characteristic surface retraction time after its initial expansion in native and coagulated liver samples. For explanation on the error bars, see Sec. 4.1.3. 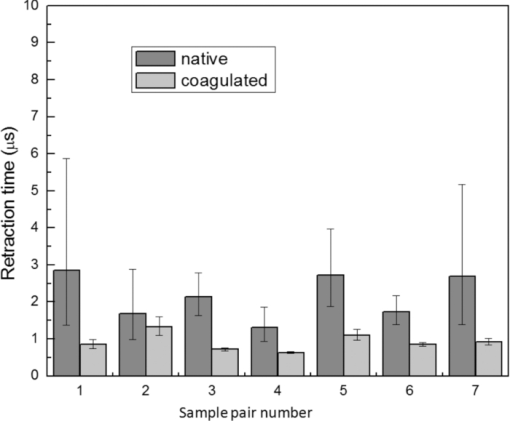 The magnitude of the surface retraction [parameter a in Eq. 7] is another parameter that can be used to demonstrate the differences in the expansion dynamics between two groups of the native and coagulated tissue samples. Relative magnitudes of sample surface retractions to the maximum displacements were calculated and their averages are shown as a bar chart in Fig. 9. Again, a significant difference (t = −3.76, P< 0.01) between the retraction magnitudes of the native and coagulated tissue surfaces is measured. The native tissue samples show an average retraction of 19% relative to their maximum displacements, while this value for the coagulated tissues on average is 30%. To relate this behavior of the tissue samples with their optical and thermomechanical properties, thermoelastic deformation of the tissue samples after their irradiation by laser pulses is studied using numerical solution of Eq. 3. 4.2.Thermoelastic Deformation of TissuesNumerical solutions of the equation of thermoelastic expansion are obtained using the method previously described in Sec. 3.4. The physical properties of tissues were varied to simulate thermoelastic deformations observed through our experiments. The numerical solutions are not used in an iterative way to find the best fit for the experimental data, since it requires excessive computation times. Parameters of Eq. 3 are set in such a way that the longitudinal speed of sound remains 1500 m/s in all cases. This requires modifying Poisson's ratio when the elastic modulus is modified [see Eq. 5]. However, the necessary variations of Poisson's ratio are limited to a very small range of values around 0.5, which is expected for mostly incompressible soft tissues.37 The geometrical properties of the laser-induced heat profile in the target can be determined by using the method previously described in Sec. 4.1.1. For the sake of simplicity, the cross section of the pump laser beam on the target was assumed circular with a radius of 2500 μm. The optical attenuation depth for the typical signals presented in Fig. 1 was calculated by fitting the initial rise to a function in the form of Eq. 6 (560 and 300 μm for the native and coagulated sample, respectively). Also, a thermal expansion coefficient of 5×10–4 K–1 for both samples was assumed. Using these values, the numerical solution of thermoelastic expansion of samples with different values of elastic modulus was computed. Then, these solutions of the surface displacement after their initial rises were fitted to a function in the form of Eq. 7. The characteristic retraction times and the extent of the surface retraction were compared to those values for the typical experimental signals shown in Fig. 2. These comparisons for the characteristic retraction time and extent of surface retraction are presented in Figs. 10 and 11, respectively. Fig. 10Characteristic surface retraction times predicted by numerical solutions of the wave equation for different values of elastic modulus. Thermal expansion coefficient is assumed to be β = 5×10−5 K−1 and the laser beam radius w = 2500 μm. The numerical solutions are computed for to two sets of parameters corresponding to two signals presented in Fig. 1 (circles): D = 560 μm, T 0 = 0.16°C, and (triangles): D = 300 μm and T 0 = 0.62°C. The obtained values for the two experimental signals are shown with black stars. 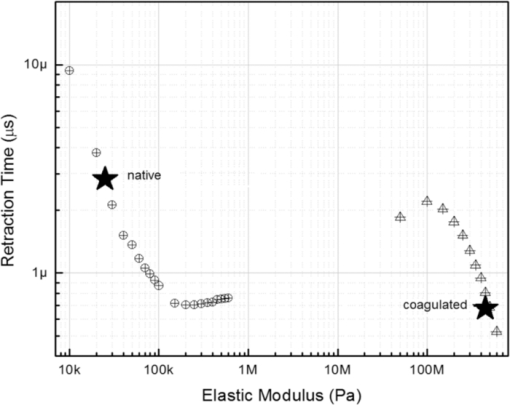 Fig. 11Extent of the surface retraction relative to its maximum displacement predicted by numerical solutions of the wave equation for different values of elastic modulus. Thermal expansion coefficient is assumed to be β = 5×10−5 K−1 and the laser beam radius w = 2500 μm. The numerical solutions are computed for two set of parameters corresponding to two signals presented in Fig. 1 (circles): D = 560 μm, T 0 = 0.16°C, and (triangles): D = 300 μm and T 0 = 0.62°C. The obtained values for the two experimental signals are shown with black stars. 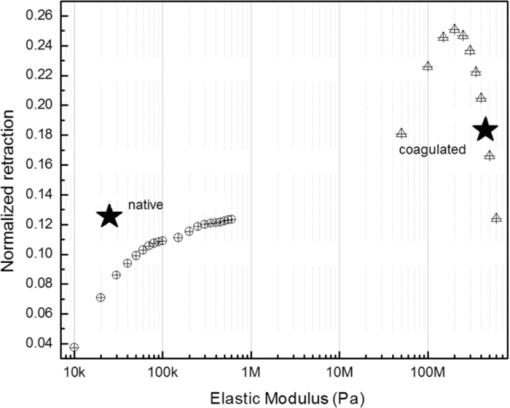 Using a value of 25 kPa for the elastic modulus and peak temperature rise [T 0 in Eq. 3] of 0.16°C, the numerical solution to the surface displacement agrees with the experimentally measured data for the native tissue. On the other hand, the experimental signal for the coagulated tissue generates a better match to the theoretical curve when assuming a value of 450 MPa for the elastic modulus and a peak temperature rise of 0.62°C. Surface displacements calculated using these values are compared against the experimental signals in Fig. 12. The disparity between the extent of the surface retraction relative to its maximum displacement measured experimentally, and the numerical solution prediction for the native liver sample can be attributed to the fact that the experimental signal has noise at higher frequencies, and that when the retraction time is very long, the quality of fit between the measured signal and Eq. 7 is worse. The numerical calculations are not used for finding a best fit to the experimental data. Only a few sets of parameters are examined to find the agreement between experimental data and predictions of the numerical solutions, using, however, accepted values for the physical constants used in the equations. The prominent retraction of the sample surface after expansion can be attributed to greater values of the elastic modulus of the coagulated liver tissue samples compared to native ones. Other studies also have shown stiffening and increase of elastic modulus of tissue samples following coagulation.14, 38, 39 Fig. 12Predictions of the numerical solutions for the thermoelastic expansions of targets agree with the experimental signals presented in Fig. 1. 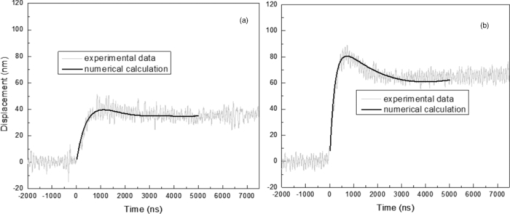 Elastic moduli that are estimated in the current study are higher than the values reported in the other studies. This can be attributed to the fact that these references deal with the estimation of the tissue's mechanical properties at lower loading frequencies. For example, Kiss, Varghese, and Hall performed experiments over a frequency range from 0.1 to 400 Hz,14 and reported values of 2 and 27 kPa for the elastic modulus of native and coagulated ex-vivo canine liver samples, respectively. It is known that the elastic modulus of tissue samples is frequency dependent and increases with higher frequencies.40 In other studies with higher values of mechanical pressure and greater deformations of the native tissue target, values for the elastic modulus ranging from 110 kPa to 1.02 MPa were measured.41 By studying the second derivative of the surface displacement, which is a measure of acceleration and hence the forces on the target surface, one can estimate the rate by which the thermal stresses in the tissue sample are working. The second derivative of the typical signals shown in Fig. 2 are calculated and shown in Fig. 13. It shows that peak-to-peak changes in surface stresses are of the order of 770 and 820 ns for these two signals. These variations correspond to frequencies higher than 1 MHz and therefore the measurement is probing moduli at these timescales. On the other hand, in our experiments the deformation of the target tissue samples due to these stresses is very small (of the order of nanometers). To the best of our knowledge, the current work is among very few experiments that provide measurements on deformation of soft tissues at high frequencies and low deformations. 5.ConclusionThe study of thermoelastic deformation of tissue samples after incidents of short laser pulses on them can provide a wealth of information about their optical and thermomechanical properties. Interferometric measurements are performed to study these types of deformations in native and coagulated ex-vivo bovine liver samples. A key advantage of this technique is that from the same dataset, both optical and thermomechnical properties can be derived. Therefore, the property measurements are both spatially and temporally coincident, something that cannot be achieved easily with other techniques. A decrease of the optical attenuation depth for the coagulated liver samples compared to native ones is observed. One can estimate the Grüneisen coefficient of tissue samples by using the slope of linear fit to the equilibrium displacement of the target surface as a function of laser pulse energy. The natural variability of the biological samples (e.g., variations in fat and connective tissue content in the small liver areas irradiated) and sensitivity of our measurements to noise prevented us from finding a significant difference in the Grüneisen coefficient between the two groups. However, dynamics of thermoelastic deformations for the two groups are distinctively different. The surface of the coagulated tissue samples shows a more pronounced retraction after its initial expansion compared to native liver samples. Comparison of the experimental data with the numerical solution of a thermoelastic wave equation show that this behavior can be explained assuming an increase in the elastic modulus of the coagulated tissue samples compared to the native one. The results suggest that optoacoustic imaging can be used for guidance and monitoring of thermal therapies by exploiting the changes that in the optical and thermomechanical properties of tissues occur while they undergo thermal treatment. AcknowledgmentThe authors would like to gratefully acknowledge Arthur Worthington for his assistance in the experiments. Financial support was provided by the Atlantic Innovation Fund, the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research (grant CHRPJ323745-60), and the Canada Foundation for Innovation (CFI). This research was undertaken, in part, thanks to funding from the Canada Research Chairs Program awarded to Michael Kolios. ReferencesA. N. Mirza,
B. D. Fornage,
N. Sneige,
H. M. Kuerer,
L. A. Newman,
F. C. Ames, and
S. E. Singletary,
“Radiofrequency ablation of solid tumors,”
Cancer J., 7
(2), 95
–102
(2001). Google Scholar
J. E. Kennedy,
G. R. TerHaar, and
D. Cranston,
“High intensity focused ultrasound: surgery of the future,”
Br. J. Radiol, 76 590
–599
(2003). https://doi.org/10.1259/bjr/17150274 Google Scholar
T. J. Vogl,
R. Straub,
K. Eichler,
O. Sollner, and
M. G. Mack,
“Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy-local tumor control rate and survival data,”
230
(2), 450
–458
(2004). Google Scholar
B. Quesson,
J. A. de Zwart, and
C. T. W. Moonen,
“Magnetic resonance temperature imaging for guidance of thermotherapy,”
J. Magn. Reson. Imag., 12
(4), 525
–533
(2000). https://doi.org/10.1002/1522-2586(200010)12:4 >525::AID-JMRI3 ≪3.0.CO;2-V Google Scholar
L. Chin,
M. Pop,
W. Whelan,
M. Sherar, and
A. Vitkin,
“Optical method using fluence or radiance measurements to monitor thermal therapy,”
Rev. Sci. Instrum., 74
(1), 393
–395
(2003). https://doi.org/10.1063/1.1519681 Google Scholar
S. Siebers,
U. Scheipers,
C. Welp,
J. Werner, and
H. Ermert,
“A classification system for monitoring thermal therapies,”
Ultrasonics Symp. IEEE, 2 1126
–-1129
(2005). Google Scholar
S. A. Ermilov,
T. Khamapirad,
A. Conjusteau,
M. H. Leonard,
R. Lacewell,
K. Mehta,
T. Miller, and
A. A. Oraevsky,
“Laser optoacoustic imaging system for detection of breast cancer,”
J. Biomed. Opt., 14
(2), 024007
(2009). https://doi.org/10.1117/1.3086616 Google Scholar
X. Wang,
Y. Pang,
G. Ku,
X. Xie,
G. Stoica, and
L. V. Wang,
“Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,”
Nature Biotechnol, 21
(7), 803
–806
(2003). https://doi.org/10.1038/nbt839 Google Scholar
R. I. Siphanto,
K. K. Thumma,
R. G. M. Kolkman,
T. G. van Leeuwen,
F. F. M. de Mul,
J. W. van Neck,
L. N. A. van Adrichem, and
W. Steenbergen,
“Serial noninvasive photoacoustic imaging of neovascularization in tumor angiogenesis,”
Opt. Express, 13
(1), 89
–95
(2004). https://doi.org/10.1364/OPEX.13.000089 Google Scholar
R. G. M. Kolkman,
J. H. G. M. Klaessens,
E. Hondebrink,
J. C. W. Hopman,
F. F. M. de Mul,
W. Steenbergen,
J. M. Thijssen, and
T. G. van Leeuwen,
“Photoacoustic determination of blood vessel diameter,”
Phys. Med. Biol., 49 4745
–4756
(2004). https://doi.org/10.1088/0031-9155/49/20/006 Google Scholar
A. A. Oraevsky,
S. L. Jacques, and
F. K. Tittel,
“Measurement of tissue optical properties by time-resolved detection of laser-induced transient stress,”
Appl. Opt, 36
(1), 402
–415
(1997). https://doi.org/10.1364/AO.36.000402 Google Scholar
C.-T. Germer,
A. Roggan,
J. P. Ritz,
C. Isbert,
D. Albrecht,
G. Müller, and
H. J. Buhr,
“Optical properties of native and coagulated human liver tissue and liver metastases in the near infrared range,”
Lasers Surg. Med., 23
(4), 194
–203
(1998). https://doi.org/10.1002/(SICI)1096-9101(1998)23:4**194::AID-LSM2**3.0.CO;2-6 Google Scholar
J. P. Ritz,
A. Roggan,
C. Isbert,
G. Mller,
H. J. Buhr, and
C. T. Germer,
“Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm,”
Lasers Surg. Med., 29
(3), 205
–212
(2001). https://doi.org/10.1002/lsm.1134 Google Scholar
M. Z. Kiss,
T. Varghese, and
T. J. Hall,
“Viscoelastic characterization of in vitro canine tissue,”
Phys. Med. Biol., 49
(18), 4207
–4218
(2004). https://doi.org/10.1088/0031-9155/49/18/002 Google Scholar
I. V. Larina,
K. V. Larin, and
R. O. Esenaliev,
“Real-time optoacoustic monitoring of temperature in tissues,”
J. Phys. D: Appl. Phys., 38
(15), 2633
–2639
(2005). https://doi.org/10.1088/0022-3727/38/15/015 Google Scholar
K. V. Larin,
I. V. Larina, and
R. O. Esenaliev,
“Monitoring of tissue coagulation during thermotherapy using optoacoustic technique,”
J. Phys. D: Appl. Phys., 38
(15), 2645
–2653
(2005). https://doi.org/10.1088/0022-3727/38/15/017 Google Scholar
C. Richter,
G. Spirou,
A. A. Oraevsky,
W. M. Whelan, and
M. C. Kolios,
“Examination of contrast mechanisms in optoacoustic imaging of thermal lesions,”
Proc. SPIE, 6086 60861
(2006). Google Scholar
J. Kandulla,
H. Elsner,
R. Birngruber, and
R. Brinkmann,
“Noninvasive optoacoustic online retinal temperature determination during continuous-wave laser irradiation,”
J. Biomed. Opt., 11
(4), 041111
(2006). https://doi.org/10.1117/1.2236301 Google Scholar
R. F. Castelino,
W. M. Whelan, and
M. C. Kolios,
“Photoacoustic detection of protein coagulation in albumen-based phantoms,”
Proc. SPIE, 6856 685626
(2008). Google Scholar
D. Albagli,
M. Dark,
C. Von Rosenberg,
L. Perelman,
I. Itzkan, and
M. S. Feld,
“Laser-induced thermoelastic deformation: a three-dimensional solution and its application to the ablation of biological tissue,”
Med. Phys., 21
(8), 1323
–1331
(1994). https://doi.org/10.1118/1.597202 Google Scholar
I. Itzkan,
D. Albagli,
M. L. Dark,
L. T. Perelman,
C. Von Rosenberg, and
M. S. Feld,
“The thermoelastic basis of short pulsed laser ablation of biological tissue,”
Proc. Natl. Acad. Sci. USA, 92 1960
–1964
(1995). https://doi.org/10.1073/pnas.92.6.1960 Google Scholar
B. Soroushian,
W. Whelan, and
M. Kolios,
“Assessment of opto-mechanical behavior of biological samples by interferometry,”
Proc. SPIE, 7177 71771X
–6
(2009). Google Scholar
M. L. Dark,
L. T. Perelman,
I. Itzkan,
J. L. Schaffer, and
M. S. Feld,
“Physical properties of hydrated tissue determined by surface interferometry of laser-induced thermoelastic deformation,”
Phys. Med. Biol., 45 529
–539
(2000). https://doi.org/10.1088/0031-9155/45/2/318 Google Scholar
J. P. Harkin and
D. A. Flavin,
“Interferometric displacement tracking based on Hilbert transform processing,”
Proc. SPIE, 4204 89
–98
(2001). Google Scholar
D. H. Huang,
C. K. Liao,
C. W. Wei, and
P. C. Li,
“Simulations of optoacoustic wave propagation in light-absorbing media using a finite-difference time-domain method,”
J. Acoust. Soc. Am., 117
(5), 2795
–2801
(2005). https://doi.org/10.1121/1.1893305 Google Scholar
C. T. Schröder and
W.R. Scott Jr.,
“On the stability of the FDTD algorithm for elastic media at a material interface,”
IEEE Trans. Geosci. Remote Sens., 40
(2), 474
–481
(2002). https://doi.org/10.1109/36.992813 Google Scholar
X. B. Tian,
I. B. Kang,
G. Y. Kim, and
H. S. Zhang,
“An improvement in the absorbing boundary technique for numerical simulation of elastic wave propagation,”
J. Geophys. Eng., 5
(2), 203
–209
(2008). https://doi.org/10.1088/1742-2132/5/2/007 Google Scholar
B. P. Payne,
V. Venugopalan,
B. B. Mikic, and
N. S. Nishioka,
“Optoacoustic determination of optical attenuation depth using interferometric detection,”
J. Biomed. Opt., 8
(2), 264
–272
(2003). https://doi.org/10.1117/1.1559731 Google Scholar
U. Techavipoo,
T. Varghese,
Q. Chen,
T. A. Stiles,
J. A. Zagzebski, and
G. R. Frank,
“Temperature dependence of ultrasonic propagation speed and attenuation in excised canine liver tissue measured using transmitted and reflected pulses,”
J. Acoust. Soc. Am., 115
(6), 2859
–2865
(2004). https://doi.org/10.1121/1.1738453 Google Scholar
D. Zhu,
Q. Luo, and
J. Cen,
“Effects of dehydration on the optical properties of in vitro porcine liver,”
Laser Surg. Med., 33 226
–231
(2003). https://doi.org/10.1002/lsm.10215 Google Scholar
M. P. Otternsmeyer,
A. E. Kedok,
R. D. Howe, and
S. L. Dawson,
“The effects of testing environment on the viscoelastic properties of soft tissues,”
Int. Symp. Med. Sim., Springer-Verlag, Berth
(2004). Google Scholar
NCRP,
“Report No. 113: exposure criteria for medical diagnostic ultrasound: I. Criteria based on thermal mechanisms Report No. 113,”
53
(1992). Google Scholar
K. Giering,
I. Lamprecht,
O. Minet, and
A. Handke,
“Determination of the specific heat capacity of healthy and tumorous human tissue,”
ThermochimicaActa, 251 199
–205
(1995). https://doi.org/10.1016/0040-6031(94)02047-R Google Scholar
S. Nakamura,
Y. Nishiwaki,
S. Suzuki,
S. Sakaguchi,
Y. Yamashita, and
K. Ohta,
“Light attenuation of human liver and hepatic tumors after surgical resection,”
Lasers Surg. Med., 10
(1), 12
–15
(1990). https://doi.org/10.1002/lsm.1900100105 Google Scholar
N. G. Shrive,
“Soft tissue strain measurement,”
Optical Measurement Methods in Biomechanics, 156
–172 Eds.Chapman and Hall, London
(1997). Google Scholar
J. Olivier,
W. D. Johnson, and
G. D. Marshall,
“The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them,”
Ann. Allergy, Asthma Immunol., 100
(4), 333
–337
(2008). https://doi.org/10.1016/S1081-1206(10)60595-9 Google Scholar
C. Chui,
E. Kobayashi,
X. Chen,
T. Hisada, and
I. Sakuma,
“Combined compression and elongation experiments and non-linear modelling of liver tissue for surgical simulation,”
Med. Biol. Eng. Computi., 42
(6), 787
–798
(2004). https://doi.org/10.1007/BF02345212 Google Scholar
S. Bharat,
U. Techavipoo,
M. Z. Kiss,
W. Liu, and
T. Varghese,
“Monitoring stiffness changes in lesions after radiofrequency ablation at different temperatures and durations of ablation,”
Ultrasound Med. Biol., 31
(3), 415
–422
(2005). https://doi.org/10.1016/j.ultrasmedbio.2004.12.020 Google Scholar
M. Zhang,
B. Castaneda,
J. Christensen,
W. Saad,
K. Bylund,
K. Hoyt,
J. G. Strang,
J. G. Strang,
D. J. Rubens, and
K.J. Parker,
“Real-time sonoelastography of hepatic thermal lesions in a swine model,”
Med. Phys., 35
(9), 4132
–4141
(2008). https://doi.org/10.1118/1.2968939 Google Scholar
D. Valtorta and
E. Mazza,
“Dynamic measurement of soft tissue viscoelastic properties with a torsional resonator device,”
Med. Image Anal., 9
(5 SPEC. ISS.), 481
–490
(2005). https://doi.org/10.1016/j.media.2005.05.002 Google Scholar
H. Saraf,
K. T. Ramesh,
A. M. Lennon,
A. C. Merkle, and
J. C. Roberts,
“Mechanical properties of soft human tissues under dynamic loading,”
J. Biomechan., 40
(9), 1960
–1967
(2007). https://doi.org/10.1016/j.jbiomech.2006.09.021 Google Scholar
P. Parsa, S. L. Jacques, and
N. S. Nishioka,
“Optical properties of rat liver between 350 and 2200 nm,”
Appl. Opt., 28
(12), 2325
–2330
(1989). https://doi.org/10.1364/AO.28.002325 Google Scholar
T. J. Pfefer,
L. S. Matchette,
C. L. Bennett,
J. A. Gall,
J. N. Wilke,
A. J. Durkin, and
M.N. Ediger,
“Reflectance-based determination of optical properties in highly attenuating tissue,”
J. Biomed. Opt., 8
(2), 206
–215
(2003). https://doi.org/10.1117/1.1559487 Google Scholar
P. G. Charette,
I. W. Hunter, and
P. J. Hunter,
“Large deformation mechanical testing of biological membranesusing speckle interferometry in transmission. I: experimental apparatus,”
Appl. Opt., 36
(10), 2238
–2245
(1997). https://doi.org/10.1364/AO.36.002238 Google Scholar
|