|
|
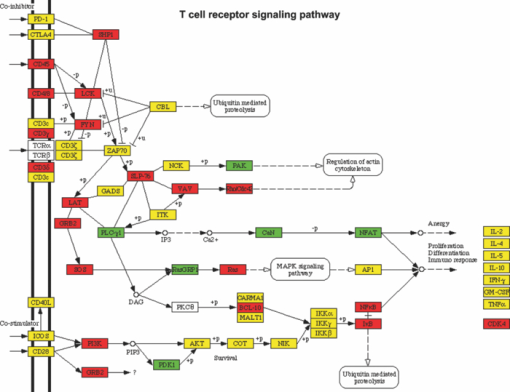
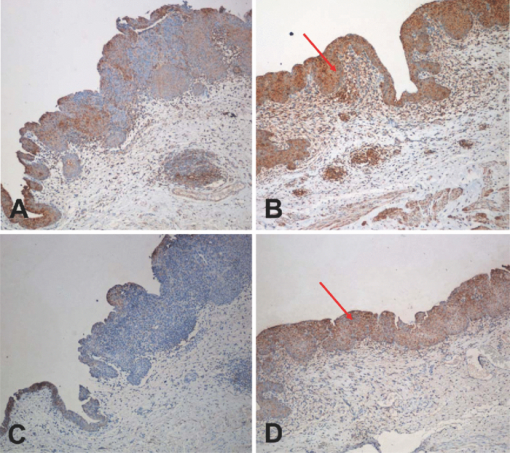
1.IntroductionBladder cancer is the second most frequently diagnosed genitourinary tumor after prostate cancer. Despite aggressive therapies, patients with invasive bladder cancer have a five-year survival rate of ∼50%.1 Patients with noninvasive bladder cancer treated with transurethral resection of bladder tumor (TUR-B) are at high risk of recurrence (60–70%) and death from disease (10–30%).2 The majority of malignant tumors arising in the urinary bladder are transitional cell carcinomas (TCC). Clinically, noninvasive bladder tumors (stages Ta and Tis) account for 75–85% of neoplasms, whereas the remaining 15–25% are invasive (T1–T4) or metastatic lesions at the time of initial presentation.3 Currently, a standard modality for the treatment of high-risk Ta, T1 bladder cancer, and carcinoma in situ (CIS) of the bladder is intravesical instillation of Bacillus Calmette-Guérin (BCG).4, 5 BCG-induced antitumor activity is believed to be dominated by a local nonspecific immunological reaction reflecting the activity of immunocompetent cells.6 The mode of action of BCG, ranging from its introduction into the bladder to killing of residual tumor cells, is a complex sequence of processes. No definite statements have yet been made about the actual effector cell(s), but a crucial cytotoxic role of natural killer (NK) cells has been proposed. In addition, some of the cytokines, and BCG itself, may exhibit a direct cytotoxic effect on tumor cells.7 Although it is effective, BCG therapy is associated with a considerable side-effect profile and ∼30% of patients either fail to respond to the treatment or suffer recurrent disease within five years.8, 9, 10, 11 It was reported that the complication rates were 17.6% for patients who were <70 years old and 48–53% for patients who were ≥70 years old.12 Therefore, alternative or adjuvant treatment, such as photodynamic therapy (PDT), is needed. Hexyl 5-aminolevulinate (HAL)-photodynamic diagnosis (PDD) has received international interest due to studies showing that it improves visualization of bladder tumors and thereby prolongs time to recurrence after TUR-B.13, 14, 15 In fact, PDT has already been established as an alternative therapy for the treatment of various types of malignant disorders, including oesophageal, brain, lung, and skin cancer.16, 17, 18, 19 This treatment involves the administration of a photosensitizer followed by its activation with light of a specific wavelength. Because cancer cells have a higher accumulation of photosensitizer cells than normal cells, PDT is to some degree a tumor-selective treatment modality.20 The precise mechanism of antitumor action by PDT is unclear, but it has been suggested that the photoactive sensitizer triggers a series of photochemical and photobiological processes, in the presence of tissue oxygen, that lead to cancer cell damage, tumor microvascular occlusion, and host immune response.21, 22, 23 Recently, we have used optical spectroscopy to monitor HAL-induced PDT in a rat model of bladder cancer (pT1 stage) and found a reduced tissue oxygenation and protoporphyrin (PpIX) fluorescence photobleaching, which was associated with tumor disappearance or size reduction.24 In the present study, we have further analyzed the tissue responses to HAL-PDT and attempted to clarify the underlying mechanisms of PDT. 2.Materials and Methods2.1.Rat Model of Bladder Cancer and Photodynamic TherapyThe rats with orthotopic bladder cancer (pT1) used in the present study were the same as those in our previous studies.24, 25 HAL-PDT treatment was given 14 days after AY-27 implantation at the time point when the tumor stage has in our previous studies as well as other studies been described as pT1.26 In brief, female Fischer F344 rats (purchased from Möllegard, Skensvend, Denmark) were inoculated with a syngeneic bladder cancer cell line (AY-27) and were subjected to PDT or microarray analysis two weeks after AY-27 cell inoculation. PDT was performed by instillation of HAL (8 mM, 0.3 ml) (Photocure ASA, Oslo, Norway) in the bladder for 1 h, followed 2 h later with light treatment (fluence rate = 20 mW/cm2) corresponding to the peak PpIX accumulation in the bladder tissue.24 The irradiation source consisted of an Argon-pumped (Innova 70, Coherent, Santa Clara, California) dye laser (model 375, Spectra-Physics, Mountain View, California) centered at 635 nm. Light was coupled into a 200-μm fiber applicator with an isotropic diffuser tip (PDT Systems, Santa Barbara, California) and with a total light fluence of 20 J/cm2.24, 27 PDT-treated rats were sacrificed one week later for analysis. 2.2.Study DesignAs reported in our previous study, three of six bladder cancer rats had no sign of tumor after PDT.24 The remaining three PDT-treated rats, together with control groups, including untreated, PDT-treated normal rats, and untreated cancer rats, were used for further morphological analysis (see Sec. 2.3). Additional six bladder cancer rats and six sham-operated rats without PDT were used for analysis of gene expression profiling, especially with regard to T-cell receptor-signaling pathway, For information about the sample preparation and data analysis, see Ref. 25. In brief, the differentially expressed genes were tested for overrepresentation in Kyoto Encyclopaedia of Genes and Genomes (KEGG)28 using Fishers exact test, and p-values of <0.01 was taken as significant. The KEGG pathways are compiled from multiple literature sources and integrate individual components into unified pathways that detect changes that are not apparent on a single-gene basis. All animal experiments were approved by the Norwegian national animal research authority. 2.3.Morphological AnalysisThe entire bladder from each rat was removed, opened, and immersed in 4% formaldehyde for 8–12 h at 4°C. The tissues were dehydrated and embedded in a paraffin block. Tissue sections were cut through the whole bladder (>300 sections/bladder at a 4-μm thickness) in a microtome, and every fifth section was thawed onto superfrost glass slides. The sections were subjected to a routine hematoxylin-eosin-saffron staining and immunostaining by incubating with antisera to proliferating cell nuclear antigen (PCNA) (code M0879, 1:100, Dako, Denmark), caspase-3 (code 9662, 1:20, Cell Signal, Danvers, MA), CD3 (Code Ab5690; 1:50, pH6, Abcam) and CD45RA (code Ab22363, 1:200, pH6, Abcam). The stained sections were examined under a microscope (Olympus BX50, Tokyo, Japan). Quantification of immunoreactive cells was performed according to the principle of stereology, measured by the point count method and expressed as volume density in percentage. The values are expressed as means ± SEM. Statistical comparison (t test) between two groups (i.e., PDT versus no PDT) was made using PASW Statistics 17 software. A p < 0.05 (two-tailed) was considered statistically significant. For electron microscopy, >10 small tissue specimens were taken from different sites of each rat bladder and immediately immersed in a mixture of 2% glutaraldehyde in (0.1 M) PBS, pH 7.2. After 6 h, the specimens were transferred to OsO4 (1%) and postfixed (1 h), dehydrated in graded acetone, and embedded in epoxy (TAAB Laboratories, Berkshire, United Kingdom). Ultrathin sections of 60–80 nm thickness were cut on a LKB MK III Ultratome (LKB, Bromma, Sweden), contrasted with uranyl acetate and lead citrate and examined under a transmission electron microscope (Joel, Tokyo, Japan). 3.Results3.1.Histological, Immunohistochemical, and Ultrastructural AppearancesIn comparison to untreated rats, PDT caused little or no changes in rat bladder without tumor but potentiated the inflammatory changes noted in rat bladders with tumor (Fig. 1). Macroscopically, no differences were noted between the 12 PDT-treated animals and the six control animals. Microscopically increased lymphocytes and mononuclear cell infiltration were noted in the tumor, and in the tumor-free tissue there were no apparent fibrotic changes [Fig. 1d]. Fig. 1Representative micrographs showing transitional epithelium of bladders of rats: (a) Normal rat, (b) PDT-treated normal rat, (c) rat with orthotopic bladder cancer at T2 stage (indicated by asterisk) (three weeks after AY-27 cell inoculation), and (d) rat with bladder cancer at T1 stage (asterisk) (three weeks after AY-27 cell inoculation and one week after PDT). Note: lymphocytes and mononuclear cell infiltration (arrows). Hematoxylin-eosin-safron staining. 100×. 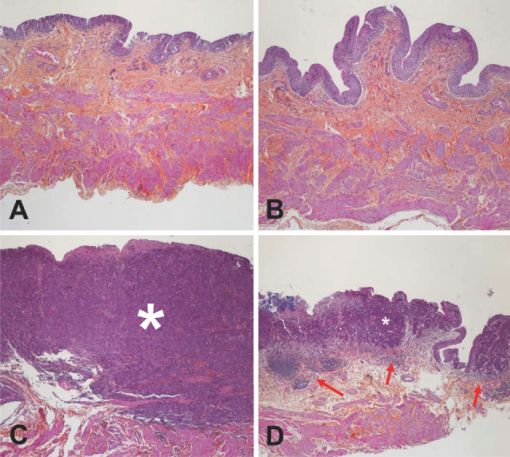 Immunohistochemistry revealed that PDT did not affect PCNA expression, a marker for cell proliferation, in the rat bladder without tumor (6.6 ± 0.3 versus 7.4 ± 1.2, p > 0.05) [Figs. 2a and 2b]. The PCNA expression was reduced after PDT in the rat bladder with tumor (98.7 ± 1.3 versus 4.7 ± 0.8, p < 0.01), which was apparently due to the reduced size of the tumor [Figs. 2c and 2d]. In fact, an increased PCNA expression was observed in the tumor area in comparison to the adjacent normal area regardless of PDT [Figs. 2c and 2d] and did not differ between rats with and without PDT (data not shown). PDT increased caspase-3 expression, a marker for apoptosis, in normal urothelium of bladder (2.7 ± 0.3 versus 26.2 ± 5.7, p < 0.01) [Figs. 3a and 3b], as well as in the tumors compared to the tumors that were not treated with PDT (35.5 ± 4.9 versus 6.2 ± 1.2, p < 0.01) [Figs. 3c and 3d]. Fig. 2Representative micrographs showing transitional epithelium of bladders of rats: (a) Normal rat, (b) PDT-treated normal rat, (c) rat with orthotopic bladder cancer at T2 stage (indicated by asterisk) (three weeks after AY-27 cell inoculation), and (d) rat with bladder cancer at T1 stage (asterisk) (three weeks after AY-27 cell inoculation and one week after PDT). Note: Immunostaining in tumor areas (arrows). PCNA immunostaining. 100×. 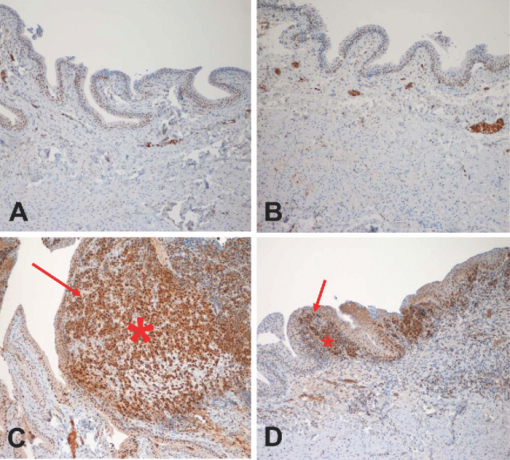 Fig. 3Representative micrographs showing transitional epithelium of bladders of rats: (a) Normal rat, (b) PDT-treated normal rat, (c) rat with orthotopic bladder cancer at T2 stage (indicated by asterisk) (three weeks after AY-27 cell inoculation), and (d) rat with bladder cancer at T1 stage (asterisk) (three weeks after AY-27 cell inoculation and one week after PDT). Note: Immunostaining markedly in (b) and moderately (d) (arrows). Caspase-3 immunostaining. 100×. 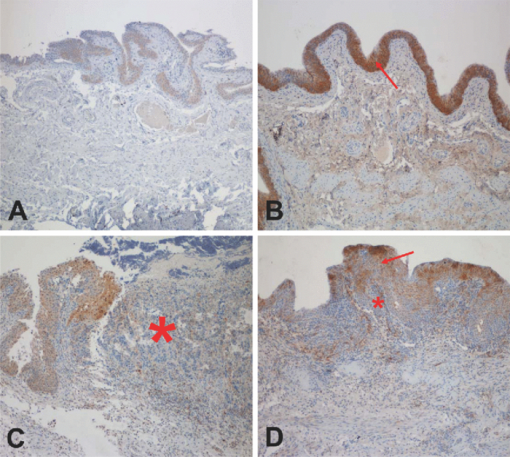 Electron microscopy showed that the bladder cancer cells (i.e., AY-27 cells in vivo) displayed a poorly differentiated appearance with numerous microvilli in rats without PDT [Fig. 4a]. In PDT-treated rats, severe mitochondrial damage (characterized by disappearance of critae), apoptotic bodies, vacuoles, and lipofuscin bodies were observed [Fig. 4b and 4c]. It should also be noted that microvillus-formed niches, which were occasionally found in untreated bladder cancer,29 were not observed in any of the samples from PDT-treated rats [Fig. 4d]. Fig. 4Representative electron micrographs showing bladder cancer cells three weeks after AY-27 cell inoculation (a) without PDT and (b–d) with PDT. Note: numerous microvilli in (a) (indicated by arrows), severe mitochondrial damage (arrow) and apoptotic bodies (framed arrow) in (b), and vacuoles (arrow) and lipofuscin body (asterisk) in (c). Transmission electron microscopy, 6000×. 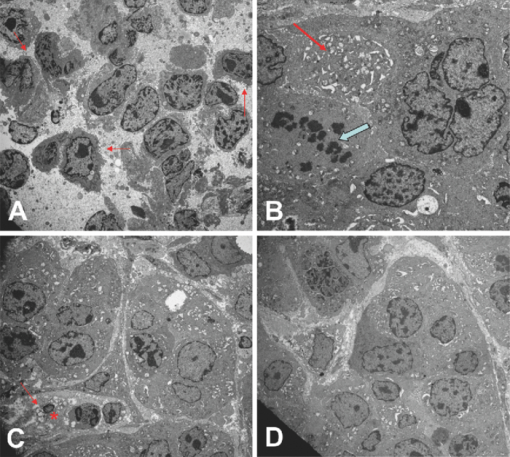 3.2.T-Cell Receptor Signaling Pathway and T CellsIn our previous study, we reported that the host immune system seemed to be fully active in the form of immunoserveillance and, yet, the tumors still progressed in this animal model.25 In the present study, further analysis by a means of bioinformatics revealed an activation of T-cell receptor signaling pathway (reflected by upregulation of 19/58 genes in this pathway) in rats with bladder cancer without PDT (Fig. 5). It should be noted that CD3 (particularly, CD3γ) and CD45 were among the upregulated genes in this pathway. PDT further increased expressions of CD3 (4.4 ± 0.9 versus 19.0 ± 4.3, p < 0.01) [Figs. 6a and 6b] and CD45RA expression (6.1 ± 0.4 versus 30.4 ± 3.3, p < 0.01) [Fig. 6c and 6d] (revealed by immunohistochemistry), especially in the tumor area. 4.Discussion and ConclusionsWe and others have shown that the orthotopic bladder cancer cell model in inbred rats mimics human bladder cancer with respect to urothelial tumorigenesis and progression (from pTa to pT3 with increased proliferation and reduced apoptosis) and with respect to molecular features involving tumorigenesis in general.30, 31 Utilizing this animal model, we were able to demonstrate the therapeutic effect of HAL-induced PDT.24 In the present study, we further showed that the underlying mechanisms may involve direct tumor cell kill via apopototic and autophagic pathways and indirect immunologic effects mediated by T cells. The relatively selective effect of HAL for malignant tissue has previously been documented.32, 33, 34, 35 The synthesis of PpIX is known to take place in the mitochondria of cells. The reasons for the preferential accumulation of PpIX in both premalignant and malignant tissues are not clearly understood. Some factors, such as higher proliferative rates, leaky vasculature, decreased degradation of photosensitizer, and decreased lymphatic drainage, have been implicated. For the bladder, in particular, one could speculate that with intravesical treatment the uromucosal barrier is less intact over malignant tissue compared to normal bladder urothelium. It is also well documented that PDT has a direct effect on cancer cells, producing cell death by apoptosis and/or necrosis; an indirect effect on the tumor vasculature, whereby illumination and reactive oxygen species (ROS) production cause the shutdown of vessels and subsequently deprives the tumor of oxygen and nutrients, and it has a significant effect on the immune system, which can be either immunostimulatory or immunosuppressive.36 The results of the present study support the view that a mode of action of HAL-induced PDT is mediated by the mitochondrial apoptotic pathway. In fact, the importance of mitochondria as targets for the initiation of apoptosis by PDT has been well demonstrated.37 PDT-induced cell death might also take place in nonapoptotic cells, presumably via other major cell death subroutines (e.g., necrosis, autophagic pathway). Moreover, our findings suggest that the autophagic pathway is also involved, as indicated by the appearance of vacuoles and lipofuscin bodies in the cytoplasm. Lipofuscin bodies are formed by oxidized cross-linked protein materials, lipids, sugars, and metals, including mercury, aluminum, iron, copper, and zinc, and is associated with accelerated cellular dysfunction, decreased viability, and hence, negatively correlated with the life expectancy of the individual cell.38 The importance of lipofuscin generated by HAL-PDT, or PDT in general, has not previously been fully elucidated. Clearing the cytosol of proteins damaged by oxidation and keeping the protein pool working are functions of the lysosomal and proteasomal systems. Cellular capacity to remove mildly oxidized proteins by the proteasomal and lysosomal proteolytic pathways may be overwhelmed by the sudden increase in the amount of oxidized protein substrates, resulting from PDT. In addition peroxidation of lysosomal membranes will cause lysosomal dysfunction. Therefore, both the acute increase of oxidized protein substrates as well as lysosomal dysfunction may lead to accumulation of lipofusin bodies. A recent study showed an increased efficacy (photodamage) by increasing iron accumulation via inhibiting recycling of iron with quinolone agents.39 Indeed, previous studies have suggested that certain doses of PDT induce autophagy.40, 41, 42 It is conceivable that high-dose PDT initially damages proteins needed for proper functioning of the lysosomal and proteosomal pathways and instead induce cellular necrosis. At moderate PDT doses, the cells will survive but the increased amounts of oxidized proteins will overwhelm lysosomal and proteosomal pathways and lead to accumulation of lipofuscin bodies. With low levels of photodamage, the autophagic pathway is able to recycle damaged constituents thereby normalizing the damaged cell and subsequently enhancing cell survival.40, 42 Dosing of PDT is relatively precise when using in vitro cell culture studies, but when it comes to in vivo studies the actual doses both of photosensitizer and light will naturally vary a great deal, resulting in myriad different responses. This fact should be considered when interpreting the results of animal studies and human data. The immune system is critically involved in the control of tumor survival and, ultimately, progression. It is now over 20 years since the U.S. Food and Drug Administration approved the treatment of noninvasive bladder cancer with BCG, and this experience has clearly demonstrated that by manipulating/stimulating the immune system one can potentially generate a tumor-specific immune response.43, 44, 45 HAL-PDD has received a great deal of attention due to studies showing that it improves visualization of bladder tumors and thereby prolongs time to recurrence after TUR-B.13, 14, 15, 46 In our previous and present studies, we have shown that the immune system in the bladder tissues with cancer is fully active.25 The present study suggests that HAL-PDT could potentially enhance the immune activity of the host. In fact, the results of the present study might indicate that PDT enhanced further the activation of the T-cell receptor singling pathway, resulting in increased tumor-infiltrating lymphocytes (TILs) as manifested by the increased protein expression of CD3 and CD45RA. Further investigations are needed to better define the degrees of T-cell receptor singling pathway activation and TILs with the optimal HAL-PDT dose by adjusting light fluence, pH, oxygen concentrations, penetration depth of photosensitizer, and exposure time to photosensitizer. PDT immunotherapy with a maintenance schedule could lead to a treatment modality with potentially fewer complications than BCG and high-dose PDT (Fig. 5). For HAL-PDT-immunotherapy to have long-term efficacy, studies looking at maintenance schedules should be addressed and not just one-time treatments.47 In addition, it was our impression that vasculogenesis takes place in the tissue surrounding the tumor after PDT. This observation does not indicate whether it was a result from PpIX formation in the vessels or reaction to the tumor response to PDT. However, this observation should be verified with a quantitative method and the cellular location of PpIX should be further identified in the future. Recently, we reported a unique ultrastructure displaying microvillus-formed niches in the rat model as well as in human bladder cancer. We suggested that these niches harbor cancer cells (stem cells), thereby preventing tumor eradication by the host immune system.29 In the present study, we did not find such niches in PDT-treated rats. We only had three of six animals with residual tumor after PDT; therefore, future studies confirming this finding need to be performed. However, if the future studies do indeed confirm a lack of these niches, it would suggest a novel mechanism for PDT that breaks the niche thereby allowing host response cells (e.g., T cells) access to the niche and a more complete eradication of all cancer cells. In conclusion, the results of the present study suggest several possible pathways that mediate the action of HAL-PDT in syngeneic orthotopic rat bladder cancer model. The pathways include, at least, activation of mitochondrial apoptosis, formation of autophagic vacuoles and lipofuscin bodies, enhancement of T cell activation, and perhaps breakdown of cancer stem cell niches. We believe that a better understanding of these mechanisms can potentially lead to improvements in PDT and thereby potentially enhance photokilling via apoptotic or autophagic pathways, and/or potentiating immunosurveillence of the host. AcknowledgmentsThis study was supported by grants from the Joint Program of Medical Faculty of NTNU and St. Olavs’ Hospital, St. Olavs’ Hospital Foundation for Cancer Research, Norwegian Functional Genomics (Midt-Norge). The syngeneic bladder cancer cell line (AY-27) was provided by Professor S. Selman at Department of Urology, Medical College of Ohio, Toledo, Ohio. ReferencesD. Raghavan, D. Quinn, D. G. Skinner, and
J. P. Stein,
“Surgery and adjunctive chemotherapy for invasive bladder cancer,”
Surg. Oncol., 11
(1-2), 55
–63
(2002). https://doi.org/10.1016/S0960-7404(02)00007-5 Google Scholar
S. Holmang, H. Hedelin, C. Anderstrom, and
S. L. Johansson,
“The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years,”
J. Urol., 153
(6), 1823
–1826
(1995). https://doi.org/10.1016/S0022-5347(01)67321-X Google Scholar
M. Castillo-Martin, J. Domingo-Domenech, O. Karni-Schmidt, T. Matos, and
C. Cordon-Cardo,
“Molecular pathways of urothelial development and bladder tumorigenesis,”
Urol. Oncol., 28
(4), 401
–408
(2010). https://doi.org/10.1016/j.urolonc.2009.04.019 Google Scholar
M. Babjuk, W. Oosterlinck, R. Sylvester, E. Kaasinen, A. Bohle, and
J. Palou-Redorta,
“EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder,”
Eur. Urol., 54
(2), 303
–314
(2008). https://doi.org/10.1016/j.eururo.2008.04.051 Google Scholar
M. C. Hall, S. S. Chang, G. Dalbagni, R. S. Pruthi, J. D. Seigne, E. C. Skinner, J. S. Wolf Jr., and P. F. Schellhammer,
“Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update,”
J. Urol., 178
(6), 2314
–2330
(2007). https://doi.org/10.1016/j.juro.2007.09.003 Google Scholar
A. B. Alexandroff, A. M. Jackson, M. A. O'Donnell, and
K. James,
“BCG immunotherapy of bladder cancer: 20 years on,”
Lancet, 353
(9165), 1689
–1694
(1999). https://doi.org/10.1016/S0140-6736(98)07422-4 Google Scholar
R. F. Bevers, K. H. Kurth, and
D. H. Schamhart,
“Role of urothelial cells in BCG immunotherapy for superficial bladder cancer,”
Br. J. Cancer, 91
(4), 607
–612
(2004). https://doi.org/10.1038%2Fsj.bjc.6602026 Google Scholar
S. J. Dovedi and
B. R. Davies,
“Emerging targeted therapies for bladder cancer: a disease waiting for a drug,”
Cancer Metastasis Rev., 28
(3-4), 355
–367
(2009). https://doi.org/10.1007/s10555-009-9192-9 Google Scholar
W. H. Rawls, D. L. Lamm, B. A. Lowe, E. D. Crawford, M. F. Sarosdy, J. E. Montie, H. B. Grossman, and
P. T. Scardino,
“Fatal sepsis following intravesical bacillus Calmette-Guerin administration for bladder cancer,”
J. Urol., 144
(6), 1328
–1330
(1990). Google Scholar
J. A. Gonzalez, B. R. Marcol, and
M. C. Wolf,
“Complications of intravesical bacillus Calmette-Guerin: a case report,”
J. Urol., 148
(6), 1892
–1893
(1992). Google Scholar
F. W. Leebeek, R. J. Ouwendijk, A. H. Kolk, A. Dees, J. C. Meek, J. E. Nienhuis, and
A. M. Dingemans-Dumas,
“Granulomatous hepatitis caused by Bacillus Calmette-Guerin (BCG) infection after BCG bladder instillation,”
Gut, 38
(4), 616
–618
(1996). https://doi.org/10.1136/gut.38.4.616 Google Scholar
J. G. Heiner and
M. K. Terris,
“Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guerin therapy,”
Urol. Oncol., 26
(2), 137
–140
(2008). https://doi.org/10.1016/j.urolonc.2007.04.005 Google Scholar
S. Denzinger, M. Burger, B. Walter, R. Knuechel, W. Roessler, W. F. Wieland, and
T. Filbeck,
“Clinically relevant reduction in risk of recurrence of superficial bladder cancer using 5-aminolevulinic acid-induced fluorescence diagnosis: 8-year results of prospective randomized study,”
Urology, 69
(4), 675
–679
(2007). https://doi.org/10.1016/j.urology.2006.12.023 Google Scholar
I. Kausch, M. Sommerauer, F. Montorsi, A. Stenzl, D. Jacqmin, P. Jichlinski, D. Jocham, A. Ziegler, and
R. Vonthein,
“Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies,”
Eur. Urol., 57
(4), 595
–606
(2009). https://doi.org/10.1016/j.eururo.2009.11.041 Google Scholar
J. A. Witjes, J. P. Redorta, D. Jacqmin, F. Sofras, P. U. Malmstrom, C. Riedl, D. Jocham, G. Conti, F. Montorsi, H. C. Arentsen, D. Zaak, A. H. Mostafid, and
M. Babjuk,
“Hexaminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: review of the evidence and recommendations,”
Eur. Urol., 57
(4), 607
–614
(2010). https://doi.org/10.1016/j.eururo.2010.01.025 Google Scholar
S. A. Gross and
H. C. Wolfsen,
“The role of photodynamic therapy in the esophagus,”
Gastrointest. Endosc. Clin. N. Am., 20
(1), 35
–53
(2010). https://doi.org/10.1016/j.giec.2009.07.008 Google Scholar
M. S. Eljamel,
“Brain photodiagnosis (PD), fluorescence guided resection (FGR) and photodynamic therapy (PDT): past, present and future,”
Photodiagnos. Photodyn. Ther., 5
(1), 29
–35
(2008). https://doi.org/10.1016/j.pdpdt.2008.01.006 Google Scholar
J. Usuda, S. Ichinose, T. Ishizumi, H. Hayashi, K. Ohtani, S. Maehara, S. Ono, N. Kajiwara, O. Uchida, H. Tsutsui, T. Ohira, H. Kato, and
N. Ikeda,
“Management of multiple primary lung cancer in patients with centrally located early cancer lesions,”
J. Thorac. Oncol., 5
(1), 62
–68
(2010). https://doi.org/10.1097/JTO.0b013e3181c42287 Google Scholar
M. Triesscheijn, P. Baas, J. H. Schellens, and
F. A. Stewart,
“Photodynamic therapy in oncology,”
Oncologist, 11
(9), 1034
–1044
(2006). https://doi.org/10.1634/theoncologist.11-9-1034 Google Scholar
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and
Q. Peng,
“Photodynamic therapy,”
J. Natl. Cancer Inst., 90
(12), 889
–905
(1998). https://doi.org/10.1093/jnci/90.12.889 Google Scholar
M. F. Zuluaga and
N. Lange,
“Combination of photodynamic therapy with anti-cancer agents,”
Curr. Med. Chem., 15
(17), 1655
–1673
(2008). https://doi.org/10.2174/092986708784872401 Google Scholar
S. O. Gollnick, B. Owczarczak, and
P. Maier,
“Photodynamic therapy and anti-tumor immunity,”
Lasers Surg. Med., 38
(5), 509
–515
(2006). https://doi.org/10.1002/(ISSN)1096-9101 Google Scholar
M. Korbelik and
G. J. Dougherty,
“Photodynamic therapy-mediated immune response against subcutaneous mouse tumors,”
Cancer Res., 59
(8), 1941
–1946
(1999). https://doi.org/cancerres.aacrjournals.org/content/59/8/1941 Google Scholar
E. L. Larsen, L. L. Randeberg, O. A. Gederaas, C. J. Arum, A. Hjelde, C. M. Zhao, D. Chen, H. E. Krokan, and
L. O. Svaasand,
“Monitoring of hexyl 5-aminolevulinate-induced photodynamic therapy in rat bladder cancer by optical spectroscopy,”
J. Biomed. Opt., 13
(4), 044031
(2008). https://doi.org/10.1117/1.2967909 Google Scholar
C. J. Arum, E. Anderssen, K. Tommeras, S. Lundgren, D. Chen, and
C. M. Zhao,
“Gene expression profiling and pathway analysis of superficial bladder cancer in rats,”
Urology, 75
(3), 742
–749
(2010). https://doi.org/10.1016/j.urology.2009.03.008 Google Scholar
K. Hendricksen, J. Molkenboer-Kuenen, E. Oosterwijk, C. A. Hulsbergen-van de Kaa, and
J. A. Witjes,
“Evaluation of an orthotopic rat bladder urothelial cell carcinoma model by cystoscopy,”
BJU Int., 101
(7), 889
–893
(2008). https://doi.org/10.1111/bju.2008.101.issue-7 Google Scholar
S. El Khatib, J. Didelon, A. Leroux, L. Bezdetnaya, D. Notter, and
M. D'Hallewin,
“Kinetics, biodistribution and therapeutic efficacy of hexylester 5-aminolevulinate induced photodynamic therapy in an orthotopic rat bladder tumor model,”
J. Urol., 172
(5 Pt 1), 2013
–2017
(2004). https://doi.org/10.1097/01.ju.0000135816.46544.74 Google Scholar
M. Kanehisa, S. Goto, M. Furumichi, M. Tanabe, M. Hirakawa,
“KEGG for representation and analysis of molecular networks involving diseases and drugs,”
Nucl. Acids Res., 38 D355
–60
(2010). https://doi.org/10.1093/nar/gkp896 Google Scholar
M. Kanehisa, S. Goto,
“KEGG: Kyoto Encyclopaedia of Genes and Genomes,”
Nucl. Acids Res., 28 27
–30
(2000). https://doi.org/10.1093/nar/28.1.27 Google Scholar
C. J. Arum, E. Anderssen, T. Viset, Y. Kodama, S. Lundgren, D. Chen, and
C. M. Zhao,
“Cancer immunoediting from immunosurveillance to tumor escape in microvillus-formed niche: a study of syngeneic orthotopic rat bladder cancer model in comparison with human bladder cancer,”
Neoplasia, 12
(6), 434
–442
(2010). https://doi.org/10.1593/neo.91824 Google Scholar
R. Oyasu,
“Epithelial tumours of the lower urinary tract in humans and rodents,”
Food Chem. Toxicol., 33
(9), 747
–755
(1995). https://doi.org/10.1016/0278-6915(95)00042-Z Google Scholar
P. A. Oliveira, A. Colaco, P. L. De la Cruz, and
C. Lopes,
“Experimental bladder carcinogenesis-rodent models,”
Exp. Oncol., 28
(1), 2
–11
(2006). Google Scholar
N. Lange, P. Jichlinski, M. Zellweger, M. Forrer, A. Marti, L. Guillou, P. Kucera, G. Wagnieres, and
H. Van Den Bergh,
“Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: a pilot study,”
Br. J. Cancer, 80
(1-2), 185
–193
(1999). https://doi.org/10.1038/sj.bjc.6690338 Google Scholar
P. Hillemanns, X. Wang, H. Hertel, V. Andikyan, M. Hillemanns, H. Stepp, and
P. Soergel,
“Pharmacokinetics and selectivity of porphyrin synthesis after topical application of hexaminolevulinate in patients with cervical intraepithelial neoplasia,”
Am. J. Obstet. Gynecol., 198
(3), 300.e1–300.e7
(2008). https://doi.org/10.1016/j.ajog.2007.07.045 Google Scholar
S. Gronlund-Pakkanen, T. M. Pakkanen, M. Talja, V. M. Kosma, M. Ala-Opas, E. Alhava,
“The morphological changes in rat bladder after photodynamic therapy with 5-aminolaevulinic acid-induced protoporphyrin IX,”
BJU Int., 86
(1), 126
–132
(2000). https://doi.org/10.1046/j.1464-410x.2000.00718.x Google Scholar
N. Solban, I. Rizvi, and
T. Hasan,
“Targeted photodynamic therapy,”
Lasers Surg. Med., 38
(5), 522
–531
(2006). https://doi.org/10.1002/(ISSN)1096-9101 Google Scholar
A. P. Castano, P. Mroz, and
M. R. Hamblin,
“Photodynamic therapy and anti-tumour immunity,”
Nat. Rev. Cancer, 6
(7), 535
–545
(2006). https://doi.org/10.1038/nrc1894 Google Scholar
R. Hilf,
“Mitochondria are targets of photodynamic therapy,”
J. Bioenerg. Biomembr., 39
(1), 85
–89
(2007). https://doi.org/10.1007/s10863-006-9064-8 Google Scholar
T. Jung, N. Bader, and
T. Grune,
“Lipofuscin: formation, distribution, and metabolic consequences,”
Ann. N.Y. Acad. Sci., 1119 97
–111
(2007). https://doi.org/10.1196/annals.1404.008 Google Scholar
Y. Ohgari, Y. Miyata, T. Thanh Chau, S. Kitajima, Y. Adachi, and
S. Taketani,
“Quinolone compounds enhance {delta}-aminolevulinic acid (ALA)-induced accumulation of protoporphyrin IX and photosensitivity of tumor cells,”
J. Biochem.,
(2010). https://doi.org/http://www.ncbi.nlm.nih.gov/pubmed/20961864 Google Scholar
D. Kessel, M. G. Vicente, and J. J. Reiners Jr,
“Initiation of apoptosis and autophagy by photodynamic therapy,”
Lasers Surg. Med., 38
(5), 482
–488
(2006). https://doi.org/10.1002/(ISSN)1096-9101 Google Scholar
D. Kessel, J. J. Reiners Jr,
“Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage,”
Photochem. Photobiol., 83
(5), 1024
–1028
(2007). https://doi.org/10.1111/php.2007.83.issue-5 Google Scholar
D. Kessel and
N. L. Oleinick,
“Initiation of autophagy by photodynamic therapy,”
Methods Enzymol., 453 1
–16
(2009). https://doi.org/10.1016/S0076-6879(08)04001-9 Google Scholar
R. J. Sylvester, A.P.M. van der Meijden, and
D. L. Lamm,
“Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials,”
J. Urol., 168
(5), 1964
–1970
(2002). https://doi.org/10.1016/S0022-5347(05)64273-5 Google Scholar
R. F. Han and
J. G. Pan,
“Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials,”
Urology, 67
(6), 1216
–1223
(2006). https://doi.org/10.1016/j.urology.2005.12.014 Google Scholar
D. Lamm, A. Bohle, J. Palou, R. Persad, M. Brausi, M. Colombel, H. Akaza, and
R. Buckley, Per-Uno Malmstrom, Richard J. Sylvester, David E. Crawford, P-U. Malmstom, R.J. Sylvester, D.E. Crawford, M. Friedrich, S. Krege, E. Rintala, E. Solsona, S.M. Di Stasi, J. A. Witjes,
“An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin c versus bacillus calmette-guerin for non-muscle-invasive bladder cancer,”
Eur. Urol., 56 247
–256
(2009). https://doi.org/10.1016/j.eururo.2009.04.038 Google Scholar
D. Lamm, A. Bohle, J. Palou, R. Persad, M. Brausi, M. Colombel, H. Akaza, and
R. Buckley, Per-Uno Malmstrom, Richard J. Sylvester, David E. Crawford, P-U. Malmstom, R.J. Sylvester, D.E. Crawford, M. Friedrich, S. Krege, E. Rintala, E. Solsona, S.M. Di Stasi, J. A. Witjes, Eur. Urol., 57
(2), e7
–9
(2010). https://doi.org/10.1016/j.eururo.2009.10.033 Google Scholar
D. Jocham, H. Stepp, and
R. Waidelich,
“Photodynamic diagnosis in urology: state-of-the-art,”
Eur. Urol., 53
(6), 1138
–1148
(2008). https://doi.org/10.1016/j.eururo.2007.11.048 Google Scholar
D. Jocham, J. von Wietersheim, H. Pfluger, H. Steiner, C. Doehn, H. Buttner, A. Bohle, and
I. Kausch,
“BCG versus photodynamic therapy (PDT) for nonmuscle invasive bladder cancer-a multicentre clinical phase III study,”
Aktuelle Urol., 40
(2), 91
–99
(2009). https://doi.org/10.1055/s-0028-1098741 Google Scholar
|